Simultaneous Electrochemical Determination of Paracetamol and Folic Acid at Pregabalin Modified Carbon Paste Electrode: A Cyclic Voltammetric Study
Received: 07-Mar-2017 / Accepted Date: 07-Mar-2018 / Published Date: 12-Mar-2018 DOI: 10.4172/2469-9764.1000124
Abstract
In this study, the electrochemical sensor was fabricated for the detection of paracetamol and folic acid by modifying the carbon paste electrode using Pregabalin drug. The fabricated carbon paste electrode was characterized by cyclic voltammetry; the result shows an excellent enhanced elctrocatalytic activity of the modified electrode towards paracetamol and folic acid with combined selectivity and sensitivity. Several kinetic parameters such as area of electrode, nature of electrode process and limit of detection were calculated. The modified carbon paste electrode can successfully use for the determination of paracetamol and folic acid individually and collectively. The limit of detection was found to be 0.093 × 10-5 M, 0.201 × 10-5 M for paracetamol and folic acid respectively. The fabricated electrode can successfully have applied for the detection of paracetamol in pharmaceutical preparations.
Keywords: Paracetamol; Folic acid; Pregabalin; Cyclic voltammetry; Differential pulse voltammetry
Introduction
Fabrications of electrochemical sensors are gaining more prominence in recent years, since they satisfy many requirements such as sensitivity, selectivity, specificity, quick response and simple fabrication. The electrochemical sensors play a vital role in the detection of neurotransmitters and drug analysis. The above therapeutic levels of drugs cause a wide variety of adverse effects, so their fast detection method make significant contribution in providing early treatment to patients and helps in correct to diagnosis of diseases. Paracetamol is also known as acetaminophen, is a medication used to treat pain and fever. It is a class of antipyretics and analgesics drug. Paracetamol is a white crystalline powder with melting point of 169°C it is a weak acid having pKa value of 9.5. It is an orally administered drug and rapidly gets absorbed in the gastrointestinal tract, gets distributed soon and its metabolites are excreted through urine [1]. Paracetamol effectively reduces body temperature in fever by inhibiting the sedating hypothalamic heat regulating centre [2]. It is a non-carcinogenic drug used in osteoarthritis treatment and ovarian cancer [3]. Paracetamol is suited to those patients who are sensitive to aspirin [4], in medication for cold and influenza paracetamol is the foremost ingredient [5,6]. However, overdose of paracetamol causes liver and kidney damage and it may lead to death [7]. Risk gets enhanced by alcohol consumption [8,9], hence the detection of Paracetamol is very important. Paracetamol contains phenolic hydroxyl group, which is electrochemically active and can be easily oxidized. Numerous methods are employed for the determination of paracetamol which includes high performance liquid chromatography (HPLC) spectrophotometry and capillary electrophoresis [10-12].
Folic acid (FN-[p-{[(2-amino-4-hydroxy-6-pteridinyl) methyl] amino} benzoyl]-l glutamic acid) also known as vitamin M and folacin or folate, which comes under Vitamin B category. It is water-soluble vitamin, found in most of the vegetables. Folate is found in vegemite or marmite with an average part (5 g) containing 100 μg and it is also synthesized by bacteria. It plays considerably an important role in human health by participating in cell division, growth, gene expression and nucleotide synthesis. Deficiency of folic acid is a common cause for anaemia and also increases heart attack and stroke [13]. Folic acid is one of the important nutrient for women especially planning for pregnancy. The lack of folic acid during pregnancy is a marker of neural tube defects, sufficient intake of folate during preconception helps to protect against a number of congenital malformations [14,15]. Hence, the analysis for folic acid is of significant importance. The Recommended Dietary Allowance (RDA) suggests 600–800 μg of folate for pregnant women and 400 μg for non-pregnant women, and also folic acid is very essential for men who are planning on fathering children, to reduce birth defect risks [13]. Various methods are reported for the detection of folic acid including HPLC, colorimetry, microbial method, spectrophotometry and flow inoculation, capillary electrophoresis and electrochemical techniques [16-19]. Folic acid, the synthetic form of B vitamin folate works primarily in the brain and nervous system and it is necessary for the production of Norepinephrine and serotonin in the nervous system. Ingestion of few substances like aspirin, ibuprofen and paracetamol can inhibit the absorption of folic acid by the body. Paracetamol and other antiinflammatory medications when taken for long increase the need for folic acid [20]. Hence conventional method is necessary for the direct determination of paracetamol and folic acid. Electrochemical methods have certain advantages like high sensitivity, selectivity, and quick response, cost effective and simplicity. The use of carbon paste as an electrode was initially reported in 1958 by Adams, carbon paste electrodes are widely used in electroanalysis due to their low cost, chemical inertness and good electron transfer. It is a particular type of assorted carbon electrode consisting of combination of graphite powder and silicon oil as a binder. Electrocatalytic activity of the carbon paste electrode could be improved by modify the carbon paste electrode. These modifiers improve electrode kinetics and impart selectivity and sensitivity. The modification of traditional electrodes was done by using various materials like organic polymers dyes surfactants transition metal complexes metal and metal oxide nanoparticles [21-26]. The major significant property of the customized carbon paste electrodes are they have ability to catalyze the electrode process through significant decrease of over potential in respect of relatively selective interaction of the electron mediator with the target analyte in a coordinated fashion. The adapted carbon paste electrodes significantly improve the selectivity in electroanalytical methods [27,28].
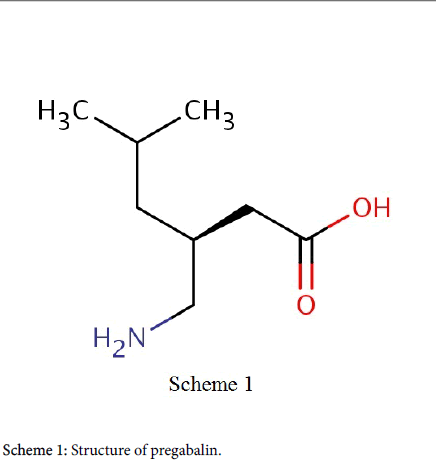
Pregabalin is a class of anti-epileptic drug. It is being used in the cure of diabetic neuropathy and post-herpetic neuralgia [29]. Preclinical and clinical study has proved the efficiency of pregabalin in supervision the neuropathic twinge. Clinical studies have too exposed the worth and magnitude dependent effect of pregabalin moreover as monotherapy or in amalgamation with analgesics in relieves pain and related symptom [30,31]. Structure of pregabalin was shown in Scheme 1.
The aspire of present work was to develop a sensitive and simple sensor; the freshly fabricated pregabalin modified carbon paste electrode was successfully employed for the determination of paracetamol and folic acid with low detection limit, good selectivity.
Experimental Section
Apparatus and chemicals
The electrochemical experiment be performed using a model CHI-660c (CH Instrument-660 electrochemical terminal). The entire experiment was carried out with a conformist three electrode cell. The bare carbon paste electrode (BCPE) Pregabalin adapted carbon paste electrode (MCPE), model calomel electrode and platinum wire were used as operational, reference, and counter electrode, respectively. Pregabalin was got from Srini pharmaceuticals ltd (AP, India), disodium hydrogen phosphate (Na2HPO4), sodium dihydrogen orthophosphate (Na2HPO4), silicone oil, and paracetamol were procured from Himedia Chemicals. Folic acid, graphite powder and NaOH were buying from Merck and all were of analytical grade quality. Folic acid was prepared in 0.1 M NaOH solution. Paracetamol, potassium ferro cyanide and KCl solutions were prepared using double distilled water.
Preparation of electrode
The Pregabalin customized carbon paste electrode was equipped by manual grinding 3 mg of Pregabalin drug, graphite powder and silicon oil at a ratio of 70:30 (w/w) in a mortar using pestle until a homogeneous paste was attained. The paste was then tightly packed in to the hole of a household electrode, and after that refined the exterior by rubbing on a weighing paper to get homogeneous glittery emergence. The bare carbon paste electrode was equipped in the similar method devoid of adding Pregabalin drug to the carbon paste.
Results and Discussion
Outcome of modifier concentration on the electrochemical response of paracetamol
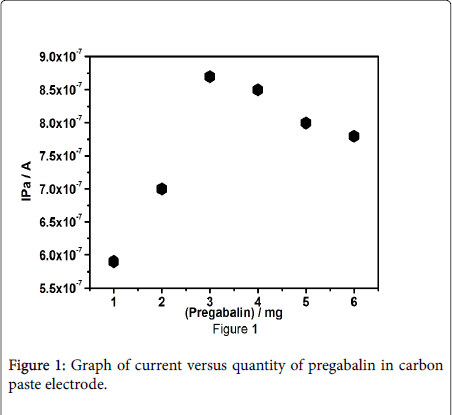
The concentration of the modifying species is extremely significant part in the elctrocatalytic nature of the modified carbon paste electrode. The core purpose for the insertion of modifying species to the bare carbon paste electrode was to increase the efficiency of elctrocatalytic activity. Pregabalin modified carbon paste electrode was prepared by the addition of different measure of pregabalin to the carbon paste electrode (Figure 1). The concentration of pregabalin was increased from 1 to 6 mg, the pregabalin modified carbon paste electrode with 3 mg demonstrate extreme anodic peak current signal, that's why 3 mg Pregabalin was selected to fabricate the electrode, the fabricated Pregabalin electrode was employed for further analysis of paracetamol.
Electrochemical performance of potassium ferrocyanide at Pregabalin modified carbon paste electrode
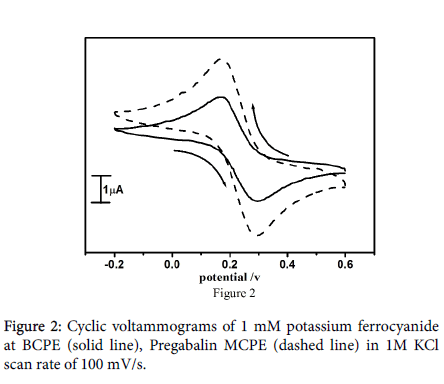
Potassium ferrocyanide is forever employed as an electrochemical uncertainty to assess the electrochemical properties of the fabricated carbon paste electrode. The electrochemical characterization of pregabalin modified carbon paste electrode was carried out by using standard potassium ferrocyanide to evaluate the electrocatalytic aptitude of the pregabalin modified electrode and to compute the area of the modified electrode. The cyclic voltammograms at Pregabalin modified carbon paste electrode in 1mM potassium ferrocyanide and 1M KCl as a supporting electrolyte with scan rate of 100 mV/s as shown in Figure 2.
The voltammetric response was enhanced at the Pregabalin modified carbon paste electrode as contrast to the bare carbon paste electrode (solid line) which is shown by the reinforcement of both anodic and cathodic peak current (dashed line). The surface area accessible for the electron transfer process was calculated by using Randles-Sevick’s equation (1) [32,33].
Ip=2.69 × 105 n3/2 A D1/2 C0 ν1/2 (1)
where, Ip is the peak current in A. C0 is the concentration of the electroactive species (mol cm3), n is the number of electrons exchanged, D is the diffusion coefficient in cm2 S-1, and υ is the scan rate (Vs-1), A is the electroactive area (cm2). The surface area of the bare carbon paste electrode and modified carbon paste electrode were found to be 0.0240 cm2 and 0.0280 cm2 respectively.
Electrochemical behaviour of paracetamol and folic acid at pregabalin adapted carbon paste electrode
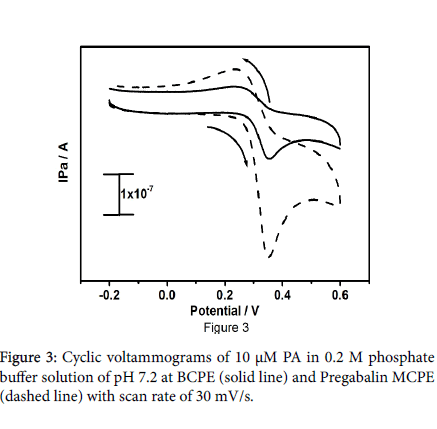
Figure 3 symbolize the cyclic voltammetric responses for the oxidation of 10 μM paracetamol at bare carbon paste electrode (solid line) and Pregabalin modified carbon paste electrode (dashed line) with a scan rate of 30 mV/s in 0.2 M PBS at pH 7.2. The pregabalin adapted carbon paste electrode illustrate considerably better redox peak current of paracetamol as contrast to the bare carbon paste electrode, the paracetamol at pregabalin modified carbon paste electrode point out the enhanced redox kinetics at modified carbon paste electrode.
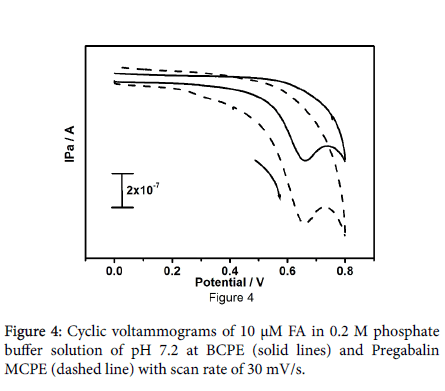
The redox peak current of paracetamol at pregabalin modified carbon paste electrode be doubled as contrast to the bare carbon paste electrode, and it attest increased surface area of the pregabalin modified electrode. The result provides more evidence for that the pregabalin drug induces the electrocatalytic activity to the modified carbon paste electrode. The electrochemical response of folic acid was examined by cyclic voltammetry at pregabalin modified carbon paste electrode. Figure 4 shows the cyclic voltammograms of 10 μM folic acid in 0.2 M PBS at pH 7.2.
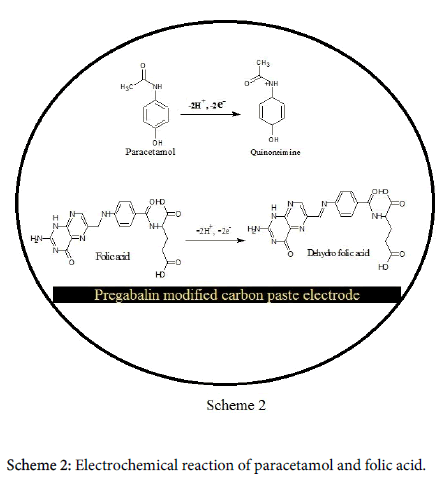
The bare carbon paste electrode (solid line) and pregabalin modified carbon paste electrode (dashed line). At the bare carbon paste electrode oxidation peak potential arise at 661 mV and at 659 mV for modified pregabalin carbon paste electrode beneath identical conditions. The anodic peak current enhanced to a great extent at pregabalin modified carbon paste electrode and it offer a clear indication for that the pregabalin drug enhanced the electrocatalytic effect of the modified electrode towards folic acid. The electrochemical mechanism involved in the paracetamol and folic acid as shown in Scheme 2 [34].
Effect of sweep rate
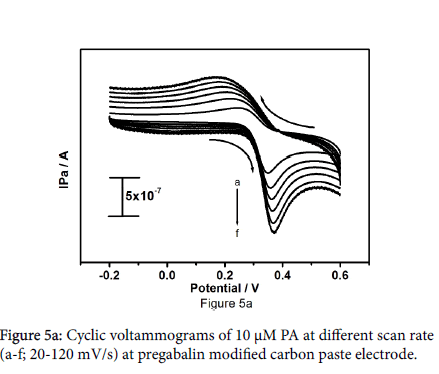
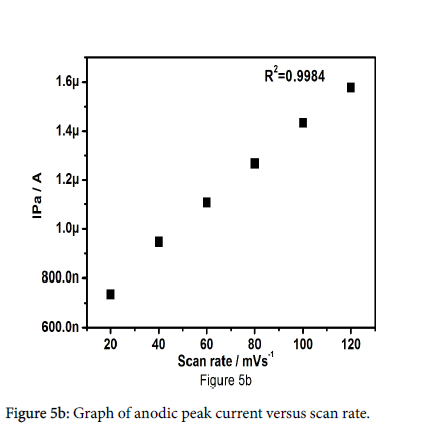
The effect of diverse sweep rates for the oxidation of paracetamol was studied to inspect the kinetics of electrode reactions. Figure 5a shows the cyclic voltammograms of 10 μM paracetamol in 0.2 M PBS at pregabalin modified carbon paste electrode. The surveillance explains that the anodic peak current and cathodic peak current progressively increases with increase in sweep rate from 20-120 mV/s. The anodic peak current increases from 7.36 × 10-7A to 1.57 × 10-6 A with increase in sweep rate from 20-120 mV/s, anodic peak potential somewhat change towards positive direction and cathodic peak potential shifts towards negative direction. The plot of anodic peak current (Ipa) versus sweep rate as shown in Figure 5b the correlation coefficient was found to be 0.9990, which point out that the electron transfer reaction of paracetamol at pregabalin modified carbon paste electrode was adsorption guarded process.
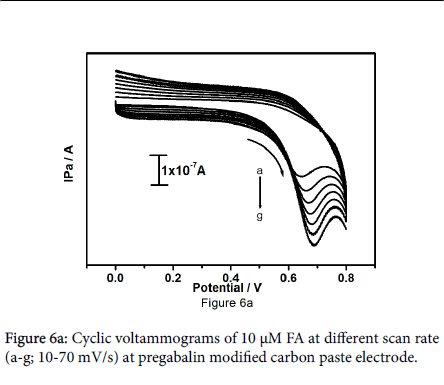
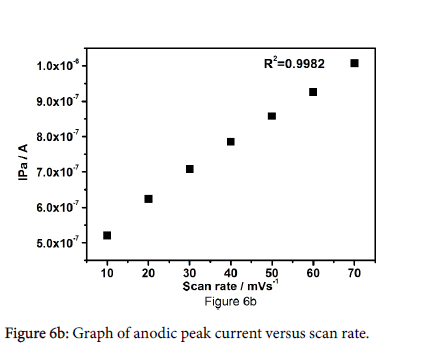
Figure 6a shows the cyclic voltammograms of 10 μM FA at pregabalin modified carbon paste electrode at different sweep rates in the range of 10-70 mV/s. The results show that with increase in sweep rate the oxidation peak current gets increased steadily. The positive shift was observed for Epa. The plot of anodic peak current versus sweep rate as shown in Figure 6b. The graph was attaining with good linearity between sweep rate and anodic peak current with correlation coefficient 0.9991, which indicates that the electrode reaction process was adsorption controlled.
Effect of concentration of paracetamol and folic acid
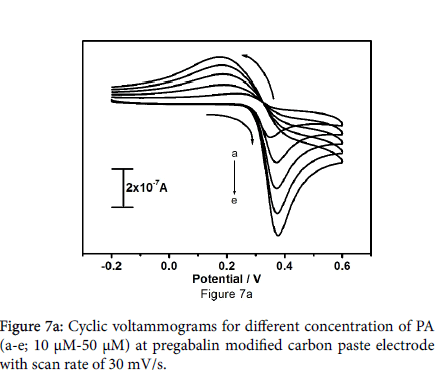
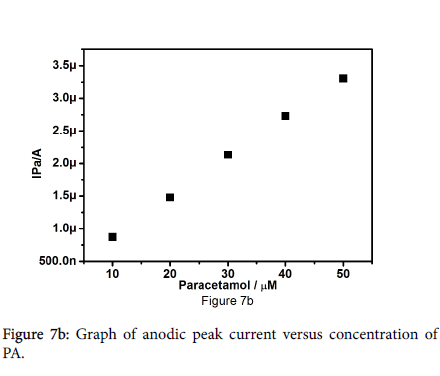
The electrocatalytic behaviour of pregabalin modified carbon paste electrode was studied by altering the concentration of paracetamol. Figure 7a shows the cyclic voltammograms of 10 to 50 μM paracetamol in 0.2 M PBS at pH 7.2 with scan rate of 30 mV/s. It reveals that the anodic peak current and cathodic peak current increases gradually with increasing the concentration of paracetamol, Positive shifts for Epa and negative shifts for Epc were observed. Figure 7b shows the graph of anodic peak current versus concentration of paracetamol, plot demonstrates the linear relationship of anodic peak current and different concentration of paracetamol with correlation coefficient of 0.9997.
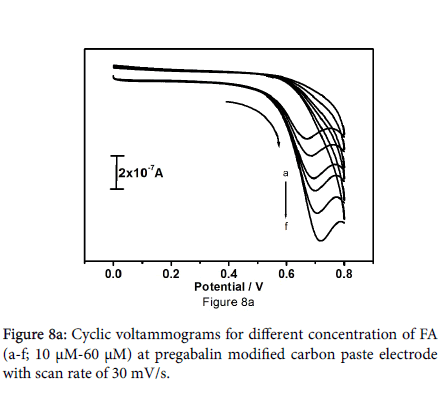
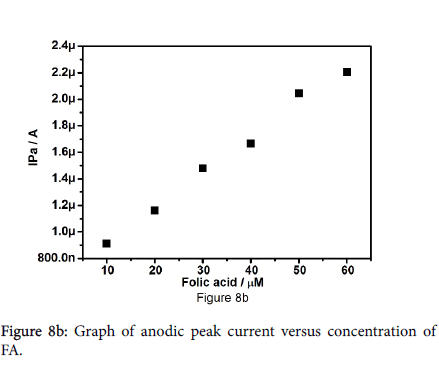
Figure 8a shows the cyclic voltammograms for different concentrations of folic acid from 10 to 60 μM at pregabalin modified carbon paste electrode in 0.2 M PBS at pH 7.2 with scan rate of 30 mV/s. The results reveal that anodic peak current increases linearly with increase in the concentration of folic acid along with anodic potential shifts towards positive side. Figure 8b represents the plot of anodic peak current versus different concentration of folic acid. Plot shows that the concentration of folic acid is proportional to anodic peak current; Linearity was established between the anodic peak current and concentration of folic acid with correlation coefficient of 0.9962.
Effect of pH
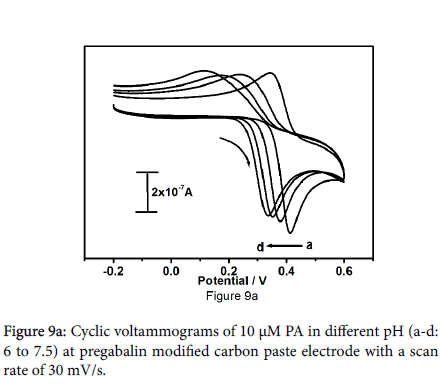
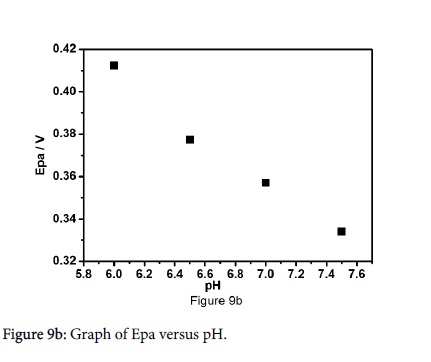
The pH of the supporting electrolyte plays a main role in electrochemical oxidation of paracetamol, that's why the influence of peak current and peak potential was cautiously examined over pH range from 6 to 7.5. Figure 9a shows the cyclic voltammograms of 10 μM paracetamol at different pH from 6 to 7.5 in 0.2 M PBS with a scan rate of 30 mV/s. Habitually redox potential shift towards the lower potential side at higher pH, the negative shifts for anodic peak potential were observed with increase in pH value due to rate of oxidation of paracetamol is high at higher pH values. The potential diagram was constructed by plotting a graph of anodic peak potential values as function of pH as shown in Figure 9b. The slope of Epa versus pH is 51.14 mV/pH is compared to the theoretical value of 59 mV, which indicates that the number of protons and electrons involved in the electrochemical reaction is equal [35-37].
Concurrent determination of paracetamol and folic acid
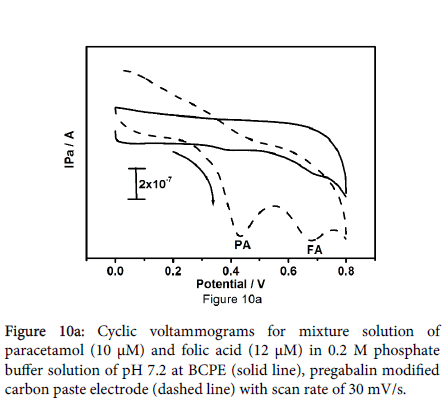
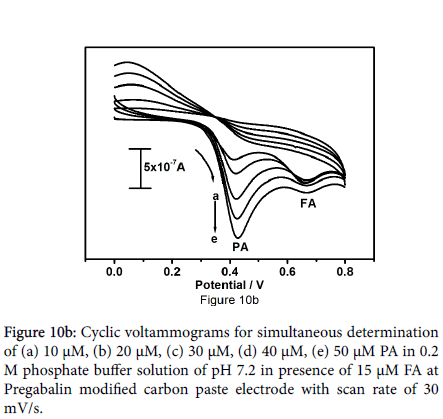
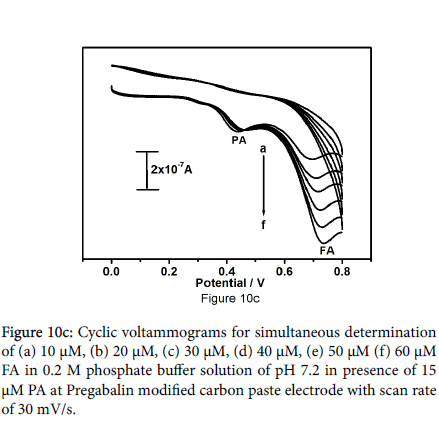
Figure 10a symbolizes the cyclic voltammograms for combination of paracetamol (10 μM) and folic acid (12 μM) at the bare carbon paste electrode (solid line) pregabalin modified carbon paste electrode (dashed line). The peaks obtained at bare carbon paste electrode were broad, less rational for paracetamol and folic acid though; pregabalin modified carbon paste electrode results two well distinct sharp peaks with superior current as compared to the bare carbon paste electrode with anodic peak potentials 433 mV and 680 mV for paracetamol and folic acid respectively. It is very important to characterize the sensitivity and selectivity of the fabricated electrode; as a result, the electrochemical performance of modified electrode was studied by altering the concentration of one species and keeping the concentration of other species constant. Concurrent determination of paracetamol and folic acid at pregabalin modified carbon paste electrode has been achieved by using cyclic voltammetry. From the Figure 10b it can be observed that the peak current of paracetamol was proportional to its concentration, which was increased from 10 to 50 μM at constant concentration of folic acid (15 μM FA). The varying concentration of paracetamol did not show any significant influence on the peak current of folic acid. Correspondingly Figure 10c demonstrates oxidation peak current of folic acid increased linearly with increase in concentration of folic acid from 10 to 60 μM in presence of 15 μM paracetamol as steady.
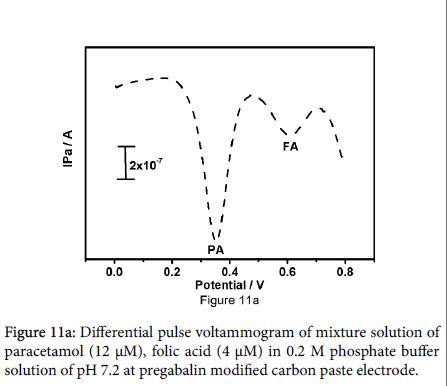
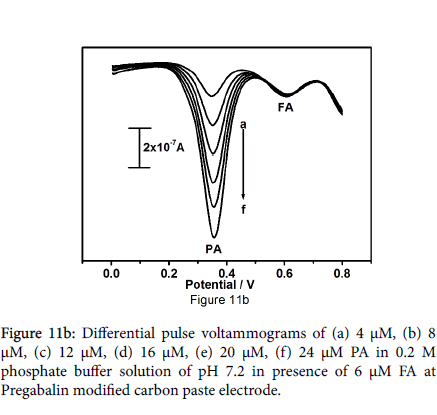
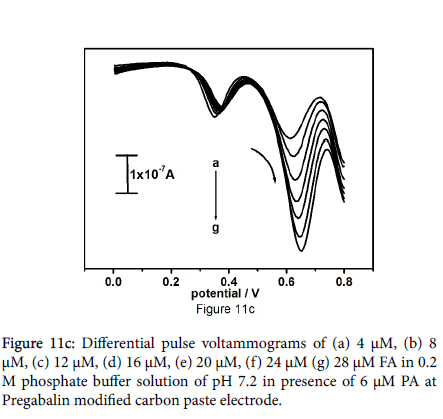
Outcome of the study marked the selectivity of fabricated electrode. Differential pulse voltammetric technique bid a great deal of interest because of their high current sensitivity and better resolution, The DPV result shows two well distinguished sharp peaks at peak potential 353 and 602 mV analogous to the oxidation of paracetamol and folic acid respectively in Figure 11a. The DPV results showed more peak partition between the paracetamol and folic acid along with welldefined sharp peaks as compared to the peaks obtained by CV technique. Figure 11b be evidence for that the oxidation peak current is dependent on the concentration of paracetamol, paracetamol concentration was varied from 4 to 24 μM with fixed concentration of folic acid (6 μM). Figure 11c shows degree of differential pulse voltammograms for 4 μM to 28 μM folic acid in presence of 6 μM paracetamol in 0.2 M phosphate buffer solution at pH 7.2.
The peak current was amplified linearly with increase in concentration of folic acid; increase in concentration of folic acid does not explain any significant effect on the peak current and peak potential of paracetamol. These points specify that the oxidation of paracetamol and folic acid are independent, therefore concurrent determination of paracetamol and folic acid is achievable without any intrusion. The overall studies disclose that drugs can also use widely as modifiers for electrochemical sensors for the analysis of drugs and neurotransmitters. The limit of detection the fabricated electrode was evaluated by using equation (2) [38] where S is the standard deviation and M is the slope obtained from the calibration plot. LOD=3S/M (2). The limit of detection was found to be 0.093 × 10-5M for paracetamol and 0.201 × 10-5M for folic acid (Table 1).
| Sl.No | Electrode | Detection limit | Techniques | Reference |
|---|---|---|---|---|
| 1 | MCPE/PR | 0.53 x10-6 M | DPV | [39] |
| 2 | PEDOT/GCE | 1.13 x10-6 M | DPV | [40] |
| 3 | Bi2O3/GCE | 0.20 x10-6 M | DPV | [41] |
| 4 | Dipyrromethene–Cu(II) monolayer modified gold electrode | 1.2x 10-4 M | DPV | [42] |
| 5 | Pregabalin/MCPE | 0.093 x10-5 M | DPV | This work |
Table 1: Comparison of limit of detection of pregabalin modified carbon paste electrode with other working electrodes.
Detection of paracetamol in pharmaceutical dosage
The applicability of the fabricated electrode was appraised by analyzing the commercially available paracetamol tablet by standard addition method. The electrochemical response at the fabricated pregabalin electrode for the spiked standard solution of paracetamol were recorded and calculated %recovery, the results were tabulated in Table 2. The obtained results were acceptable and screening that the fabricated pregabalin modified carbon paste electrode might be efficiently used for the determination paracetamol in pharmaceutical preparations, with revival in the range from 97 -102%.
| Sample | Added (M) | Found | Recovery % |
|---|---|---|---|
| 1 | 0.1x 10-4 | 0.972x 10-4 | 97.28 |
| 2 | 0.2x 10-4 | 0.198x 10-4 | 99.18 |
| 3 | 0.3x 10-4 | 0.306x 10-4 | 102.2 |
| 4 | 0.4x 10-4 | 0.406x 10-4 | 101.7 |
| 5 | 0.5x 10-4 | 0.499x 10-4 | 99.96 |
Table 2: Detection of paracetamol in pharmaceutical dosage (n= 3).
Conclusion
This present work reveals the successful application of pregabalin drug modified carbon paste electrode for the selective and sensitive determination of paracetamol and folic acid. The fabricated electrode has good electrocatalytic ability, sensitivity, selectivity, easy preparation along with low detection limit. The fabricated electrode could fruitfully pertain for the determination of paracetamol in pharmaceutical preparations. The pleasing results of fabricated pregabalin drug modified carbon paste electrode will develop its application towards electrochemical analysis of other drugs and neurotransmitters.
References
- Fan Y, Liu JH, Lu HT, Zhang Q (2011) Electrochemical behaviour and voltammetric determination of paracetamol on Nafion/TiO2–graphene modified glassy carbon electrode. Colloids Surf B 85: 289-292.
- Muralidharan B, Gopu G, Vedhi C, Manisankar P (2009) Determination of analgesics in pharmaceutical formulations and urine samples using nano polypyrrole modified glassy carbon electrode. J Appl Electrochem 39: 1177-1184.
- Atta NF, Galal A, Abu-Attian FM, Azab SM (2011) Simultaneous determination of paracetamol and neurotransmitters in biological fluids using a carbon paste sensor modified with gold nanoparticles. J Mater Chem 21:13015-13024.
- Clayton BD, Stock YN (2001) Basic pharmacology for nurses. Mosby Inc., Harcourt Health Sciences Company, St. Louis. Int J Electrochem Sci.
- Shang Guan X, Zhang H, Zheng J (2008) Electrochemical behaviour and differential pulse voltammetric determination of paracetamol at a carbon ionic liquid electrode. Anal Bioanal Chem 391: 1049-1055.
- Atta NF, Galal A, Azab SM (2011) Electrochemical Determination of Paracetamol Using Gold Nanoparticles – Application in Tablets and Human Fluids. Int J Electrochem Sci 6: 5082-5096.
- Daly FF, Fountain JS, Murray L, Graudins A, Buckley NA (2008) Guidelines for the management of paracetamol poisoning in Australia and New Zealand-explanation and elaboration. Med J Aust 188: 296-301.
- Larson AM, Polson J, Fontana RJ (2005) Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 42: 1364-1372.
- Bertolini A, Ferrari A, Ottani A, Guerzoni S, Tacchi R, et al. (2006) Paracetamol: new vistas of an old drug. CNS Drug Rev 12: 250-275.
- Ishii Y, Iijima M, Umemura T, Nishikawa A, Iwasaki Y, et al. (2006) Determination of nitrotyrosine and tyrosine by high-performance liquid chromatography with tandem mass spectrometry and immunohistochemical analysis in livers of mice administered acetaminophen. J Pharm Biomed Anal 41: 1325-1331.
- Hanaee J (1997) Simultaneous determination of acetaminophen and codeine in pharmaceutical preparations by derivative spectrophotometry. Pharm Acta Helv 72: 239-241.
- Chu Q, Jiang L, Tian X, Ye J (2008) Rapid determination of acetaminophen and p-aminophenol in pharmaceutical formulations using miniaturized capillary electrophoresis with amperometric detection. Anal Chim Acta 606: 246-251.
- Hoegger D, Morier P, Vollet C, Heini D, Reymond F, et al. (2007) Disposable microfluidic ELISA for the rapid determination of folic acid content in food products. Anal Bioanal Chem 387: 267-275.
- Milunsky A, Jick H, Jick SS, Bruell CL, MacLaughlin DS, et al. (1989)Â Multivitamin/folic acid supplementation in early pregnancy reduces the prevalence of neural tube defects. J Am Med Assoc 262: 2847-2852.
- Mulinare J, Cordero JF, Erickson JD, Berry RJ (1988) Periconceptional Use of Multivitamins and the Occurrence of Neural Tube Defects. J Am Med Assoc 260: 3141-3145.
- Mazloum-Ardakani M, Beitollahi H, Sheikh Mohseni MA, Benvidi A, Naeimi H, et al. (2010) Simultaneous determination of epinephrine and acetaminophen concentrations using a novel carbon paste electrode prepared with 2,2′- [1,2] butanediylbis (nitriloethylidyne)]-bis-hydroquinone and TiO2nanoparticles. Colloids Surf B 76: 82-87.
- Ali Ensafi A, Karimi-Maleh H (2010) Modified multiwall carbon nanotubes paste electrode as a sensor for simultaneous determination of 6-thioguanine and folic acid using ferrocenedicarboxylic acid as a mediator. J Electroanal Chem 640: 75-83.
- Beitollahi H, MazloumArdakani M, Ganjipour B, Naeimi H (2008) Novel 2,2′-[1,2-ethanediylbis(nitriloethylidyne)]-bis-hydroquinone double-wall carbon nanotube paste electrode for simultaneous determination of epinephrine, uric acid and folic acid. Biosens Bioelectron 24: 362-368.
- Zhaoa SL, Yuan HY, Xie C, Xiao D (2006) Determination of folic acid by capillary electrophoresis with chemiluminescence detection. J Chromatogr A 1107: 290-293.
- Paul RTP, McDonnell AP, Kelly CB (2004) Folic acid: neurochemistry, metabolism and relationship to depression. Hum Psychopharmacol Clin Exp 19: 477-488.
- Bahloul A, Nessark B, Habelhames F, Julien CM (2011) Preparation and characterization of polybithiophene/β-MnO2 composite electrode for oxygen reduction. Ionics 17: 239-246.
- Thiemann S, Hartung R, Guth U, Schönauer U (1996) Chemical modifications of au-electrodes on YSZ and their influence on the non-Nernstian behavior. Ionics 2: 463-467.
- Forzani ES, Rivas GA, Solis VM (1995) Amperometric determination of dopamine on an enzymatically modified carbon paste electrode. J Electroanal Chem 382: 33-40.
- Nasri Z, Shams E (2009) Application of silica gel as an effective modifier for the voltammetric determination of dopamine in the presence of ascorbic acid and uric acid. Electrochim Acta 54: 7416-7421.
- Prasad BB, Srivastava S, Tiwari K, Sharma PS (2009) Trace-level sensing of dopamine in real samples using molecularly imprinted polymer-sensor. Biochem Eng J 44: 232-239.
- Raj CR, Okajima T, Ohsaka T (2003) Gold nanoparticle arrays for the voltammetric sensing of dopamine. J Electroanal Chem 543: 127-133.
- Wang SF, Xie F, Hu RF (2007) Carbon-coated nickel magnetic nanoparticles modified electrodes as a sensor for determination of acetaminophen. Sens Actuators B Chem 123: 495-500.
- Wan QJ, Wang XW, Yu F, Wang XX, Yang NJ (2009) Poly (taurine)/MWNT-modified glassy carbon electrodes for the detection of acetaminophen. J Appl Electrochem 39: 785-790.
- Wang J, Martinez T, Yaniv DR, McCornick L (1990) Characterization of the microdistribution of conductive and insulating regions of carbon paste electrodes with scanning tunneling microscopy. J Electroanal Chem 286: 265-272.
- Rice ME, Galus Z, Adams RN (1983) Graphite paste electrodes: Effects of paste composition and surface states on electron-transfer rates. J Electroanal Chem 143: 89-102.
- Tassone DM, Boyce E, Guyer J, Nuzum D (2007) Pregabalin: a novel gamma-aminobutyric acid analogue in the treatment of neuropathic pain, partial-onset seizures, and anxiety disorders. Clin Ther 29: 26-48.Â
- Pacios M, Valle MD, Bartroli J, Esplandiu MJ (2008) Electrochemical behavior of rigid carbon nanotube composite electrodes. J Electroanal Chem 619:Â 117-124.
- Salinas-Torres D, Huerta F, Montilla F, Morallon E (2011) Study on electroactive and elctrocatalytic surfaces of single walled carbon nanotube-modified electrodes. Electrochim Acta 56: 2464-2470.
- Arvand M, Dehsaraei M (2013) A simple and efficient electrochemical sensor for folic acid determination in human blood plasma based on gold nanoparticles–modified carbon paste electrode. Mater Sci Eng C 33: 3474-3480.
- Chandra U, Kumara Swamy BE, Gilbert O, Sherigara BS (2010) Voltammetric resolution of dopamine in the presence of ascorbic acid and uric acid at poly (calmagite) film coated carbon paste electrode. Electrochim Acta 55: 7166-7174.
- Adam RN (1996) Electrochemistry at Solid Electrodes. Marcel Dekker, New York 73: 1098.
- Chandra U, Kumara Swamy BE, Gilbert O, Pandurangachar M, Sherigara BS (2009) Voltammetric Resolution of Dopamine in presence of Ascorbic Acid at Polyvinyl Alcohol Modified Carbon Paste Electrode. Int J Electrochem Sci 4:1479-1488.
- Kutluay A, Aslanoglu M (2013) Modification of electrodes using conductive porous layers to confer selectivity for the voltammetric detection of paracetamol in the presence of ascorbic acid, dopamine and uric acid. Sensors and Actuators B 185: 398-404.
- Chandra P, Son NX, Noh HB, Goyal RN, Shim YB (2013) Investigation on the down regulation of dopamine by acetaminophen administration based on their simultaneous determination in urine. Biosens Bioelectron 39: 139-144.
- Mehretie S, Admassie S, Hunde T, Tessema M, Solomon T (2011) Simultaneous determination of N-acetyl-p-aminophenol and p-aminophenol with poly (3,4- ethylenedioxythiophene) modified glassy carbon electrode. Talanta 85: 1376-1382.
- Umasankar Y, Unnikrishnan B, Chen SM, Ting TW (2012) Effective Determination of Acetaminophen Present in Pharmaceutical Drug Using Functionalized Multi-Walled Carbon Nanotube Film. Int J Electrochem Sci 7: 484-498.
- Saraswathyamma B, Grzybowska I, Orlewska C, Radecki J, Dehaen W, et al. (2008) Electroactive Dipyrromethene-Cu(II) Monolayers Deposited onto Gold Electrodes for Voltammetric Determination of Paracetamol. Electroanalysis 20: 2317-2323.
Citation: Tanuja SB, Kumara Swamy BE and Vasantakumar Pai K (2018) Simultaneous Electrochemical Determination of Paracetamol and Folic Acid at Pregabalin Modified Carbon Paste Electrode: A Cyclic Voltammetric Study. Ind Chem 4:123. DOI: 10.4172/2469-9764.1000124
Copyright: © 2018 Tanuja SB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 8236
- [From(publication date): 0-2018 - Dec 20, 2025]
- Breakdown by view type
- HTML page views: 6955
- PDF downloads: 1281