Skeletal Muscle Metastases Arising from Renal Cell Carcinoma in a 58-Year Old Male: A Case Report
Received: 25-Jun-2020 / Accepted Date: 15-Jul-2020 / Published Date: 25-Jul-2020 DOI: 10.4172/2472-016X.1000139
Abstract
Purpose: Renal cell carcinoma (RCC) is the most common malignant kidney tumor, commonly metastasizing to the lung, lymph nodes, bones, and brain. Here, we present a rare case of renal cell skeletal muscle metastases (SMM), accounting for only <1% of all RCC metastases.
Methods: This is a descriptive report on the clinical course, diagnostic investigations, and surgical treatment of a case of SMM in a patient previously diagnosed with RCC.
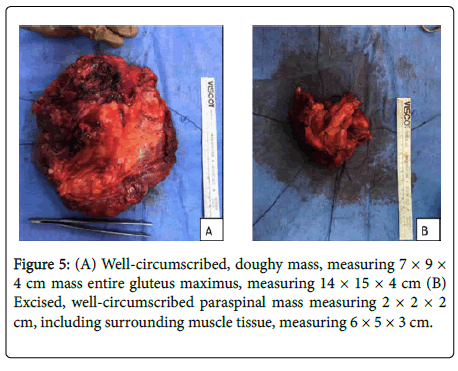
Results: This is a 58-year old male who previously underwent radical nephrectomy for RCC, presenting with a 5- month history of a rapidly enlarging left gluteal mass. The mass was confirmed to be renal clear cell metastasis via percutaneous biopsy. On magnetic resonance imaging (MRI), two heterogeneously enhancing lesions in the left gluteal muscle and right paralumbar muscles at the level of L4 and L5 were noted. Positron-emitted tomography (PET) scan confirmed no other metastases. He underwent wide excision of the right paraspinal mass and buttockectomy for the left gluteal mass.
Conclusion: SMM in RCC is rare, thus tissue diagnosis and imaging is deemed necessary to rule out any other primary sarcoma. In these cases, patients may benefit from metastasectomy. Regular follow up and surveillance is recommended for these patients to rule out recurrence.
Keywords: Renal cell carcinoma; Metatasis; Muscle metastasis; Extraskeletal metastasis; Buttockectomy
Introduction
Renal cell carcinoma is the most common form of kidney malignancy in adults. It is derived from the lining of the proximal convoluted tubules and is usually hypervascularized. It is the 14th most common malignancy worldwide, with an overall prevalence of 2%-3% of new cases per year. Its incidence is at 15 per 100,000, and is more common in males than in females [1].
It usually has an unpredictable metastatic pattern despite undergoing curative nephrectomy. The most common sites of renal cell carcinoma metastases are the lungs, lymph nodes, bones, liver and brain. Skeletal muscle metastases in renal cell carcinoma are considered rare, accounting for<1% of metastases [1]. In documented cases, they have been detected several years after radical nephrectomy [2].
We describe a case of skeletal muscle metastases of RCC in a male patient 3 years after undergoing radical nephrectomy.
Case Report
This is a case of a 58-year old male who previously diagnosed with Stage IV renal cell carcinoma who underwent radical nephrectomy, right, and right hemicolectomy for gastrointestinal metastatic disease 3 years prior to consult. He also finished 1 cycle of chemotherapy and 5 cycles of immunotherapy 1 year prior to admission. Patient underwent regular follow-ups for the past 3 years and was declared disease-free.
He consulted due to left gluteal discomfort since 5 months prior to admission. He described the pain as intermittent, aching pain, worse when sitting and with direct pressure. He also noted a slowlyenlarging, minimally tender, palpable mass on the superolateral aspect of the left gluteal area. This prompted consult 1 month prior to admission.
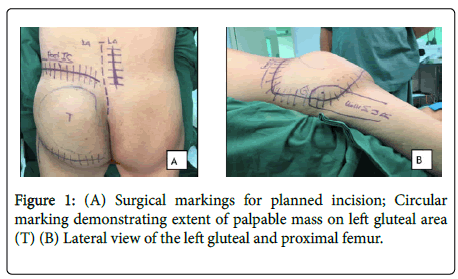
The patient is able to ambulate independently, but noted to have antalgic gait. On palpation, there was a 15 × 15 cm, well-demarcated, doughy, non-movable, tender, round mass covering the entire left gluteal area (Figure 1). There were no noted gross skin changes, ulcerations, or erythema. There was noted full range of motion of the left hip, but with discomfort towards the end range of left hip extension. There were no sensory deficits, with full pulses on the left lower extremity. There were no palpable masses on the right paralumbar area. No noted motor and sensory deficits at the levels of L2-S1, bilaterally.
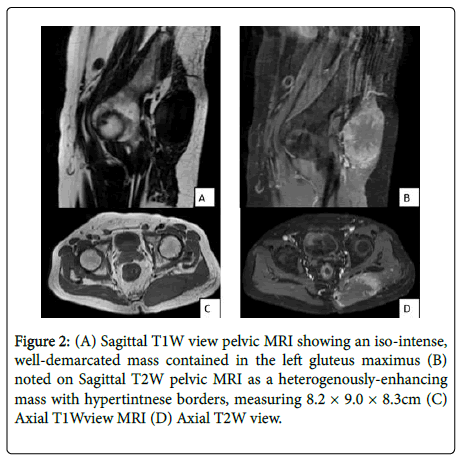
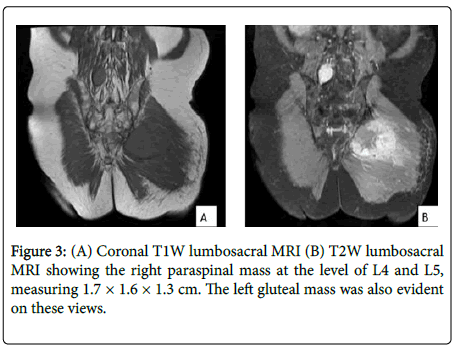
He underwent magnetic resonance imaging (MRI). T2-weighted magnetic resonance imaging (MRI) sequence of the lumbosacral spine showed a heterogenously-enhancing, well-demarcated mass contained on the left gluteal muscle, measuring 8.2 × 9.0 × 8.3 cm. Note of hyperintense signals on its periphery highly suggests presence of hypervascularity in the left gluteal mass. This mass was seen as a homogenous, iso-intense signal on T1-weighted MRI images (Figure 2). There is also note of a hyperintense signal on the right paralumbar muscles at the level of L4 and L5, measuring 1.7 × 1.6 × 1.3 cm (Figure 3). No noted infiltration of the surrounding bony structures, such as the iliac bone and lumbar spine. Computed tomography-guided core needle biopsy of the left gluteal mass, with histopathology results consistent with renal cell metastatic disease.
After confirming presence of metastases, positron-emitted tomography (PET) scan was done to evaluate for presence of other metastatic lesions. There were no other areas with increased uptake aside from the previously mentioned left gluteal and right paralumbar masses. There were no noted recurrence of the primary lesion on PET scan.
Operation performed and post-operative course
Pre-operatively, he underwent angiogram of the left gluteal mass, followed by embolization of the arterial branches of the inferior gluteal artery, which was noted to be the main vascular supply of the lesion.
He then underwent left buttockectomy and wide excision of the right paraspinal mass. Patient was placed on prone position under general anesthesia. A curvilinear incision was done from the medial border of the posterior iliac spine, going laterally, then curving inferiorly to the posterolateral border of the proximal femur, extending superomedially up to the gluteal crease.
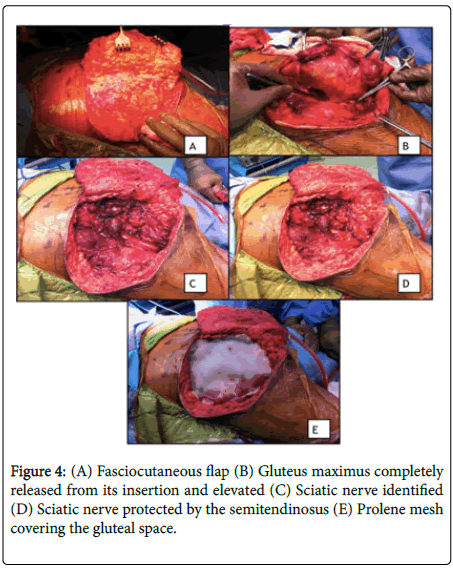
A fasciocutaneous flap was developed by dissecting through the plane between the subcutaneous and fascial layers of the left gluteal area, starting laterally, extending medially (Figure 4A). The origin of the left gluteus maximus muscle in the posterior iliac crest, and its insertion to the iliotibial band and proximal femur were exposed and released (Figure 4B). The gluteus maximus was then lifted from the external rotators. The mass was noted to be adherent to the superior gluteal artery and was dissected free. Some branches of the superior gluteal artery were ligated and cut. The left sciatic nerve was then identified and isolated from its attachment to the pseudocapsule of the tumor (Figure 4C). The gluteus muscle was then released completely from these landmarks. Exposed areas of the sciatic nerve was protected by fixing the most proximal part of the semimembranosus to the iliotibial band (Figure 4D). A prolene mesh, measuring 15 × 5 cm, was applied to cover the gluteal space, acting as a fascia. (Figure 4E) Two Jackson-Pratt drains were placed superficially over the mesh.
Intraoperatively, a well-circumscribed, doughy mass, measuring 7 × 9 × 4 cm mass, was fully excised, including the entire gluteus maximus, measuring 14 × 15 × 4 cm (Figure 5A). A right paramedian incision was done over the area of L4 to S1, confirmed via image intensifier. The paralumbar muscles were elevated from their spinal attachments and completely excised, including the lumbodorsal fascia extending from L4 to S1. There was a well-circumscribed, doughy mass, measuring 2 × 2 × 2 cm noted on the excised portion of the paralumbar muscles, grossly resembling the excised mass on the left gluteal area (Figure 5B).
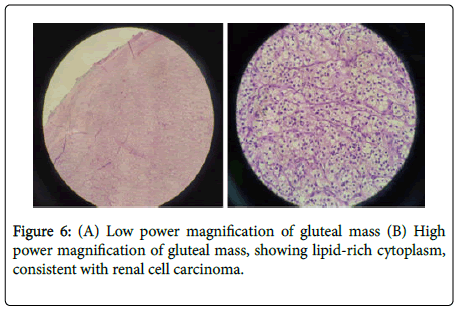
Histopathology revealed metastatic clear cell renal carcinoma, with positive tumor margins noted on the volar aspect of the excised left gluteal mass (Figure 6). The paralumbar mass margins tested negative for tumor. While admitted, the wound was regularly inspected for signs of necrosis or infection. Patient was continued on antithrombotics to facilitate blood flow and healing. Direct pressure was avoided over the left gluteal area by use of a donut pillow and by encouraging right lateral decubitus.
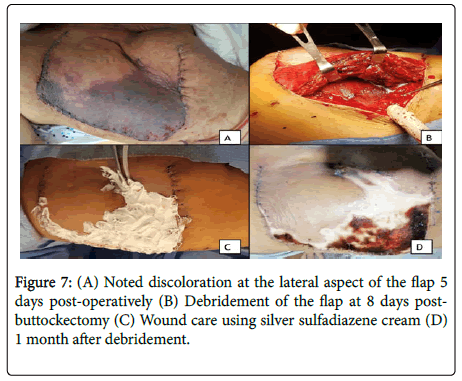
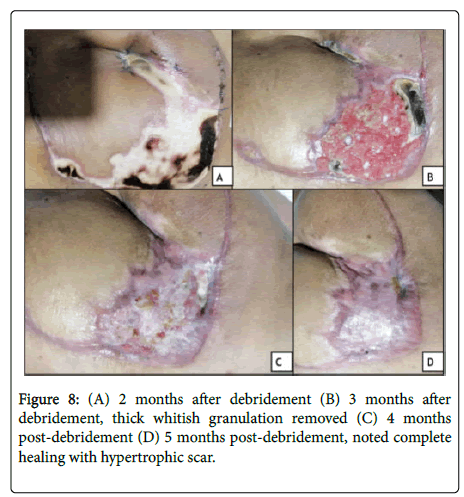
During the 5th day post-operatively, there was noted hyperpigmentation over lateral aspect of the flap (Figure 7A). Debridement was done on the 8th day post-buttockectomy. Intraoperatively, epidermolysis was noted, with intact fasciocutaneous flap. Regular wound care done using silver sulfadiazene (Flammazine) cream (Figures 7B and 7C). Patient was then discharged. He underwent regular monthly follow-ups. Wound care continued using wound healing cream (Dermlin) and hydrogel wound dressing (SoloSite) applied daily. On the 3rd month post-surgery, thick granulation tissue was debrided, and continued on the previous wound regimen (Figures 8A and 8B). Noted progression of reepithelialization, until 5 months post-surgery with noted healing with hypertrophic scar on the superolateral aspect of the surgical site (Figures 8C and 8D).
Discussion
Metastatic disease occurs as a sequential process via hematogenous or lymphatic dissemination of cancer cells from the primary lesion. Malignant solid tumors rarely metastasize in skeletal muscles alone. Although skeletal muscle is highly-vascular, metastasis is unlikely in these areas due to the following hypotheses: (1) high hydrostatic pressure related to exercise-induced blood flow, making it a poor environment for tumor growth (2) inhibition of tumor genesis due to presence of lactic acid, muscle-derived peptidic factors, and protease inhibitors (3) antitumor activity of lymphocytes and natural killer cells [3].
A retrospective study by Surov et.al in 2010 included 5170 patients with metastatic solid tumors confirmed by CT scan. Only 61 cases (1.2%) among the subjects presented with skeletal muscle metastases (SMMs). In 5 cases (<0.01%), skeletal muscles were the only metastatic sites. Renal cell carcinoma, was listed as the 4th most common primary malignancies associated with skeletal muscle metastases (3.2%). Overall, the most common site of skeletal muscle metastases (SMMs) for RCC is the iliopsoas muscle (27.5%), followed by paravertebral muscles (25%) and gluteal muscles (16.3%). This pattern was similar to our case.
Moreover, the pattern of metastases in renal cell carcinoma is unpredictable, attributing to its complex lymphatic drainage. Several experimental studies show peculiar metastatic pattern in mice, wherein migration of cancer cells were noted in pre-selected organs in order to establish an optimal environment for the tumor cells to grow [4]. This model was postulated as a possible cause of the unusual pattern of metastases in renal cell carcinoma. 20%-30% of patients with RCC treated by nephrectomy were noted to develop distant metastases [5]. The common areas of metastases of RCC include the lungs, lymph nodes, gastrointestinal organs, bones, and brain.
Renal cell carcinoma rarely metastasizes to skeletal muscles (<1%). Current studies consist of autoptic and case reports. The largest study to date was a comprehensive literature review including 37 patients obtained from case reports, combined with a retrospective review of 21 patients with skeletal muscle metastases arising from RCC [6]. They described the demographics, clinical course, radiographic presentation and outcome of patients with RCC who presented with skeletal muscle metastases. The average age of the subjects upon diagnosis of the first skeletal muscle metastases was 58.1 years. Most of them were males (86%), with clear cell RCC as histopathological subtype (81%). In both groups, they analysed a total of 116 muscle metastases. However, distribution of location and symptoms were different between the 2 groups. In the study group, majority of SMMs were incidental findings on CT scan, most commonly seen in trunk muscles, followed by upper extremities, and lower extremities. In the literature review group, most were seen in the lower extremities and initially presented as painful masses on the onset. Only 7 reports showed pure muscle metastases as initial complaint without a concomitant primary tumor. In the retrospective group however, most of the lesions were only incidental findings only on CT Scan. Our case falls similarly to the described subsets of patients in terms of age, histopathology findings. He initially presented with a painful, rapidly-growing mass without recurrence of the primary tumor, similar to the literature group. They postulated that the cohort represent typical findings of RCC patients undergoing routine monitoring, whereas the literature group, consisting of case reports, describe unusual presentations of SMM [7]. Also, the study identified that cases of SMMs in RCC may be underreported, as these may only be incidental findings.
Establishing a tissue diagnosis of a skeletal mass is important, as a new-onset benign tumor may have a different treatment approach compared to a metastatic lesion or a new primary malignant mass. An open or percutaneous needle biopsy may be necessary prior to treatment planning. In the case presented, this was deemed important, given a previous history of RCC, and a new growth located in an atypical location.
Imaging studies may also aid in accurate and timely diagnosis. In a study by Sakamoto, et al. (2007), they described MRI findings that may be helpful in distinguishing metastatic RCC to skeletal muscles from other tumors. They usually present with regular borders, with hyperintensity in both T1 and T2-weighted images. In contrast, primary soft-tissue tumors usually present with iso or hypointense lesions on T1-weighted images. However, this study emphasized that doing MRI alone may only be beneficial in distinguishing between benign soft tissue masses from malignant tumors, as metastatic RCC may resemble other sarcomas (clear cell, alveolar soft-part), and that a tissue diagnosis is still recommended. These findings were also seen in another study [6] and added that other skeletal metastases typically present with iso or hypo-intense signals on T1 weighted images compared to the hyperintensity in RCC SMM. In our case, presence of a hyperintense signal on T2, correlated with a hypointense appearance on T1 may mimic other skeletal muscle metastases, or even primary sarcomas. Hence, tissue diagnosis is still vital, with imaging as adjunct in pre-operative planning, as well as in checking for presence of other metastatic lesions.
In CT scan, contrast is required because SMMs are very subtle on plain CT, and may resemble normal surrounding skeletal muscle tissue. SMMs usually present with peripheral enhancement (83%), but may present either with homogenous or heterogenous pattern, without predominance of one over the other [7]. PET Scan may be of great value to evaluate presence of other asymptomatic metastatic lesions that are small or too deep.
Generally, renal cell carcinoma is relatively resistant to chemotherapy. The mainstay of treatment of RCC is nephrectomy followed by immunotherapy. Nephrectomy is useful in reducing tumor-load, decreasing the tumor-derived factors and T-cell inhibitory factors. By doing nephrectomy for RCC alone, survival may be improved by 3-6 months (Crispen et.al 2012). In cases of stage IV RCC with oligometastatic site, as in our case, the recommended primary treatment is nephrectomy combined with surgical metastectomy [8]. In a study by Russo, et al., excision of solitary RCC metastasis was deemed useful for relatively young patients with solitary, non-CNS lesions detected more than 12 months after initial diagnosis, with a 5- year survival of 30% [9]. Factors identified as predictive of poor response to metastectomy include hypoalbuminemia, elevated lactate dehydrogenase (LDH) levels, tumor stage T3 and above, liver metastasis and retroperitoneal or supradiaphragmatic lymph node involvement. Patients satisfying at least 4 of these factors were shown to not benefit from metastasectomy. Based on these factors, our patient is a good candidate for wide excision of metastases.
Based on the existing evidence, radiotherapy is not yet established as part of the treatment regimen for metastatic RCC. In a recent metaanalysis, post-nephrectomy radiotherapy reduced risk of local or regional recurrence significantly by 53% [10]. However, this did not translate to disease-free survival or overall survival. Stereotactic body radiotherapy (SBRT) is a new technique, utilizing short courses of intensive, but highly-focused radiation delivered locally to metastatic lesions. It is associated with excellent local tumor control, as seen on PET scan as lack of tumor activity, or<20% expansion of tumor size on follow up imaging) [11]. For our case, this is the planned regimen after noting positive tumor margin on the volar aspect of the gluteal specimen. Ideally, this should have been done earlier on the postoperative course. However, due to the problems related to woundhealing, planned radiotherapy was delayed until a satisfactory postsurgical site was achieved.
For follow-up of patients with metastatic RCC, regular history and physical examination every 6-16 weeks is recommended [8]. CT scan with contrast of the thorax and abdomen, as well as the previous metastatic site, is the most frequently used imaging modality [12-14]. MRI is also an acceptable alternative for patients with contraindication to IV contrast. There have been multiple conflicting guidelines with regards to imaging follow up. However, it is recommended to do intensive imaging follow up during the first 3 years. Bases on the current guideline of the National Comprehence Cancer Network (NCCN), CT or MRI of the chest, abdomen, and pelvis is recommended at baseline, then follow-up imaging may be done in 6-16 week intervals. However, frequency of follow up may be altered, depending on the discretion of the surgeon and clinical status of the patient. One of the most widely-accepted guideline is from the American College of Radiology, which recommends the following regimen for a Stage T4 RCC (such as in our case):chest radiography every 6-12 months, and abdominal CT with contrast every 3-6 months for the 1st 3 years then annually up to 5 years. No specific guideline was provided for patients presenting with atypical distal metastases.
Conclusion
In conclusion, we present a case of a 58 year old male diagnosed with metastatic RCC to the left gluteal and right paraspinal muscles 3 years after initial diagnosis and nephrectomy. He underwent wide excision of the right paraspinal muscle and left buttock ectomy and presented with signs of flap epidermolysis post-operatively within the 1st week. Biopsy is still the gold-standard in diagnosis to distinguish if a skeletal muscle mass is benign or malignant, primary or metastatic. Imaging studies are also important to aid in pre-operative planning and to check for other areas of metastases. In cases of oligometastases or solitary metastatic lesions, meta stasectomy may improve survival. Post-surgical radiotherapy may be of benefit to prevent local metastatic recurrence. Furthermore, regular imaging follow-up is recommended for cases of metastatic RCC to monitor recurrence.
Acknowledgement
Institute of Pathology-St. Luke's Medical Center Quezon City for the histopathology slide images.
References
- Graves A, Hessamodini H, Wong G, Lim WH (2013) Metastatic renal cell carcinoma: update on epidemiology, genetics, and therapeutic modalities. ImmunoTargets and Therapy 2:73-90.
- Sakamoto A, Yoshia T, Matsuura S, Tanaka K, Matsuda S, et.al. (2007) Metastasis to the gluteus maximus muscle from renal cell carcinoma with special emphasis on MRI features. World J Surg Onco 5:88.
- Goger Y, Piskin M, Balasar M, Kilinc M (2013) Unusual Presentation of Renal Cell Carcinoma: Gluteal Metastasis. Case Reports in Urology: 1-3.
- Surov A, Hainz M, Holzhausen HJ, Arnold D, Katzer M, et al. (2010) Skeletal muscle metastases: Primary tumours, prevalence, and radiological features. European J Radiology 20:649-658.
- Sountoulides P, Metaxa L, Cindolo L (2011) Atypical presentations and rare metastatic sites of renal cell carcinoma: a review of case reports. J Med Case Reports 5:429.
- Haygood TM, Sayyouh M, Wong J, Lin J, Matamoros A, et al. (2015) Skeletal Muscle Metastasis from Renal Cell Carcinoma. Sultan Qaboos University Med J 15:327-337.
- Ramchandani P (2008) Post-treatment surveillance of renal cancer renal cell. In: Patel U, ed. Carcinoma of the kidney, 1st edition Cambridge:Cambridge University Press 2008:185-202.
- National Comprehensive Cancer Network. (2018) Kidney cancer (version 4.2018). Retrieved from https://www2.tri-kobe.org/nccn/guideline/urological/english/kidney.pdf
- Russo P (2012) Multi-modal treatment for metastatic renal cancer: the role of surgery. World J Urology 28:295-301.
- Kenney PA, Wood CG (2012) Integration of surgery and systemic therapy for renal cell carcinoma. Urologic Clinics of NA 39:211-231.
- Masucci GV, Wersall P, Kiessling R, Lundqvist A, Lewensohn R (2012) Stereotactic ablative radio therapy (SABR) followed by immunotherapy a challenge for individualized treatment of metastatic solid tumours. J Trans Med 10:104.
- Patel U, Sokhi H (2012) Imaging in the follow-up of renal cell carcinoma. Amer J Radiology 198:1266-1276.
- Crispen PL, Blute ML (2012) Role of cytoreductive nephrectomy in the era of targeted therapy for renal cell carcinoma. Current Urology Reports 13:38-46.
- Culp SH, Tannir NM, Abel EJ (2010) Can we better select patients with metastatic renal cell carcinoma for cytoreductive nephrectomy? Cancer 116:3378-3388.
Citation: Moser SS, Guerzon EJ, Ragasa JC (2020) Skeletal Muscle Metastases Arising from Renal Cell Carcinoma in a 58-Year Old Male: A Case Report. J Orthop Oncol 6: 139. DOI: 10.4172/2472-016X.1000139
Copyright: © 2020 Moser SS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4008
- [From(publication date): 0-2020 - Nov 07, 2025]
- Breakdown by view type
- HTML page views: 3120
- PDF downloads: 888