Smoking Effects on Blood Antioxidants Level: Lactate Dehydrogenase, Catalase, Superoxide Dismutase and Glutathione Peroxidase in University Students
Received: 13-Nov-2017 / Accepted Date: 12-Dec-2017 / Published Date: 17-Dec-2017 DOI: 10.4172/2161-0681.1000331
Abstract
Smoking has been involved as a significant risk factor for series and establishment of diseases. The aim of our study to investigate smoking effects induced oxidative damage. We have chosen male students of Al-Iraqia University and Tikrit University, aged 20-23 years. In our study we measured activity of Lactate Dehydrogenase (LDH), Catalase (CAT), Superoxide Dismutase (SOD), and Glutathione Peroxidase (GXp). Our results showing that the level of LDH in smokers were higher than nonsmokers, and the levels of CAT was higher in group of nonsmokers ,while SOD and GXp were higher significantly in non-smokers when comparison with smoker students. Also we study the height, body weight, body mass index (BMI), and some of blood parameters red blood cells (RBCs), White blood cells (WBCs), and hemoglobin (Hb). Results showing significant differences between smokers and nonsmokers, all of these parameters were lower in smokers. These results perhaps indicate that smoker students have oxidative stress and shortness in antioxidants defense system.
Keywords: Lactate dehydrogenase; Catalase; Superoxide dismutase; Glutathione peroxidase; Blood
Introduction
Smoking considers being wide public health problem, which reached today to the level of global epidemical. It is a risk factor for variety of disease (cardiovascular disease, stroke, chronic pulmonary disease, Alzheimer's disease, Parkinson's disease) [1]. About more than 5 million people die from smoking related illnesses, as the report of World Health Organization [2], this number will be doubled by 2025. The main addictive component of smoke are Nicotine, Hydrogen cyanide, Methanol, Butan and about more than 400 other chemicals. These chemicals induced the rate of Reactive Oxygen Species (ROS), which is a part of free radicals. Free radicals are highly unstable and capable of undergoing complex interaction in biological system, make oxidative stress, which occur when there are not enough antioxidant molecules to counteract their side effects [3]. Antioxidant are natural molecules in the biological system that scavenging free radicals or protecting from its effects. They can be synthesized endogenously in the body or determined by food intake [4].
Smoking is a rich source of oxidants. It has been considering the main cause of increase production of (ROS) which may exceed the capacity of antioxidants defense system [5].
Lactate dehydrogenase (LDH)
Lactate Dehydrogenase (LDH) is an enzyme required during the process of turning sugar into energy for cells function. LDH is present in much kind of organs and tissues within the body including: liver, heart, pancreas, kidneys, skeletal muscles, lymph tissue, and blood cells. When oxidative stress or oxidative damage occurs in the body, LDH may release and raise its level in the blood [6]. High level of LDH in the blood point to acute or chronic cell damage, abnormality LDH low levels only rarely happened and usually are not consider as harmful and the high level of LDH indicate a number of conditions such as stroke, cancer, heart attack, blood flow deficiency, hemolytic anemia, hepatitis, muscle injury and tissue death [7].
Catalase (CAT)
Catalase (CAT) is an antioxidant enzyme present in all aerobic organisms. It's known to catalyze H2O2 into water and oxygen in an energy efficient manner in the cell exposed to environmental stress. Catalase is located in all major sites of Hydrogen peroxide ( H2O2) production in cellular environments such as peroxisomes, mitochondria [8,9].
Superoxide dismutase (SOD)
Superoxide Dismutase (SOD) is metalloenzyme and one of the most important antioxidative enzymes that catalyze dismutation of the superoxide anion to molecular oxygen and hydrogen peroxide and thus form a crucial part of the cellular antioxidant defense mechanism [10]. The amount of SOD present intracellular and extracellular environments is very important to prevention of the disease related to oxidative stress [11]. SOD also appears to be important in prevention of other neurodegenerative disorders such as Alzheimer's, Parkinson's and Huntington's disease. The reaction catalyzed by SOD is extremely fast and the presence of sufficient amount of the SOD in cells and tissues typically keep the concentration of superoxide anion (O2) very low [12].
Glutathione peroxidase (GXp)
Glutathione Peroxidase (GXp) is one of enzyme family of peroxidase activity whose main biological role is to protect the organism from oxidative damage [9]. The biochemical function of GXp is to reduce lipid hydro peroxides to their corresponding alcohols and reduce free hydrogen peroxide to water. The main reaction that GXp catalyzes is:
2GSH+H2O→ GS-GS+2H2O [13].
Materials and Methods
Samples
Our samples included 100 individuals selected without known bias, divided into two group50 male smoking and 50 male nonsmoking consider as control group. Samples age were 22-23 years, attending as students to AL-Iraqia University and Tikrit University Iraq. Venous blood samples were collected into tubes with heparin anticoagulants. Blood samples store freezing until the day of used.
Determination of weight, height and body mass index
Data of weight, height, and body mass index were obtained during collecting the samples of study.
Determination of blood components RBCs, WBCs, and Hb
Data of blood components were obtained after blood dropping according to laboratory methods. Blood samples were assayed in Auto Hematology Analyzer from Mindray Medical International Company China.
Determination of blood LDH activity
Principle: The principle is based on the kinetic determination of the lactate
Dehydrogenase according to the following reaction.
Pyruvate+NADH+H+ L-Lactate+NAD+
Procedure: To 2 μl of sample, 1000 μl of the reagent was added, mixed and incubated for
10 minutes at 37°C. The absorbance of the sample was measured per minute,
(ΔOD/min), during 3 min against de-ionized water at 340 nm. LDH activity (U/l)=(ΔOD/min) × 8095
Determination of superoxide dismutase (SOD) activity in blood
Blood SOD activity was determined according to the method of Mc Cord and Fridovich [14] after removing the hemoglobin.
Procedure: 0.1 ml of the heparinised blood was haemolysed by 0.9 ml of cold water (4°C).
The haemolysate was treated with 0.25 ml of Chloroform (CHCl3) and 0.5 ml of ethanol.
Vigorous mixing is done to remove the hemoglobin. The mixture was centrifuged at 15000 rpm for 60 min. 0.025 ml of the clear supernatant was used for the SOD assay as described in the section 3.2.6.6. The activity was expressed as U/g Hb.
Determination of catalase (CAT) activity in blood
Catalase activity in the blood was determined according to the method of Aebi [15].
Procedure: Erythrocyte sediment was prepared from the heparinised blood and washed 3 times with isotonic saline. A stock haemolysate containing approximately 5 g Hb/dl were prepared. By the addition of 4 parts by volume of distilled water a 1:500 dilution of this concentrated haemolysate with sodium-potassium phosphate buffer (0.05 M, pH 7) was prepared immediately before the assay. Reference cuvette contained 1 ml of buffer and 2 ml of haemolysate and test cuvette contained 2 ml distilled haemolysate. The reaction was started by addition of 1 ml of H2O2 (30 mM in the buffer) to the test cuvette, mix well and the decrease in extinction was measured at 240 nm for 1 min by 15 s Interval. Catalase activity was calculated using the formula and expressed as k/g Hb, where k is a rate constant of 1st order reaction. Catalase=2.3 × (log E1-log E2) × dil. Factor 15 × g Hb/ml of blood=0.153 × 1000 × (log E1- log E2) g Hb/ml of blood.
Where E1 is E240 at t=0 and E2 is E240 at t=15 s.
Determination of glutathione peroxidase (GPx) activity in blood
Glutathione peroxidase activity was determined according to the method of Hafemann et al. [16].
Procedure: 0.02 ml of heparinised blood was treated with 0.1 ml of 5 mM GSH, 0.1 ml of 1.25 mM H2O2, 0.1 ml of 25 mM NaN3 and phosphate buffer (0.05 mM, pH 7) in a total volume of 2.5 ml at 37°C for 10 min. The reaction was stopped by adding 2 ml of 1.65 % H3PO3 and the reaction mixture was centrifuged at 1500 rpm for 10 min. 2 ml of supernatant was used for the estimation according to the procedure given under tissue GPx determination. The result was expressed as U/g Hb.
Statistical analysis
Spss 20.0 program was used to process the data. Adopt to detect when comparing data of two variables. Results were presented as the Mean ± SD, in the case of p<0.05, and the analytic results showing significantly statistical differences.
Results and Discussion
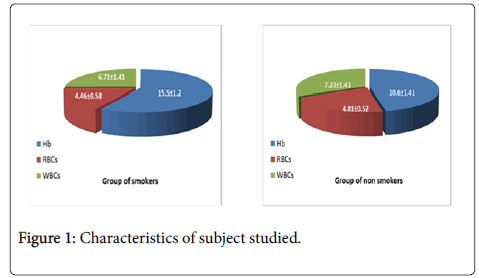
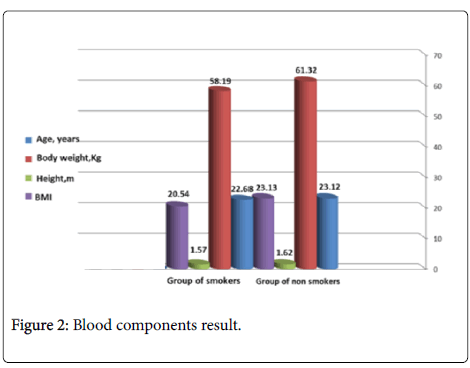
There were 50 student’s smokers and 50 students nonsmokers. The characteristics of the subject study are shown in Figure 1 (Table 1). Data are presented as mean ± SD. Results showing lowering significant differences in body weight, height and BMI in group of smokers when comparison with control group of nonsmokers.
| parameters | Group of smokers | Group of non-smokers |
|---|---|---|
| Age (years) | 22.68 ± 3.71a | 23.12 ± 4.16a |
| Body weight (Kg) | 58.19 ± 10.12a | 61.32 ± 11.56b |
| Height (m) | 1.57 ± 0.09a | 1.62 ± 0.07b |
| BMI | 20.54 ± 3.71a | 23.13 ± 4.16b |
| N=50, ± mean SD, (a,b) refers to significant differences , P<0.05 | ||
Table 1: Characteristics of subject studied.
Our results in Table 1 showed the differences of characteristics of study subject which include age, body weight and height and body mass index .Our results shown significant decrease in body weight, height and BMI.
The effects of chronic nicotine administration on appetite suppression, decreased food intake and leanness have been replicated in many studies.
We explained the reason of significant lowering of body weight, and BMI in smokers group to the effect of smoking chemicals within smoke on the appetite regulation center of the hypothalamus [17]. Other reason may return to the behavioral factors, which appear to be strong relationship between smoking habits, lifestyle, body weight, BMI and number of cigarette smoking per day [18]. Significant decrease in body weight and BMI of smokers group in our study is consistent with explained by the observations in several studies [18-21]. Our results disagree with the results of [22], which refers to significant increasing in body weight and BMI in smoker’s comparison with nonsmokers. Also may the decreased in body weight and BMI happened as a results of increase the metabolic rate [23]. Other why of this result may to the increase of would generate of free radicals in tissues because of increasing lipid peroxidation by smoking [24].
Differences between RBCs, WBCs, and Hb
The results we obtained showing increasing significant in Hb and WBCs in group of smokers when comparison with nonsmokers. RBCs showing no difference in both group smokers and nonsmokers.
Result of our study for blood components include Red blood corpuscles (RBCs), White Blood Cells (WBCs) and Hemoglobin (Hb), are shown in Table 2, as comparable between smokers and nonsmokers.
| Group of non-smokers | Group of smokers | parameters |
|---|---|---|
| 6.71 ± 1.41b | 7.23 ± 1.43a | WBCs |
| 4.46 ± 0.58a | 4.81± 0.52a | RBCs |
| 10.6 ± 1.41a | 15.5 ± 1.2b | Hb (g/dl) |
| N=50, ± mean SD, (a,b) refers to significant differences , P<0.05. | ||
Table 2: Blood components result.
In smokers group we observed significantly increased in WBCs, Hb, and no significantly change in RBCs. There are many studies supporting the role of free radicals generated by smoking which have deleterious effects on hematological parameters which causing oxidative stress (Figure 2) [25-27].
Increasing significantly in WBCs may return to the role of oxidative status result of smoking, as well as the significant increase in Hb, in smokers with respect to nonsmokers. Our explaining independent on the fact of smoke inhalation through Lung into the blood stream, it actually reacts with the hemoglobin in RBCs forming a stable coordinated complex, CO interferes with the content of Hb in blood [26]. In specific, our results showed no significant differences statistically in RBCs in both group smokers and nonsmokers. Our results agreed with the results [21,27]. Also, we agreed practically with the results of [28], about WBCs and Hb, but disagree with RBCs results.
Antioxidant enzymes
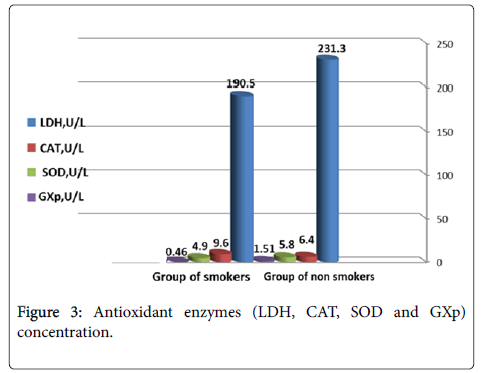
Results in Figure 3 (Table 3) are showing the antioxidant enzymes concentration (LDH, CAT, SOD and GXp). According to the results, LDH was showing significantly increasing in smokers comparable with nonsmokers while all of CAT, SOD and GXp were decreased significantly in smokers comparable with nonsmokers.
| Group of smokers | Group of non-smokers | parameters |
|---|---|---|
| 190.5 ± 1.9b | 231.3 ± 6.5a | LDH, U/L |
| 9.6 ± 1.3b | 6.4 ± 1.4a | CAT, U/L |
| 4.9 ± 1.2b | 5.8 ± 1.3a | SOD, U/L |
| 0.46 ± 0.12b | 1.51 ± 0.10a | GXp, U/L |
| N=50, ± mean SD, (a,b) refers to significant differences , P<0.05. | ||
Table 3: Antioxidant enzymes (LDH, CAT, SOD and GXp) concentration
Our results in Figure 3 (Table 3) showed the antioxidants enzymes Lactate Dehydrogenase (LDH), Catalase (CAT), Superoxide dismutase (SOD) and Glutathione peroxidase (GXp) in both of smokers and nonsmokers group.
Our study findings showed significantly increasing in LDH level of smoker’s group comparison with nonsmokers group. Lactate dehydrogenase enzyme is found in almost every tissue in the body and its level increase in blood in many tissues damage pathological disorders.
We think that the reasons for elevated of LDH significantly due to the smoke chemicals compounds may affect the respiratory tract as well as all of the cells in respiratory system [29]. The increased of LDH considered as indicator of cell necrosis or tissues damage or may be attributed on the smoking induced cell damage anywhere in the body, which may leak cellular contents along with LDH into serum [30]. Elevated of LDH levels is usually considered to reflect cell damage, and this is agree with the bad effect of smoking in generated free radicals and oxidative stress in cell which lead to release more of LDH to the blood stream by cell injury [31]. In the other hand, LDH is a ubiquitous medically significant enzyme, which exists in five isoforms compound. Interestingly the isozyme of LDH profile found in serum and saliva and both of them will be in contact and affected by smoke chemical compounds [32]. Our results appear to approve many other studies findings, about the role of smoking in increase LDH level in blood stream [33,34]. Similar studies have observed increased level of LDH in blood of smokers [35]. We think there is another reason help in explaining the higher LDH level, which is the ability of smoke compounds to generate free radicals and oxidative stress in many cell and tissues of the body [29]. In other hand, we suggested that the decreased in CAT level in smokers group due to accumulation of hydrogen peroxide, which inhibit this enzyme or alter enzymatic conformation. Also this decreased CAT activity could be due to inactivation of CAT by the cross linking or the impairment of nitric oxide (NO) synthase, which can bind reversible to ferric iron and inhibiting afterwards CAT activity [36-38]. Our findings about CAT activity were disagreeing with previous results [39], which observed increased level of CAT significantly in smokers. Our observation about CAT, SOD and GXp confirm the results of many studies [40]. Our result disagrees with other studies [41], which observed that about SOD and GXp, which decreased in group of smokers as compared to nonsmokers. The significantly differences of oxidative enzymes levels CAT, SOD and GXp may be due to nicotine increased generation of superoxide anion and hydrogen peroxide, which in turn results in generation of hydroxyl free radicals. Generation of these free radicals have been shown to participate in many toxic reaction and increased production of superoxide and hydrogen peroxide may cause deleterious injury to alveolar macrophages by causing release of proteolytic enzymes [42].
Our results in Figure 3 showed significantly lowering in Catalase (CAT) levels in blood of smokers group when comparable with nonsmokers. Enzymes may take form of free radicals scavengers such as CAT, SOD, and GXp in smokers the obligatory use of reserve antioxidant to detoxify the excess free radicals, result in alternation of the levels of different antioxidants enzymes [43]. We have demonstrated the activity of CAT was significantly lowering in smokers than nonsmokers and this result may be due to structural features of CAT or environmental factors, increased of SOD and GXp levels were occurred as a result of increase lipid peroxidation [44]. In normal conditions CAT, SOD and GXp consider are among most important antioxidants enzymes as free radical scavengers. In our study, we investigated and compared these enzymes concentration in blood in group of smokers and nonsmokers. We have demonstrated the SOD and GXp activity, which were significantly higher in smokers than nonsmokers. This observation suggests oxidative stress induction following smoking [45]. Our results were similar to finding in some of studies [46,47], differences the activity of these enzymes have been reported. We think that the damage caused by ROS of smoking occur as consequence of imbalance between the generation and detoxification of these species, defense against oxidative stress is provided by oxidative enzymes such as CAT, SOD and GXp which are the first line of cellular defense against oxidative damage [49-51].
Conclusion
The present study evaluated the effect of smoking on serum antioxidants (LDH, CAT, SOD and GXp). The study group was classified based on age and heavy smoking consumed per day to evaluate the harmful effects of smoking on the serum levels of antioxidant enzymes and resultant oxidative stress. The result showed a significant decreasing and increasing in serum antioxidants enzymes concentrations when compared to the control group of nonsmoker. This study also highlights the effects of smoking at a young age, suggesting a progressive depletion and a sequential accumulation of antioxidants. Conclusively, smoking depletes many serum antioxidants required to scavenge excess free radicals, thus increasing the rate of lipid peroxidation.
Recommendations
Efforts should be made to increase the number of future studies on the relations between heavy smoking and their effects on antioxidants defense in the body, to assess the relationship between smoking and its harmful effects.
References
- Gboyega EA, Adesegun JK, Chikezie UE (2013) Tobacco smoking and awareness of smoking cessation products in a University community. J Public Health Epidemiol 5: 351-356.
- World Health Organization (WHO 2002) The World Health Organization Report 2002 reducing risks, promoting health life. Geneva, Switzerland: World Health Organization.
- Gandhi KK, Foalds J, Steinberg MB (2009) Lower Quit rates among African American and Latino menthol cigarette smokers at a tobacco treatment clinic. Int J Clin Pract 63: 360- 367.
- Akpotuzor JO, Udoh AE, Etukudo H (2012) Total antioxidant status and other antioxidant agent levels in children with P. falciparum infection in Calabar , Nigeria .Int J Biomed Lab Sci 1: 35-39.
- Chundru VN, Ram MM (2013) Study of oxidant enzymes superoxide dismutase and glutathione peroxidase levels in tobacco chewers and smokers: A pilot study. J Cancer Res Ther 9: 210-214.
- Chernecky CC, Berger BJ (2013) Laboratory tests and diagnostic procedures. 16th ed St. Louis MO: Elssvier Saunders.
- Masella R, Di Benedotto R, Vari R, Filesi C, Ginovannini C (2005) Novel mechanism s of natural antioxidant compounds in biological system: Involvement of glutathione and glutathione reductase enzymes. J Nutr Biochem 16: 577-586.
- Chelikhani P, Fita I, Loewen PC (2004) Diversity of structures and properties among catalase. Cell Mol Life Sci 61: 192-208.
- Yang S, Jensen MK, Rimm EB, Willett W, Wu T (2014) Erythrocyte superoxide dismutase , glutathione peroxidase and catalase activity and risk of coronary heart disease in generally healthy women. Am J Epidemiol 180: 901-908.
- Maier CM, Chan PH (2002) Role of superoxide dismutase in oxidative damage and neurodegenerative disorder. Neuroscientist 8: 323-334.
- Longfeng S, Xiaofei LI, Gang LI, Bing D, Wei T (2017) Actinide Chinese's blanch .Improves the indices of antioxidants and anti- inflammation status of type 2 diabetes mellitus by activating Keap1 and NrF2 Via the upper gulation of Micro RNA 424. J Oxid Med Cellular Longevity 1-14.
- Cases J, Romain C, Marin Pagan C, Chung LH, Rubio-Perez JM, et al. (2017) Supplementation with poly-rich extract , perfload , improves physical performance during high-intensity exercise: A Randomized, Double Blind, Gross Over , Trail. Nutrients 9: 421-433.
- Bhabak KP, Mugesh G (2010) Functional mimics of glutathione peroxidase: bioinspired synthetic antioxidants. Acc Chem Res J 43: 1408-1419.
- McCord JM, Fridovich I (1969) Superoxide dismutase: An enzymatic function for erythrocuperin (hemocuprein). J Biol Chem 44: 6049-6055.
- Abi H (1974) Catalase in: Bergmeyer HV, Editor, methods in enzymatic analysis. New York Acadamic Press 2: 674-684.
- Hafemann DG, Sunde RA, Houestra WG (1974) Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. J Nutr 104: 580-584.
- Jo YH, Talmage DA, Role LW (2002) Nicotine receptors mediated effects on appietite and food intake. J Neurobiol 53: 618- 632.
- Pisinger C , Jorgensen T (2007) Waist circumference and weight following smoking cessation in a general population: The Inter 99 Study. Prev Med 44: 290-295.
- Pragti Ch, Sunil K (2011) Effect of smoking on body mass index :A community- based study. Nat J Comm Med 2: 325-330.
- Shadrach D, Daniel FM, Jill PP (2015) Relationship between smoking and obesity A cross – sectional study of 499, 504 middle aged adults in UK general population .PLOS ONE 10: 1-12.
- Sharma KH , Shah KH , Patel I, Patel AK, Chaudhari S (2015) Do Circulating blood cells types correlate with modifiable risk factors and outcomes in patients with acute coronary syndrome(ACS). Indian Heart J 67: 444-451.
- Xiao L, Yupeng W, Zhiqiang W, Fuwen C, Biao X, et al. (2015) The mediating effect of body mass index on the relationship between cigarette smoking and atopic sensitization in Chinese adults. Int J Environ Res 12: 3381-3394.
- Dalloso HM, James WP (1984) The role of smoking in the regulation of energy balance. Int J Obes 8: 365-375.
- Hauwa'u AB, Abdullahi D, Gaddafi ID (2017) Effect of cigarette smoking on lipid peroxidation and serum antioxidant vitamins. J Pharm Biol Sci 12: 40-44.
- Sneve M, Jorde R (2008) Cross- sectional study on the relationship between mass index and smoking , and longitudinal changes in body mass index in relation to change in smoking status : The tromso study. Scand J Public Health 36: 397-407.
- Ramamurthy V, Raveendran S, Thirumoni S, Krishnaveni S (2012) Biochemical changes of cigarette smokers and cigarette non-smokers. Inter. J Adv Life Sci 1: 68-72.
- Pankaj Y , Daivd E , Gaelle R , Sandra F , Anabelle C, et al. (2015) Genetic Factors Interact With Tobacco Smoke to Modify Risk for Inflammatory Bowel Disease in Humans and Mice. Gastroenterology 153: 550-565.
- Lymperak E, Mikedou K, Iliadis S, Vagdatli E (2015) Effect of acute cigarette smoking on total blood count and markers of oxidative stress in active and passive smokers. Hippokratia 19: 293-297.
- Madole MB, Dilip B, Mamatha MT, Ankur P (2016) Study of serum lactate dehydrogenase and lipid profile in patients with chronic cough. Int J Clin Biochem Res 3: 409-412.
- Pannuru P, Vaddi DR, Nallanchakravarthula V (2009) Influence of chronic cigarette smoking on serum biochemical profile in male human volunteers. J Health Sci 55: 265-270.
- Robert L, Smith MD, Carolyn SR, Milena LL (1988) Elevated Lactate dehydrogenase values in patients with pneumocystis Carinii pneumonia. Chest 95: 987-992.
- Tajindra SS, Anita S, Anita D, Shaheen S (2016) An In Vitro analysis to ascertain whether smokeless tobacco has any detrimental effect on the diagnostic efficacy of salivary lactate dehydrogenase. Int J Med Sci Public Health 6: 583-588.
- Joseph DB, Nancy CL, Susan FW, Thomas D, Douglas WD (1998) Pulmonary toxicity I hamsters of smoke particles from Kuwaiti oil fires. J Environ Health Perspect 106: 141-147.
- Csaba R, Raymond JS, Jagdis LD, Justin RJ (1999) Cigarette smoke potentiates house dust mite Allergen- induced increase in the permeability of human Bronchial epithelial cell in vitro. Am J Respir Cell Mol Biol 20: 1238-1250.
- Omotoso GO, Adeyemi HA (2014) Effect of prenatal cigarette smoke exposure on the Architecture of the heart in Juvenile Wistar Rats. Anatomy J Af 3: 405-411.
- Bogdansk JJ , Korneti P, Todorova B (2003) Erythrocyte superoxide dismutase, glutathione peroxidase and catalase activities in healthy male subjects in republic of Macedonia. Bratisl Lek Listy 104: 108-114.
- Senthil S, Veerappan RM, Ramakrishna R, Pugalen Di KV (2004) Oxidative stress and antioxidants in patients with cardiogenic shoch complicating acute myocardial infraction .Clinc Chim Acta 384: 131-137.
- Yosri N, Abdelkader CH, Latifa CH, Bruno B, Samia E, et al. (2013) Low erythrocyte catalase enzyme activity is correlated with high serum total homocysteine level in Tunisian patients with Acute myocardial infarction. Diagn Pathol 8: 68-74.
- Omar FK, Karem HA, Mohammed BA, Arwa D, Thomas E, et al. (2012) Acute exposure to water pipe tobacco smoke induces changes in the oxidative and inflammatory markers in mouse lung. J Inhal Toxicol 24: 667-675.
- Anshu J, Flora SJS (2012) Dose related effects of nicotine on oxidative injury in young, adult and old rats. J Environ Biol 33: 233-238.
- Syed Anzar AR, Alvina T (2016) The effect of melet deposition on antioxidant enzymes of lens in smokers of Karachi – Pakistan. Asia Pac J Med Toxicol 5: 119- 123.
- Yildiz D (2004) Nicotine, its metabolism and overview of its biological effects. Toxicon 43: 619-632.
- Reejamol MK, Mythili S (2013) Estimation of lipid peroxides and anyioxidants in smokers and non-smokers with periodontitis . J Dent Sci 4: 53-56.
- Husain K, Scott BR, Reddy SK, Somani SM (2001) Chronic ethanol and nicotine interaction on rat tissue antioxidant defense system. Alcohol 25: 89-97.
- Zahraie M, Goodarzvand K, Sadeghpour HR, Kiani A (2005) Effects of cigarette smoking on erythrocyte antioxidative enzyme activities and plasma concentration of their cofactors. J Acta Medica Iranica 43: 253-258.
- Durak I, Yalcin S, Burak CMY, Buyukkocak S, Kacmaz M, et al. (1999) Effects of smoking on plasma and erythrocyte antioxidant defense system. J Toxicol Environ Health A 56: 373-378.
- Szuster-Ciesielska A, Stachura A, Slotwinska M, Kaminska T, Sniezko R , et al. (2000) The inhibitory effect of zinc on cadmium – induced cell apoptosis and reactive oxygen species ROS production in cell cultures. Toxicol 145: 159-171.
- Notara V, Panagiotakos DB, Kouroupi S, Stergiouli I (2015) Greece study investigators. Greece Tob Induc Dis 13: 38-46.
- Elena BB, Galina PZ, Svetlana MG, Lyudmila DF, Antonina IK, et al. (2010) Biomarkers of oxidative stress and smoking in cancer patients. J Cancer Res 6: 47-53.
- Pankaj J, Reena J, Mal KL, Ketan M (2014) Effect of cigarette smoking on hematological parameters: comparison between male smokers and non- smokers. Int J Sci Nat 5: 740-743.
- Sharma KH, Shah KH, Patel I, Patel AK, Chaudhari S (2015) Do Circulating blood cells types correlate with modifiable risk factors and outcomes in patients with acute coronary syndrome (ACS). Indian Heart J 67: 444-451.
Citation: Raddam QN, Moafaq MZ, Mostafa AA, Nahla KA (2017) Smoking Effects on Blood Antioxidants Level: Lactate Dehydrogenase, Catalase, Superoxide Dismutase and Glutathione Peroxidase in University Students. J Clin Exp Pathol 7: 331. DOI: 10.4172/2161-0681.1000331
Copyright: ©2017 Raddam QN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 8625
- [From(publication date): 0-2017 - Dec 21, 2025]
- Breakdown by view type
- HTML page views: 7494
- PDF downloads: 1131