Sodium Bicarbonate and N-Acetyl Cysteine in Organophosphorus Poisoning Cases of Different Severity: A Randomized Controlled Clinical Trial
Received: 20-Dec-2021 / Accepted Date: 03-Jan-2022 / Published Date: 10-Jan-2022
Abstract
Introduction: Organo Phosphorus Poisoning (OPP) remains a detrimental health problem in many parts of the world particularly in developing countries. The treatment did not change since many years, despite the increasing complications and case fatalities of the exposure. Separate clinical trials were conducted to test, if sodium bicarbonate and N-Acetylcysteine (NAC) are useful in improving the outcome and the prognosis of the OPP.
Aim: This study was conducted based on a previous clinical trial to test the efficacy of N-acetyl cysteine; the famous antioxidant, and of blood alkalization by sodium bicarbonate in improving the outcome of OPP cases, according to the severity of cases.
Methods: Seventy patients of OPP were given N-Acetylcysteine (NAC) and NaHCO3 together with the classic treatment of OPP. Serum Malondialdehyde (MDA), Glutathione Peroxidase (GPx), pH, plasma butyryl cholinesterase were measured and compared of a matched group of patients, who received the classic treatment only, on presentation and after 24 hours of the classic treatment only. The effects of the new antidotes were compared for the severe cases with the mild and moderate degrees of OPP.
Results: There were no significant differences between the two groups of the study in the initial levels of MDA, GPx, pH, plasma butyryl cholinesterase that turned highly significant after 24 hours of treatment and observation. There was a highly significant difference (P=0.001) in the Length of the Hospital Stay (LOS) between the two groups. No adverse effects of the supplements were observed. The percentage of improvement to the initial enzymes levels was higher to the severe cases than to the mild and moderate degrees of poisoning.
Conclusion: NAC and sodium bicarbonate are affordable agents and are very helpful in improving the outcome of OPP patients, particularly the severe cases.
Keywords: N-acetyl cysteine; Sodium bicarbonate; Organophosphorus poisoning; Severity; Outcome
Abbreviations
AChE: Acetylcholinesterase Enzyme; BChE: Butyrylcholiesterase Enzyme; H: Hour; IL: Illinois; NCI: Nosocomial Infection; Ops: Organophosphates; OPP: Organo Phosphorus Poisoning; OPIDN: Organo Phosphorus-Induced Delayed Neuropathy; RBC: Red Blood Cell; RPM: Round Per Minute; SD: Standard Deviation; °C: Degree Centigrade; USA: United States of America; Y: Year
Introduction
Organophosphorus compounds (OPs) are a diverse group of organic chemical compounds containing phosphorus that are used for several purposes. They include insecticides (malathion, parathion, diazinon, fenthion, dichlorvos, chlorpyrifos, and others), nerve gases (soman, sarin, tabun, and VX), and flame retardants (Polybrominated Diphenyl Ethers (PBDEs) and Chlorinated Tris (TDCPP). They are widely used for agriculture and domestic pest-control and as chemical warfare agents. Suicidal exposure to pesticides accounts for one-sixth to oneeighth of the world's suicides each year [1].
With more than 3 million exposures and 300000 deaths per year, OPP constitutes a critical care and a public health problem [2]. At the same time, the treatment of this common toxic exposure did not change since decades [3]. Moreover, OPP may be mixed with solvents toxicity that are primarily responsible for a high morbidity and mortality in many cases, where oximes, in the traditional regimen of OPP treatment, would not be expected to reduce toxicity in these cases even if given early and in the appropriate dose after the poison exposure [4].
The exposure to OPs can be occupational, accidental, or intentional. The exposure can be through dermal, inhalational, or through ingestion routes. The OPs are absorbed through all these routes, and once absorbed, it binds to the acetyl cholinesterase enzyme in red blood cells rendering the enzyme inactive. This leads to an accumulation of the acetylcholine within the synapses and neuromuscular junctions. Overstimulation of nicotinic receptors found at neuromuscular junctions leads to fasciculations and myoclonic jerks, and finally flaccid paralysis because of the depolarizing block. Nicotinic receptors in the adrenal glands may cause hypertension, sweating, tachycardia, and leukocytosis with left shift [5].
Effects on muscarinic receptors are usually slower than on the nicotinic receptors because they occur via a G-protein-coupled receptor mechanism. Muscarinic receptors are found in the parasympathetic and sympathetic nervous systems. Sweat glands within the sympathetic nervous system get overstimulated and cause large amounts of sweating. The parasympathetic effects of organophosphate poisoning can be seen in multiple systems including the heart, exocrine glands, and smooth muscles. A conformational change can occur to the AChE enzyme bound to the organophosphate compound (but not carbamates) and it renders the enzyme resistant to reactivation, until a new enzyme is synthesized in the liver, making some treatment options useless [6].
Moreover, OP poisoning may be complicated by the ‘Intermediate Syndrome’; characterized by a relapse after apparent resolution of cholinergic symptoms, and manifests by muscle paralysis, more in upper-limb muscles, neck flexors, and cranial nerves. It occurs about 24-96 hours after OP exposure and may progress to a respiratory failure [7].
Another complication of OP poisoning is the OP-induced delayed neuropathy. It results from phosphorylation and aging of at least 70% of Neuropathy Target Esterase (NTE). Cramping muscle pain in the lower limbs, distal numbness, paresthesia, followed by progressive weakness, depression of deep tendon reflexes in the lower limbs and, in severe cases, in the upper limbs. The therapeutic combination of oxime, atropine, and diazepam is well established in the treatment of OP insecticide poisoning. However, there has been controversy as to whether oximes improve morbidity and mortality in human poisoning. The explanation may be that the solvents in many formulations are primarily responsible for the high morbidity and mortality; oximes would not be expected to reduce the toxicity in these circumstances even if given early and in the appropriate dose [8]. Other complications include endocrine and thyroid disruption, immune system dysfunction, reproductive toxicity, and cancer, adverse effects on fetal and child development, disruption of microbiota [9]. Rare complications include hemorrhagic cystitis and transient diabetes insipidus [10]. Motawei and Elbiomy (2017) tried adding the famous antioxidant 'N-acetylcysteine' and blood alkalinization by Sodium bicarbonate (NaHCO3 ) to the treatment regimen of the OPP patients, and they found significant improvements in the clinical and laboratory parameters of the treated cases with these available pharmacologic agents, and significantly shorter hospital stay than the OPP cases who received the classic treatment regimen only. In addition to the lack of the side effects of these pharmacologic agents when used properly [3], there is a need for a more formulation of the use of these new drugs for the treatment of the OPP [3].
Aim of the work
This study was conducted to assess the efficacy of sodium bicarbonate and the N-acetyl cysteine in the treatment of Organophosphorus-poisoning cases in relation to the severity of the case. This is to formulate to which degree of the OPP severity will NAC and NaHCO3 will be most useful, after they have improved the outcome in a previous clinical trial by the investigators [3].
Subjects and methods
This is a prospective randomized controlled clinical trial that was carried out on 140 patients diagnosed of poisoning by Organophosphorus compounds. The patients were attending to the Toxicology Unit in Mansoura Emergency Hospital in the period from July 2018 to August 2019.
Patients were divided into 2 groups of 70 patients each; a control group which received the classic treatment protocol of the Organophosphorus poisoning and a second group that received, in addition to the classic treatment, the N-acetyl cysteine and sodium bicarbonate.
N-acetyl cysteine was given in a dose of 400 mg/day, and sodium bicarbonate was given in a dose of 2-5 mEq/kg IV infusion over 4-8 h. Subsequent doses should be based on patient's acid-base status until reaching the target arterial pH of 7.50 (range 7.45-7.55) [3].
Patients' age ranged from 17-60 years. Sixty (60) cases were female, and 80 cases were male. The median age of females was 30 years, range of 18-49 years. The median age for males was 34 years. (range 17-60 years). Excluding patients with concomitant other diseases, e.g., liver, kidney or respiratory diseases and cases with other drugs co-ingestion or exposure were also excluded.
Patient’s severity of intoxication ranged from mild to severe degrees. The selection of patients to receive the additional treatments plus the classic treatment, or the classic treatment only was done by a simple random sampling. The study was approved from the local ethical committee and informed written consents were obtained from all subjects included in this study. Forms of the approval and the consent are included later.
Diagnosis of Organophosphorus poisoning was made based on the history and the clinical manifestations. Five common clinical manifestations of OP poisoning have been selected as parameters (miosis, increased secretions, diaphoresis, bronchospasm, bradycardia) each to be assessed on a 3-point scale varying from 0-2. Poisoning can then be graded as mild (score 0-3), moderate (score 4-7) or severe (score 8-11) with the patient's first presentation. Increased secretions include salivation, lacrimation, urination, diarrhea, and vomiting [2].
Other manifestations include smell of pesticides or solvents, and reduced butyryl cholinesterase or acetyl cholinesterase activity in the blood [2]. Patients with severe Organophosphorus poisoning show reduced consciousness and poor respiration. The major differential diagnosis is carbamate poisoning, which is clinically indistinguishable [4]. Many Organophosphorus pesticides are more potent inhibitors of butyryl cholinesterase than they are of acetyl cholinesterase. So, butyryl cholinesterase assays can be used to detect the exposure to an organophosphates or carbamates pesticide [11].
Treatment of cases after exposure includes resuscitation of patients and giving oxygen, a muscarinic antagonist; atropine in a dose of 0.01-0.03 mg/kg, fluid therapy in the form of normal saline 0.9% in a dose of 10-20 ml/kg, and an acetyl cholinesterase reactivator (an oxime that dephosphorylate the acetyl cholinesterase rendering the enzyme active again). The oxime; Toxogonin is given in a dose of 3-7 mg/kg. This continued for 24 hours and better to be done in an Intensive Care Unit (ICU). Respiratory support is given, as necessary. The patient is usually vomiting, so gastric decontamination is not needed. If it will be done, it should be considered only after the patient has been fully resuscitated and stabilized and will be done considering the protective measures against aspiration. Continuous observation is mandatory, adjustment of atropine and oxime needs should be done. Worsening respiratory function because of intermediate syndrome, and recurrent cholinergic manifestations may occur with fat-soluble Organophosphorus compounds [12].
The patients were monitored in an Intensive Care Unit (ICU) setting and were compared for the clinical status and the routine laboratory tests including Arterial Blood Gases (ABG) and serum electrolytes. Blood samples were withdrawn by a simple venipuncture under sterile conditions at the time of arrival of the patient and 24 hours after the treatment for the measurements of MDA, GPx, blood pH and the plasma cholinesterase enzyme level, which was assessed, in addition, on the discharge of the cases.
Materials and Methods
Drugs
The following drugs were used.
N-acetylcysteine: “Acetylcysteine sachets” each contains 200 mg acetylcysteine. It is produced by Sedico pharmaceutical Company, 6th October City, Egypt.
NaHCO3 : Egypt Otsuka pharmaceutical Co., SAE. Ampoules of 25ml, 8.4% Sodium bicarbonate for intravenous infusion.
Kits
Malondialdehyde was assayed by kits purchased from Biodiagnostic Chemical Co., Egypt (Cat. No. MD 2529), using spectrophotometer (Model Metrolab 1600 DR, Serial Number 071216D39, Argintine) with wavelength adjusted at 534 nm. Glutathione peroxidase (GPx) was assayed by kits purchased from Biodiagnostic Chemical Co., Egypt (Cat. No. GP 2524), using spectrophotometer (Model Slim, Serial Number 327288, Italy) with wavelength adjusted at 340 nm. Butyrylcholinesterase assay kits of Sigma Aldrich (Cat. No. C1057).
Blood samples of 2 ml were withdrawn from the patients by simple venipuncture under sterile conditions. The serum was separated by centrifuging the blood sample at 3000 rpm for 5 min. Following which the serum MDA was measured using the method of Walker and Shah [13].
Glutathione peroxidase was measured by Spectrophotometer (Model Slim, Serial Number 327288, Italy) with wavelength adjusted at 340 nm. While buturylcholinesterase was measured by spectrophotometric assay of BChE titrimetrically in a 50.4 ml reaction mixture containing 4 mM butyrylcholine, 1, 600 mM MgCl 2 , 100 mM NaCl, and 30-60 units BChE at pH 8 and a temperature of 37°C [3].
Results
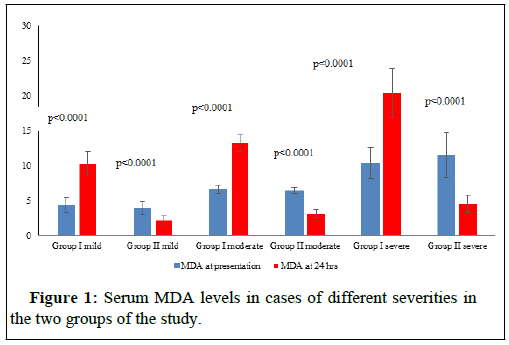
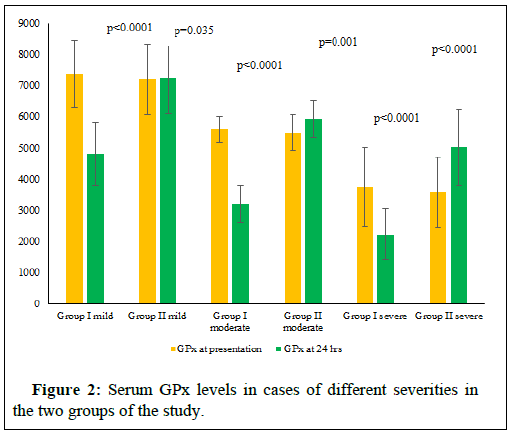
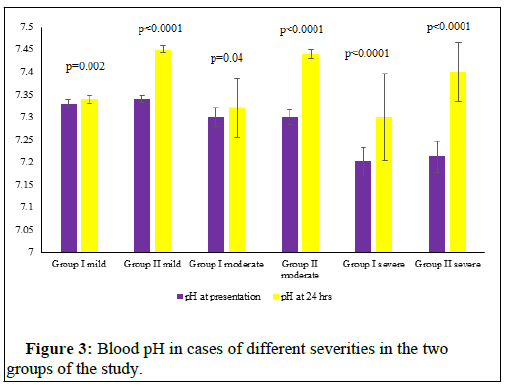
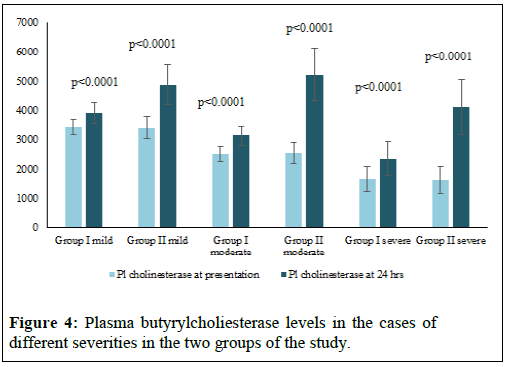
The Table 1 represents the socio- demographic data and the circumstances of poisoning in the cases of the study. Table 2 represents the different parameters of the study in the cases of different severity in the two groups on presentation. The figures (1-4) represent the different parameters of the study, measured in the two groups, and overtime, in the mild, moderate, and severe degrees of poisoning in the studied cases.
| Control group (n=70) | Treated group (n=70) | p-value | |
|---|---|---|---|
| Age | |||
| ≤25 years n. (%) | 24 | 23 | 0.9 |
| >25 years n. (%) | 46 | 47 | |
| Residence | |||
| Rural n. (%) | 40 | 39 | 0.9 |
| Urban n. (%) | 30 | 31 | |
| Gender | |||
| Male n. (%) | 35 | 37 | 0.7 |
| Female n. (%) | 35 | 33 | |
| Occupation | |||
| Farmer n. (%) | 41 | 35 | 0.3 |
| Non-farmer n. (%) | 29 | 35 | |
| Mode of poisoning | |||
| Intentional n. (%) | 25 | 27 | 0.8 |
| Accidental n. (%) | 18 | 20 | |
| Occupational n. (%) | 27 | 23 | |
| Route of exposure | |||
| Ingestion n. (%) | 35 | 37 | 0.9 |
| Inhalation n. (%) | 27 | 25 | |
| Dermal contact n. (%) | 8 | 8 | |
| Severity of toxicity | |||
| Mild n. (%) | 23 (32.86%) | 17 (24.29%) | 0.5 |
| Moderate n. (%) | 25 (35.71%) | 26 (37.14%) | |
| Severe n. (%) | 22 (31.43%) | 27 (38.57%) | |
Number (n.); Percent (%); p-value is significant at <0.05; Test used-Independent sample t-test (t)
Table 1: Socio-demographic data and the circumstances of poisoning in the cases of the study.
| Measure mean (SD) | Group I | Group II | Test of significance |
|---|---|---|---|
| Mild N=23 | Mild N=17 | ||
| MDA on presentation | 4.3 (1.1) | 3.9 (0.9) | t=0.97, p=0.3 |
| GPx on presentation | 7376.5 (1075.6) | 7192.1 (1134.6) | t=0.5, p=0.6 |
| pH on presentation | 7.33 (0.0089) | 7.34 (0.0086) | t=2.2, p=0.037 |
| Plasma cholinesterase on presentation | 3428.5 (265) | 3416.4 (378.5) | t=0.12, p=0.9 |
| LOS | 35.2 (4) | 21.6 (3.02) | t=11.7, p<0.0001 |
| Moderate N=25 | Moderate N=26 | ||
| MDA on presentation | 6.6 (0.6) | 6.4 (0.4) | t=1.7, p=0.09 |
| GPx on presentation | 5580 (420.6) | 5488.3 (567.1) | t=0.7, p=0.5 |
| pH on presentation | 7.3 (0.02) | 7.3 (0.016) | t=1.1, p=0.3 |
| Plasma cholinesterase on presentation | 2507.4 (255.8) | 2553.8 (344.6) | t=0.5, p=0.6 |
| LOS | 47.8 (1.5) | 24.2 (5.6) | t=20.5, p<0.0001 |
| Severe N=22 | Severe N=27 | ||
| MDA on presentation | 10.3 (2.2) | 11.5 (3.2) | t=1.5, p=0.1 |
| GPx on presentation | 3739.4 (1259.5) | 3568.4 (1124.7) | t=0.5, p=0.6 |
| pH on presentation | 7.202 (0.031) | 7.212 (0.035) | t=0.97, p=0.3 |
| Plasma cholinesterase on presentation | 1654.1 (435.8) | 1620.7 (473.8) | t=0.3, p=0.8 |
| LOS | 60.7 (7.8) | 28.7 (9.4) | t=12.8, p<0.0001 |
Length of Hospital Stay (LOS); Number (n); p-value (p); Test used: Independent samples t-test (t).
Table 2: Different parameters of the study in the case of different severity in two groups on presentation.
Note: MDA- Malondialdehyde; H-hours.
Note: GPx-Glutathione peroxidase, H-hours.
Note: pH- A scale to express the acidity or alkalinity of a solution (here it is the blood), H-Hours.
Note: H-Hours.
Statistical analysis
Data was tabulated, coded, and analyzed using the computer program SPSS (Statistical Package for Social Science) version 21.0. (SPSS Inc., Chicago, IL, USA). Quantitative data were described as means (SD) or medians, as appropriate, after testing for normality by Kolmogorov-Smirnov test. In the normally distributed variables, independent samples t-test was used for comparison between groups, while paired samples t-test was used for comparison between pairs within groups. In the non-normally distributed variables, Kruskal-Wallis test and Mann Whitney test were used for comparison between groups, as appropriate. "p-value ≤ 0.05" was considered statistically significant.
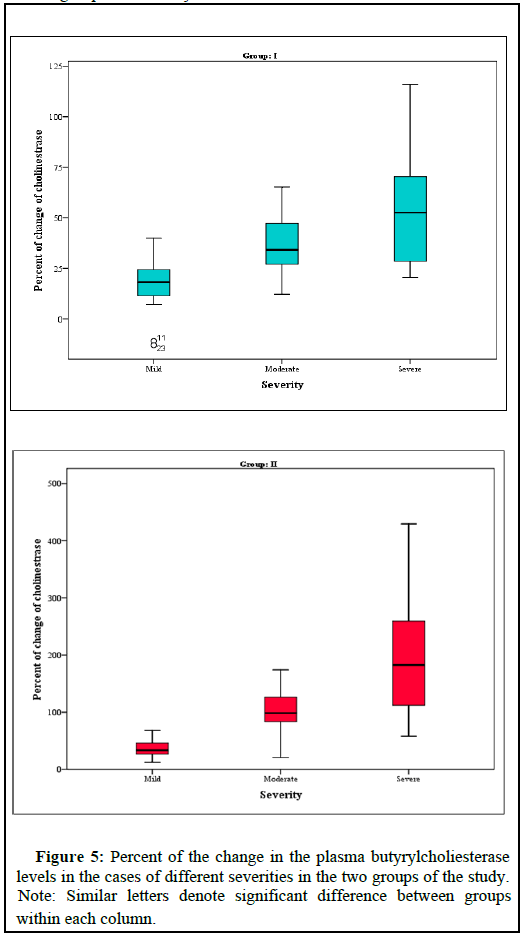
Figure (5) represents the percent of the change in the Plasma butyrylcholiesterase levels in the cases of different severities in the two groups of the study.
Discussion
Drawing a broad range of sources of exposure to OPP, it remains a major cause of poisoning mortalities and morbidities worldwide [2], and the cases fatalities are exceeding 20%, even in the most advanced treatment settings with the conventional antidotes 'atropine plus oximes' and may be benzodiazepines. This denotes the need for a new treatment regimen for OPP [14]. In a previous clinical trial by Motawei and Elbiomy, N-acetyl cysteine and sodium bicarbonate proved efficient in the treatment of acute organophosphorus-poisoned patients together with the classic antidote regimen. In this current study, we needed to formulate that NAC and NaHCO3 will be most useful to which degree of the OPP severity. In this study, N-acetyl cysteine and sodium bicarbonate were tried for the treatment of acute organophosphorus-poisoned patients together with the classic antidote regimen. This was compared with a matched group of patients who were treated with the classic antidote regimen only, without these additional therapies. The diagnosis of cases was based on history, clinical manifestations, and laboratory investigations of reduced serum cholinesterase levels of 50% less than average range of the enzyme. The patients were observed for 24 hours. and clinical and laboratory outcomes were recorded.
There were no statistical differences between the two groups of the study in the sociodemographic data and the number of cases of different severity of poisoning (Table 1). There was found no significant differences between the two groups of the study with different degrees of poisoning severity, in the levels of serum MDA, GPx and pH at the time of presentation (Table 2). Also, plasma Butyryl cholinesterase was 50% or more, less than the normal range, in both groups at the time of the patients’ presentation and there were no significant differences between its levels in the two groups (Table 2). The differences between the two groups of the study in all the above parameters turned highly significantly noticed after 24 hours, of adding the new treatment as shown in Figures 1-4.
Motawei and Elbiomy described these impressive effects of adding NAC and NaHCO3 for the protocol of treatment of OPP [3]. In this current clinical trial, we are testing the effects of these new antidotes in relation to the different degrees of severity of OPP. There is an observed non-significant difference (p=0.035) in blood levels of GPx in the mild cases of group II (who received the supplements plus the classic treatment of OPP) after 24 hours of treatment, with the onpresentation time. This can be explained that the antioxidant effects of NAC had compensated the oxidative stress happening with the OPP condition, and helped hepatic production of the antioxidant enzyme, that the group I has shown significant reduction (p=0.0001) (Figure 2).
These results go with Rambabu, et al. who stated that oxidative damage is one mechanism of the organophosphates poisoning, and that NAC proved to mitigate the toxic effects of the OPs. The authors encouraged clinical trials of large numbers of patients with OPP, to settle NAC as an antidote in the OPP treatment protocol [15]. The highly significant (p=0.0001) decrease in the length of the hospital stay in the cases of all degrees of severity, who received the NAC and the NaHCO3, than in the cases who did not receive them, means that these drugs have reduced the need for atropine and oximes for the reversal of the OPP manifestations. Other benefits of decreased LOS are the reduction in the economic costs, human efforts, and patients' risks of longer hospitalizations (e.g., getting NCI, absence from work, psychiatric effects), findings that go with Karaca and Ertaşkın [16]. We found that NAC and NaHCO3 as adjuvant therapies in cases of OPP, has improved the cholinesterase enzyme levels as compared on discharge and on presentation, more in the severe cases (p<0.0001), than in the mild and the moderate cases (Figure 5). This finding goes with Mohammadi, et al. who tried NAC in OPP and found that it helps oximes in the reactivation of plasma and RBC ChEs through a stepwise addition-elimination process [17]. Vahid S, Shetab-Boushehri believed that sodium bicarbonate increases ChE re-activation by oximes and increases survival of the OPP patients, when given early in the toxicity. Otherwise, it will increase the risk of the enzyme aging if there has been saturation of all available free ChEs active sites by OPs [18,15].
Conclusion
Authors stated that antioxidants and sodium bicarbonate are amongst the many affordable pharmacologic products that can greatly improve the organophosphate toxicity and decrease its complications like the OPIDN. The authors recommended many clinical trials to be conducted, so helpful agents can be licensed for routine clinical use in the settings of organophosphate poisoning.
Recommendations
Organophosphorus poisoning constitutes a major clinical and public health problem across the developing world. The clinical care of patients with Organophosphorus (OP) insecticide poisoning has little improved over the last six decades. N-acetyl cysteine and sodium bicarbonate are affordable pharmacologic agents that proved effective in increasing the survival of OPP cases and in decreasing the complications of poisoning. NAC is more effective in severe cases of OPPs. Larger randomized controlled trials should be made, so affordable and already licensed antidotes may find their place in the routine clinical care of OPP patients.
Conflicts of Interests
None.
References
- Adeyinka A, Muco E, Pierre L (2021) Organophosphates. StatPearls Publishing
- Robb EL, Baker MB (2017) Organophosphate toxicity. StatPearls Publishing
- Motawei SM, Elbiomy AA (2017) Sodium bicarbonate and N-acetyl cysteine in treatment of organophosphorus poisoning cases: A randomized controlled clinical trial. Toxicol Open Access 3:123.
- Adeyinka A, Kondamudi NP (2018) Cholinergic crisis. StatPearls Publishing
- Dardiotis E, Aloizou AM, Siokas V, Tsouris Z, Rikos D, et al. (2019) Paraoxonase-1 genetic polymorphisms in organophosphate metabolism. Toxicology 411:24-31.
- Jokanović M (2018) Neurotoxic effects of organophosphorus pesticides and possible association with neurodegenerative diseases in man: A review. Toxicology 410:125-131.
- Uprety AB, Pantha B, Karki L, Nepal SP, Khadka M (2019) Prevalence of intermediate syndrome among admitted patients with organophosphorous poisoning in a tertiary care hospital. J Nepal Med Assoc 57: 340-343.
- Mangas I, Estévez J, Vilanova E (2016) Esterases hydrolyze phenyl valerate activity as targets of organophosphorus compounds. Chem Biol Interact 259:358-367.
- Roman P, Cardona D, Sempere L, Carvajal F (2019) Microbiota and organophosphates. Neurotoxicology 75:200-208.
- Keyal NK, Bhujel A (2019) Transient diabetes insipidus following organophosphorus poisoning. J Crit Care Med 5:145-148.
- Hagstrom D, Hirokawa H, Zhang L, Radic Z, Taylor P, et al. (2017) Planarian cholinesterase: In vitro characterization of an evolutionarily ancient enzyme to study organophosphorus pesticide toxicity and reactivation. Arch Toxicol 91: 2837-2847.
- Hulse EJ, Haslam JD, Emmett SR, Woolley T (2019) Organophosphorus nerve agent poisoning: managing the poisoned patient. Br J Anaesth 123:457-463.
- Walker PD, Shah SV (1988) Evidence suggesting a role for hydroxyl radical in gentamicin-induced acute renal failure in rats. J Clin Invest 81:334-341.
- Eddleston M, Clutton RE, Taylor M, Thompson A, Worek F, et al. (2020) Efficacy of an organophosphorus hydrolase enzyme (OpdA) in human serum and minipig models of organophosphorus insecticide poisoning. Clin Toxicol 58:397-405.
- Rambabu L, Megson IL, Eddleston M (2020) Does oxidative stress contribute to toxicity in acute organophosphorus poisoning? A systematic review of the evidence. Clin Toxicol 58:437-452.
- Karaca O, Ertaşkın A (2020) Epidemiology of self-poisoning with drug in the Central Anatolian Region in Turkey. Cureus 12: e6962.
- Mohammadi H, Jalilian J, Karimi MY, Shetab-Boushehri SV (2017) In vitro cysteine reactivates organophosphate insecticide dichlorvos-inhibited human cholinesterases. Sultan Qaboos Univ Med J 17:e293-e300.
- Shetab-Boushehri SV (2019) Dual opposite actions of sodium bicarbonate in treatment of acute organophosphate poisoning. EXCLI J 18:677-678.
Citation: Motawei SM, Elbiomy AA , ElWafa HSA (2021) Sodium Bicarbonate and N-Acetyl Cysteine in Organophosphorus Poisoning Cases of Different Severity: A Randomized Controlled Clinical Trial. Toxicol Open Access 7: 168.
Copyright: © 2021 Motawei SM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 4256
- [From(publication date): 0-2021 - Dec 09, 2025]
- Breakdown by view type
- HTML page views: 3541
- PDF downloads: 715