Research Article Open Access
Sodium Dodecyl Sulphate/Poly(Brilliant Blue)/Multi Walled Carbon Nanotube Modified Carbon Paste Electrode for the Voltammetric Resolution of Dopamine in the Presence of Ascorbic Acid and Uric Acid
Ganesh PS and Kumara Swamy BE*
Department of PG Studies and Research in Industrial Chemistry, Kuvempu University, Jnana Sahyadri, Shankaraghatta, Shimoga, Karnataka, India
- *Corresponding Author:
- Kumara Swamy BE
Department of PG Studies and Research in Industrial Chemistry
Kuvempu University, Jnana Sahyadri, Shankaraghatta-577 451
Shimoga, Karnataka, India
Tel: +91 82822562251
Fax: +91 8282 256255
E-mail: kumaraswamy21@yahoo.com
Received date: September 03, 2015; Accepted date: October 13, 2015; Published date: October 20, 2015
Citation: Ganesh PS, Kumara Swamy BE (2015) Sodium Dodecyl Sulphate/ Poly(Brilliant Blue)/Multi Walled Carbon Nanotube Modified Carbon Paste Electrode for the Voltammetric Resolution of Dopamine in the Presence of Ascorbic Acid and Uric Acid. J Anal Bioanal Tech 6:285. doi:10.4172/2155-9872.1000285
Copyright: © 2015 Ganesh PS , et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Sodium dodecyl sulphate/poly(brilliant blue)/multi walled carbon nanotube modified carbon paste electrode was fabricated for the electroanalysis of dopamine in the presence of ascorbic acid and uric acid in phosphate buffer solution of pH 7.4. The key parameters such as sensitivity, selectivity, antifouling property and stability were achievedby the modified electrode. The redox peaks obtained at modified electrode shows good electrocatalytic activity towards the oxidation of dopamine. From the effect of scan rateand concentration the electrode phenomenon was confirmed to be adsorption-controlled process. The lower limit of detection of dopamine was 2.69 × 10-7M, and the simultaneous analysis shows a good result with peak to peak separation between dopamine and other two analytes ascorbic acid and uric acid by both cyclic voltammetry and differential pulse voltammetric techniques.
Keywords
Electroanalysis; Dopamine; Poly(brilliant blue); Multi walled carbon nanotube; Sodium dodecyl sulphate; Voltammetry
Introduction
In the last few decades the electrochemical methods are most widely studied and accepted for the determination of electroactive compounds in pharmaceutical samples and physiological fluids due to its simple, sensitive, rapid and economical properties [1,2]. Particularly the development of voltammetric sensors for both the analysis and determination of biologically important molecules such as dopamine (DA), ascorbic acid (AA) anduric acid (UA) has been received much more importance for the scientific growth of electroanalytical research [3-6]. DA is one of the naturally occurring neurotransmitter in the human brain belongs to the family of catecholamine and plays a very important role in the normal activity of the central and peripheral nervous systems [7,8]. Extreme abnormalities of DA levels are the major drawbacks of several diseases, such as Parkinsonism and Schizophrenia [9,10]. A patient suffering from this disease shows a low level of DA. UA is the primary product of purine metabolism in the human body and major nitrogenous compound inthe urine [11]. Its abnormality in human body leads to many diseases, such as gout, hyperuricaemia and Lesch-Nyan disease [12,13]. Increased urate level also leads to pneumonia and leukaemia [14]. AA is also known as vitamin-c and is water soluble compound that take part in many important life processes, it has been used as a medicament for the treatment of common cold, mental illness and cancer [15]. It can be chemically or electrochemically oxidized to dehydroascorbic acid [16]. Hence monitoring the concentration of these biological compounds is veryimportant in clinical diagnosis. Normally in the electrochemical detection of DA the major problem was the coexistence of the common interference AA and UA. Because of the similar oxidation potential of AA there is an always interference in analysing the DA electrochemically and gives overlapped and broad voltammetric response at bare carbon paste electrode (BCPE). To improve the performance of the BCPE the properties such as sensitivity, selectivity, antifouling property, reproducibility and stability are the key parameters [17-21]. Carbon nanotubes (CNTs) have been one of the most actively studied electrode material due to their unique electronic and mechanical properties and also the availability of high accessible surface area and low resistivity [22-25]. Recently carbon nanotubes modified electrodes were utilised to investigate the direct electrochemistry of several biomolecules [26-29]. A surfactant is a linear molecule with amphiphilic or amphipathic behaviour and they bear an ionicor non-ionic polar head group and a hydrophobic portion. Due to their unique molecular structure, surfactants have been employed extensively in the field of electroanalytical chemistry for various purposes [30-33]. In the present work the modification of the bare carbon paste electrode was achieved by using different quantity of multi walled carbon nanotube (MWCNT) along with carbon powder and a silicon oil binder by mechanical grinding method. In order to enhance both the sensitivity and selectivity it was further modified by immobilising the sodium dodecyl sulphate (SDS) to the surface of the electrode followed by electropolymerisation brilliant blue [34]. The fabricated electrode was employed for the electroanalysis of dopamine in presence of ascorbic acid and uric acid at physiological pH.
Experimental Section
Reagents
Multi walled carbon nanotube (110-170 nm) are received from Sigma Aldrich. Dopamine hydrochloride (DA), Uric acid (UA), Ascorbic acid (AA) were purchased from Himedia and the stock solutions of 25 × 10-4M, 25 × 10-3M and 25 × 10-3M were prepared in 0.1M perchloric acid, 0.1M NaOH and double distilled water respectively. Buffer used was 0.2M phosphate buffer solution (PBS) of pH 7.4. Graphite powder of 50 μm particle size was purchased from Merck and silicone oil from Himedia was used to prepare carbon paste electrode (CPE). All the chemicals mentioned were all of analytical grade used as received without any further purification.
Apparatus
The electrochemical experiments were performed using a model CHI-660c (CH Instrument-660 electrochemical workstation) at ambient temperature. A traditional three electrode compartment was used with a saturated calomel electrode (SCE) as a reference, a platinum counter electrode, and bare or Sodium dodecyl sulphate/poly(brilliant blue)/multi walled carbon nanotube modified carbon paste electrode (SDS/Poly(brilliant blue)/MWCNT/MCPE) as working electrode.
Preparation of the BCPE and MWCNT/MCPE
The bare carbon paste electrode (BCPE) was prepared by hand mixing of 70% graphite powder and 30% silicone oil in an agate mortar until a homogeneous paste was formed. The paste was then packed into a cavity of PVC tube of 3 mm internal diameter and smoothened on a tissue paper. The electrical contact was provided by a copper wire connected to the end of the tube. MWCNT/MCPE was prepared by homogeneously grinding different amounts of MWCNT in milligrams along with 70% graphite powder and 30% silicone oil.
Preparation of Poly(brilliant blue)/MWCNT/MCPE
The reported procedure was used for the preparation of poly(brilliant blue) film onto the MWCNT/MCPE surface [34]. Electrochemical polymerization of brilliant blue on the surface of MWCNT/MCPE was carried out using cyclic voltammetric method in aqueous solution containing 0.5mM brilliant blue in 0.1M NaOH solution. The electropolymerisation was achieved by the formation of film that grew between -0.5 V to +1.5 V at the scan rate of 0.1 Vs-1 for 10 cycles. After this the electrode was rinsed thoroughly with double distilled water.
Preparation of SDS/poly(brilliant blue)/MWCNT/MCPE
10 μL of SDS solution (0.1mM) was added by using micropipette on the surface of the poly(brilliant blue)/MWCNT/MCPE and allowed it for about 12 min at room temperature. The electrode was later thoroughly rinsed with double distilled water to remove unadsorbed SDS to get the SDS/poly(brilliant blue)/MWCNT/MCPE.
Results and Discussion
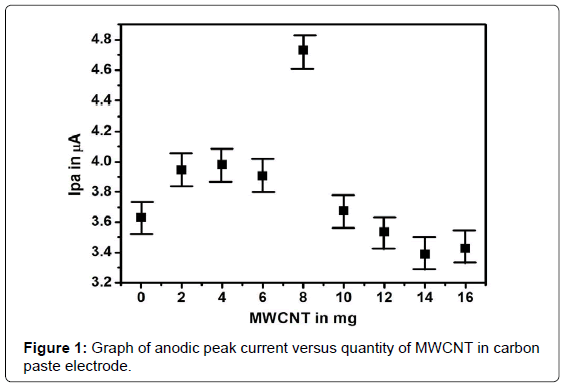
Effect of quantity MWCNT on the peak current of DA
The MWCNT MCPE was prepared by adding different amount of MWCNT to the carbon paste electrode and was employed for the oxidation of 0.1mM DA in 0.2M PBS of pH 7.4 using cyclic voltammetric (CV) technique. By increasing the quantity of MWCNT in the modification, the electrochemical cathodic peak current (Ipc) and anodic peak current (Ipa) goes on increasing at particular ratio. The modification procedure was calibrated from 2 mg to 14 mg. The redox peak currents were increased up to 8 mg MWCNT in carbon paste electrode. After this, the redox peak current was decreased as shown in Figure 1. Further increase in the quantity of MWCNTboth Ipa and Ipcwere decreased. Therefore, 8 mg MWCNT was chosen as optimum for the modification procedure. In order to enhance the sensitivity of detection, the electropolymerisation of brilliant blue on the surface of MWCNT/MCPE was carried out using CV methodin an aqueous solution containing 0.5mM brilliant blue in 0.1M NaOH solution. The electropolymerisation was achieved by the formation of film that grew between -0.5 V to +1.5 V at the scan rate of 0.1 Vs-1 for 10 cycles. After this the poly(brilliant blue)/MWCNT/MCPE electrode was rinsed thoroughly with double distilled water.
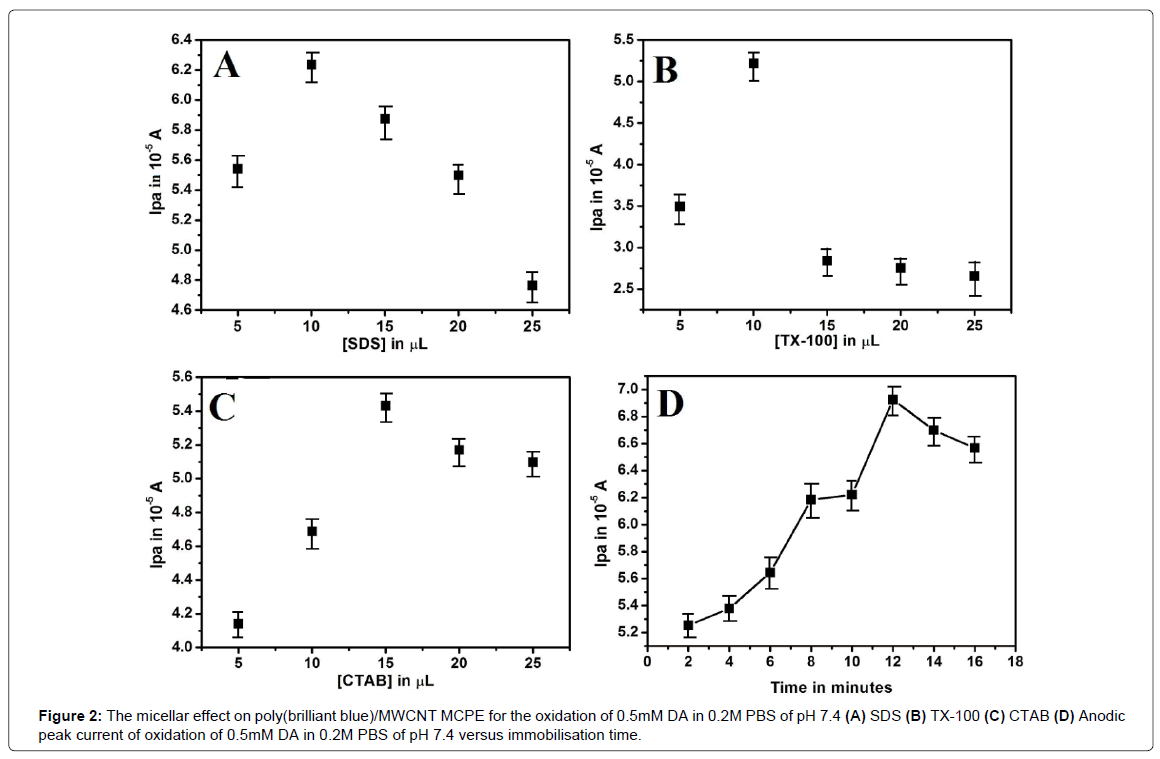
Micellar effect on Poly(brilliant blue)/MWCNT/MCPE for the oxidation of DA
Surfactants are proven to enhance the sensitivity of the electrode [35-37]. In the present study 0.1mM sodium dodecyl sulphate (SDS) an anionic, 0.1mM Triton X-100 (TX-100) a non-ionic, 0.1mM cetyl trimethyl ammonium bromide (CTAB) a cationic surfactant solutions of different concentration (5 μL to 25 μL) are immobilized on the surface of poly(brilliant blue)/MWCNT/MCPE for about 5 min and it is employed for the oxidation of 0.5mM DA in 0.2M PBSof pH 7.4 at the scan rate 0.05 Vs-1 using CV technique. All three surfactants shows noticeable enhancement in the peak currents of DA as shown in Figure 2(A), 2(B) and 2(C). However, SDS shows remarkable enhancement as compared with other two surfactantsnamely TX-100 and CTAB. At the concentration of 10 μL of SDS both anodic and cathodic peak currents was maximum as already illustrated in Figure 2(A). In order to calibrate the sensitivity of the electrode, again the influence of immobilization time was checked in the interval of 2 min each upto 16 min. The Ipa and Ipc go on increasing upto 12 min and later remains almost constant (Figure 2D). Hence the concentration of 10 μL SDS and immobilization time of 12 min was fixed as optimum to fabricate a stable working electrode to investigate all other remaining parameters.
Electrocatalytic oxidation of DA at SDS/poly(brilliant blue)/ MWCNT/MCPE
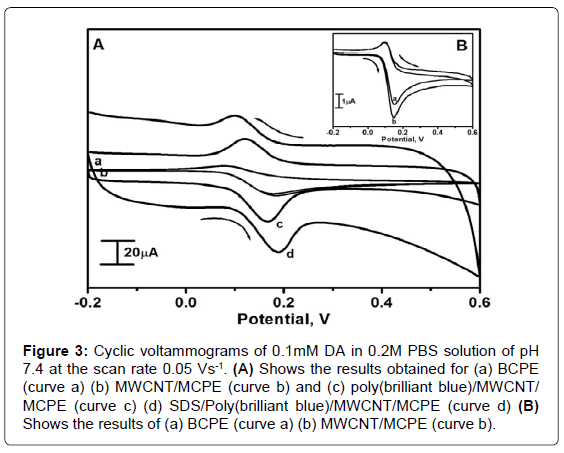
DA being an easily oxidizable electroactive catecholamine, its voltammogram was recorded in the potential range from -0.2 to 0.6 V. Figure 3A shows the cyclic voltammograms recorded for 0.1mM DA at BCPE (curve a) MWCNT/MCPE (curve b) poly(brilliant blue)/ MWCNT/MCPE (curve c) SDS/poly(brilliant blue)/MWCNT/MCPE (curve d) in 0.2M PBS of pH 7.4 with the scan rate 0.05 Vs-1. At BCPE (curve a) the oxidation potential was occurred at 0.15 V with poor voltammetric response and for the MWCNT/MCPE the oxidation occurred at 0.14 V vs. SCE as shown in inserted Figure 3B. However, at SDS/poly(brilliant blue)/MWCNT/MCPE (curve d) the oxidation potential was observed at 0.180 V with a slight shift in the oxidation potential towards positive side with the significant enhancement in the redox peak current signals. This enhancement of current signal reflects the electrocatalytic activity of SDS/poly(brilliant blue)/MWCNT/ MCPE towards the detection of DA.
Figure 3: Cyclic voltammograms of 0.1mM DA in 0.2M PBS solution of pH 7.4 at the scan rate 0.05 Vs-1. (A) Shows the results obtained for (a) BCPE (curve a) (b) MWCNT/MCPE (curve b) and (c) poly(brilliant blue)/MWCNT/ MCPE (curve c) (d) SDS/Poly(brilliant blue)/MWCNT/MCPE (curve d) (B) Shows the results of (a) BCPE (curve a) (b) MWCNT/MCPE (curve b).
Effect of scan rate
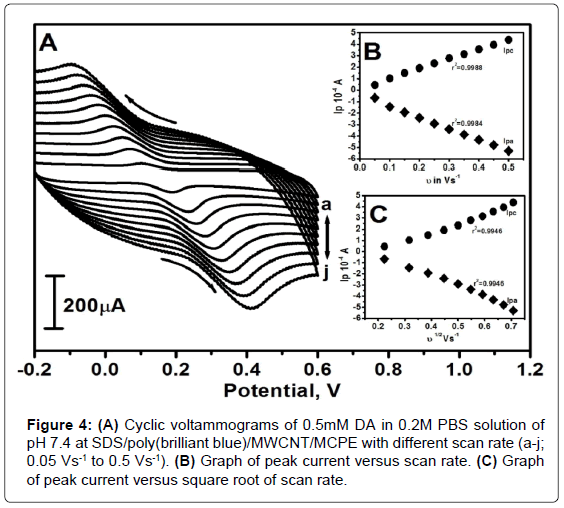
The effect of scan rate for 0.5mM DA in 0.2M PBS of pH 7.4 was studied by CV technique at SDS/poly(brilliant blue)/MWCNT/MCPE as shown in Figure 4A. The modified electrodeobeys Randles-Sevcik equation and showed increase in the redox peak currents with increase in the scan ratewith the small shifting of the redox peak potential. In order to confirm the electrode process, the graph of peak current (Ip) vs. scan rate (υ) was plotted and the obtained graph is a straight line with good linearity in the range from 0.05-0.5 Vs-1 as shown in Figure 4B the correlation coefficient (r2) was 0.9988 and 0.9984. The Ip vs. square root scan rate (υ1/2) were plotted as shown in Figure 4C with the correlation coefficient (r2) 0.9946 and 0.9946 this suggests the electrode process was adsorption controlled and in support to this logarithm of anodic peak current vs. logarithm of scan rate (Figure 5) was plotted and the determined slope was 0.8686 which confirms the electrode process was adsorption controlled process [38]. This was again supported by previously reported literatures [39].
According to an equation previously reported [40] for determining the value of heterogeneous rate constant (k0) from experimental peak potential difference (ΔEp) values, equation (1) was used.
ΔEp=201.39 log (υ/k0) - 301.78 (1)
From the experimental ΔEp values as shown in Table 1 and equation (1) the values of the k0 for the DA oxidation was determined. The value of k0 obtained at a scan rate of 0.05 Vs-1 for the SDS/poly(brilliant blue)/MWCNT/MCPE exhibits larger heterogeneous rate constant compared with those determined in other scan rate variation studies. All the parameters are tabulated in Table 1.
| υ/mVs-1 | ΔEp/mV | k0/ s-1 |
|---|---|---|
| 0.05 | 87.6 | 0.5827 |
| 0.10 | 165.4 | 0.4789 |
| 0.15 | 210.6 | 0.4286 |
| 0.20 | 256.9 | 0.3365 |
| 0.25 | 302.0 | 0.2511 |
| 0.30 | 309.2 | 0.2780 |
| 0.35 | 314.0 | 0.3069 |
| 0.40 | 311.0 | 0.3625 |
| 0.45 | 320.8 | 0.3650 |
| 0.50 | 314.0 | 0.4385 |
Table 1: Variation of the voltammetric parameters gathered from the plots shown in Figure 4 as a function of the potential scan rate.
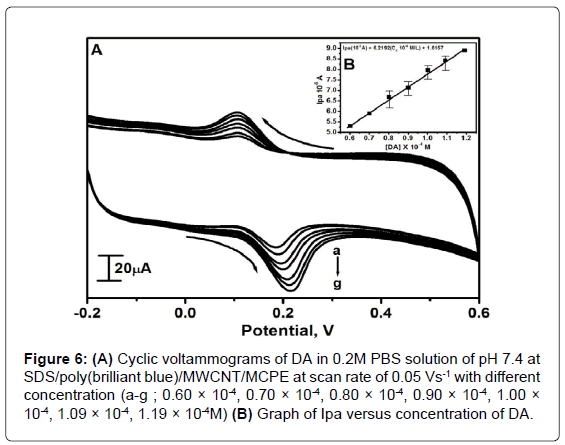
Effect of DA concentration
The electrocatalytic oxidation of DA was carried out by varying its concentration at SDS/poly(brilliant blue)/MWCNT/MCPE. Figure 6A shows by increasing the concentration of DA from 0.60 × 10-4 M to 1.19 × 10-4 M, the Ipa and Ipc goes on increasing with shifting Epa towards less positive and Epc towards least negative side. The graph of Ipa vs. concentration of DA was plotted as shown in Figure 6B it shows almost straight line with linear regression equation Ipa(10-5A)=6.2192(C010-4M/L)+1.6157, (r2=0.9975). The limit of detection was calculated [41,42] and the detection limit on the lower concentration range for DA was 2.69 × 10-7 M for the SDS/poly(brilliant blue)/MWCNT/MCPEand limit of quantification was 8.97 × 10-7M. The proposed electrode exhibited a relatively lower detection limit than those reported [43-46] as shown in Table 2.
Figure 6: (A) Cyclic voltammograms of DA in 0.2M PBS solution of pH 7.4 at SDS/poly(brilliant blue)/MWCNT/MCPE at scan rate of 0.05 Vs-1 with different concentration (a-g ; 0.60 × 10-4, 0.70 × 10-4, 0.80 × 10-4, 0.90 × 10-4, 1.00 × 10-4, 1.09 × 10-4, 1.19 × 10-4M) (B) Graph of Ipa versus concentration of DA.
| Working Electrode | Limit of Detection (mol/L) | Method | References |
|---|---|---|---|
| Bicopper complex modified GCE | 1.4 × 10-6 | DPV | [43] |
| CPE modified with SDS micelles at pH 7 | 3.70 × 10-6 | DPV | [44] |
| Ionic liquid modified Carbon paste electrode | 7.0 × 10-7 | CV | [45] |
| CTAB/CPE | 11 × 10-7 | DPV | [46] |
| SDS/poly(brilliant blue)/MWCNT/MCPE | 2.69 × 10-7 | CV | This work |
Table 2: Comparison of detection limits of different modified electrodes.
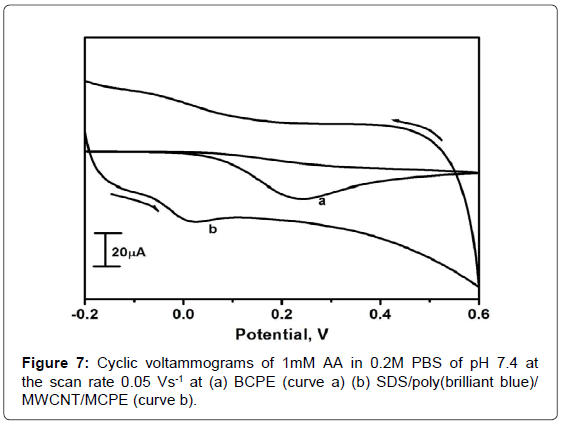
Electrochemical oxidation of AA at SDS/poly(brilliant blue)/ MWCNT/MCPE
Figure 7 showed the cyclic voltammograms of 1mM AA at the BCPE (curve a), SDS/poly(brilliant blue)/MWCNT/MCPE(curve b)in 0.2M PBS solution of pH 7.4 with the scan rate 0.05 Vs-1. At the BCPE the oxidation peak occurred at around 0.242 V and generally oxidation of AA at bare electrode was irreversible, seldom broad and required high over potential due to fouling of the electrode surface by the adsorption of oxidized product of AA. However, at the SDS/poly(brilliant blue)/ MWCNT/MCPEthe oxidation peak potential of AA was obtained at around 0.020 V which shifted to least positive potential and showed faster electron transfer kinetics of AA when compared to that of BCPE, which indicated that the SDS/poly(brilliant blue)/MWCNT/MCPE lowers the over potential and favoured the oxidation process of AA.
Figure 8A shows the cyclic voltammograms of AA at SDS/ poly(brilliant blue)/MWCNT/MCPEfor 1mM AA in 0.2M PBS of pH 7.4 in the scan raterange of 0.05 Vs-1 to 0.5 Vs-1 by increase the scan rate there was an increase in the anodic peak current (Ipa) and the oxidation peak potential was observed to shift positively with the increase in scan rate [47], in addition to this, the graph of Ipa versus υ and Ipa versus υ1/2 were plotted the graph obtained was linearly straight line shown in Figure 8B and Figure 8C respectively. A good linearity with correlation coefficients 0.9974 and 0.9970 indicated that the electrode transfer reaction was adsorption-controlled process on the SDS/poly(brilliant blue)/MWCNT/MCPEsurface.
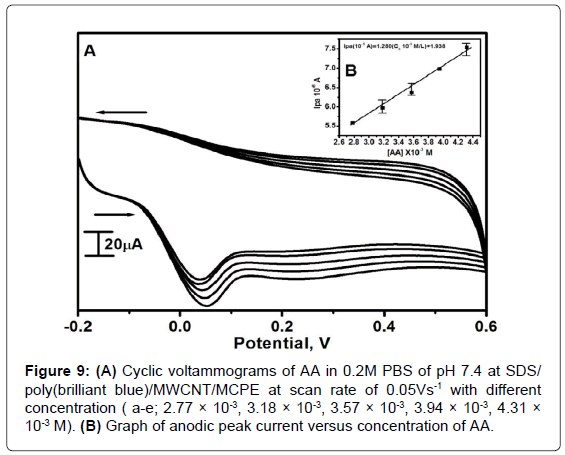
Effect of AA concentration
The electrochemical oxidation of AA was carried out by varying its concentration at SDS/poly(brilliant blue)/MWCNT/MCPE by using CV technique at scan rate 0.05 Vs-1. Figure 9A shows the voltammograms obtained for AA at different concentrations. By increasing the concentration of AA from 2.77 × 10-3M to 4.31 × 10-3M the Ipa was also increased. The graph of Ipa vs. different concentration of AA was plotted in Figure 9B the result showed linear increase in peak current with increase in the AA concentration with thelinear regression equation Ipa(10-5A)=1.280(C010-3M/L)+1.938, (r2=0.9926). The LOD and LOQ were 1.31 × 10-6 and 4.36 × 10-6 respectively.
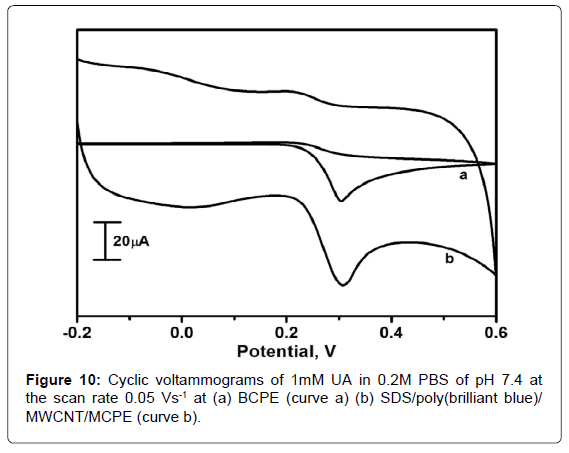
Electrochemical response of UA at SDS/poly(brilliant blue)/ MWCNT/MCPE
Figure 10 shows the cyclic voltammograms of 1mM UA for BCPE (curve a) and SDS/poly(brilliant blue)/MWCNT/MCPE(curve b)in 0.2M PBS of pH 7.4 with the scan rate 0.05 Vs-1. It is could be noticed that voltammetric peak appeared at about 0.304 V for BCPE, the peak was less sensitive rather broad suggesting slow electron transfer kinetics. However, at SDS/poly(brilliant blue)/MWCNT/MCPE the UA showed a significant increment in oxidation peak current and located at 0.307 V. By this it can be confirmed that there wasanoccurrence of electrocatalytic reaction between the SDS/poly(brilliant blue)/ MWCNT/MCPE and UA.
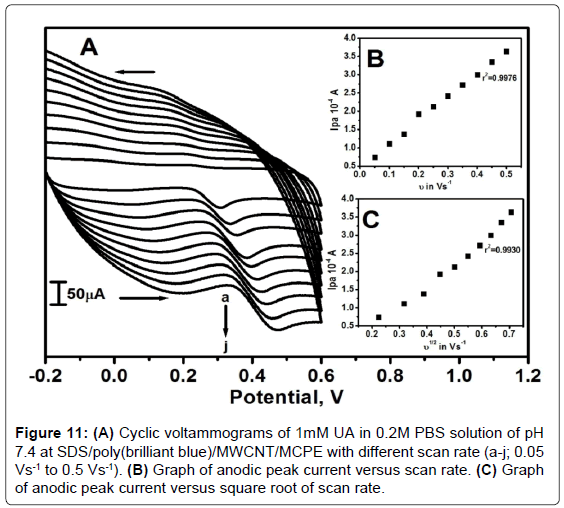
Figure 11A shows the cyclic voltammograms of UA at SDS/ poly(brilliant blue)/MWCNT/MCPE for 1mM UA in 0.2M PBS of pH 7.4 and scan rate from 0.05 to 0.5 Vs-1. The graph of Ipa versus υ was plotted in the range from 0.05 to 0.5 Vs-1 The graph obtained was linearly straight line shown in Figure 11B with correlation coefficient 0.9976. And a plot of Ipa versus υ1/2 in the same scan rate range showed correlation coefficient of 0.9930 as in Figure 11C. Therefore, it was confirmed that there was an adsorption complications of analytes on the surface of the SDS/poly(brilliant blue)/MWCNT/MCPE.
Effect of UA concentration
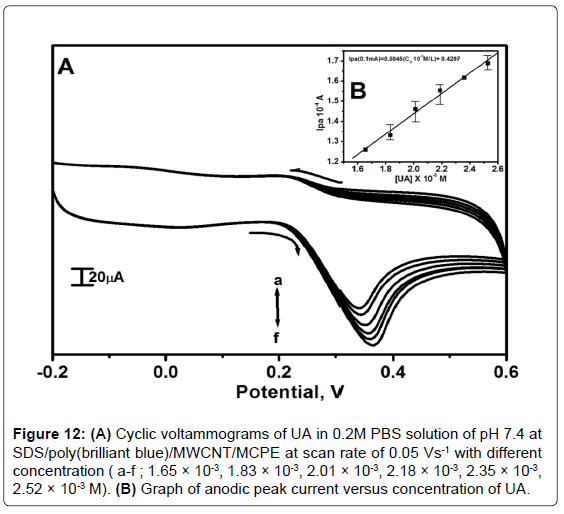
The cyclic voltammograms were recorded for the oxidation of UA with varying concentration in 0.2M PBS of pH 7.4 at scan rate 0.05 Vs-1. The cyclic voltammogram of different concentration of UA (1.65 × 10-3 M to 2.52 × 10-3 M) as shown in the Figure 12A which shows the increase in anodic peak current due to increase in the concentration of UA. The plot shown in the Figure 12B shows the linear relationship between Ipa and the concentration of UA with the linear regression equation Ipa(0.1 mA)=0.5045(C010-3M/L)+0.4297, r2=0.9945. The detection limit on the lower concentration range for UA was 4.36 × 10-6M for the SDS/poly(brilliant blue)/MWCNT/MCPE and limit of quantification was 1.10 × 10-5M.
Figure 12: (A) Cyclic voltammograms of UA in 0.2M PBS solution of pH 7.4 at SDS/poly(brilliant blue)/MWCNT/MCPE at scan rate of 0.05 Vs-1 with different concentration ( a-f ; 1.65 × 10-3, 1.83 × 10-3, 2.01 × 10-3, 2.18 × 10-3, 2.35 × 10-3, 2.52 × 10-3 M). (B) Graph of anodic peak current versus concentration of UA.
Simultaneous electroanalysis of DA, AA and UA
In mammalian brain AA and UA were present along with DA. Since the oxidation potential of both AA and UA were nearly same as that of DA results in a broad and overlapped voltammetric response at BCPE. Figure 13 shows CV recorded for 0.5 × 10-4M DA, 1.0 × 10-3M AA and 0.5 × 10-3 UA in 0.2M PBS of pH 7.4 at scan rate 0.05 Vs-1. At BCPE (curve a) the oxidative separation of all the three analytes was impossible due to fouling of the surface and gives poor voltammetric response. However, in the same condition the SDS/poly(brilliant blue)/MWCNT/MCPE(curve b) taken this task and separated all three analytes into well distinguished voltammetric signals, the oxidation potential of AA, DA and UA were located at 0.012 V, 0.173 V and 0.299 V respectively. The peak to peak separation of DA-AA was 0.161 V and that of DA-UA was 0.126 V. This result was sufficient to identify DA in presence of probable interferenceUA and AA atSDS/poly(brilliant blue)/MWCNT/MCPE.
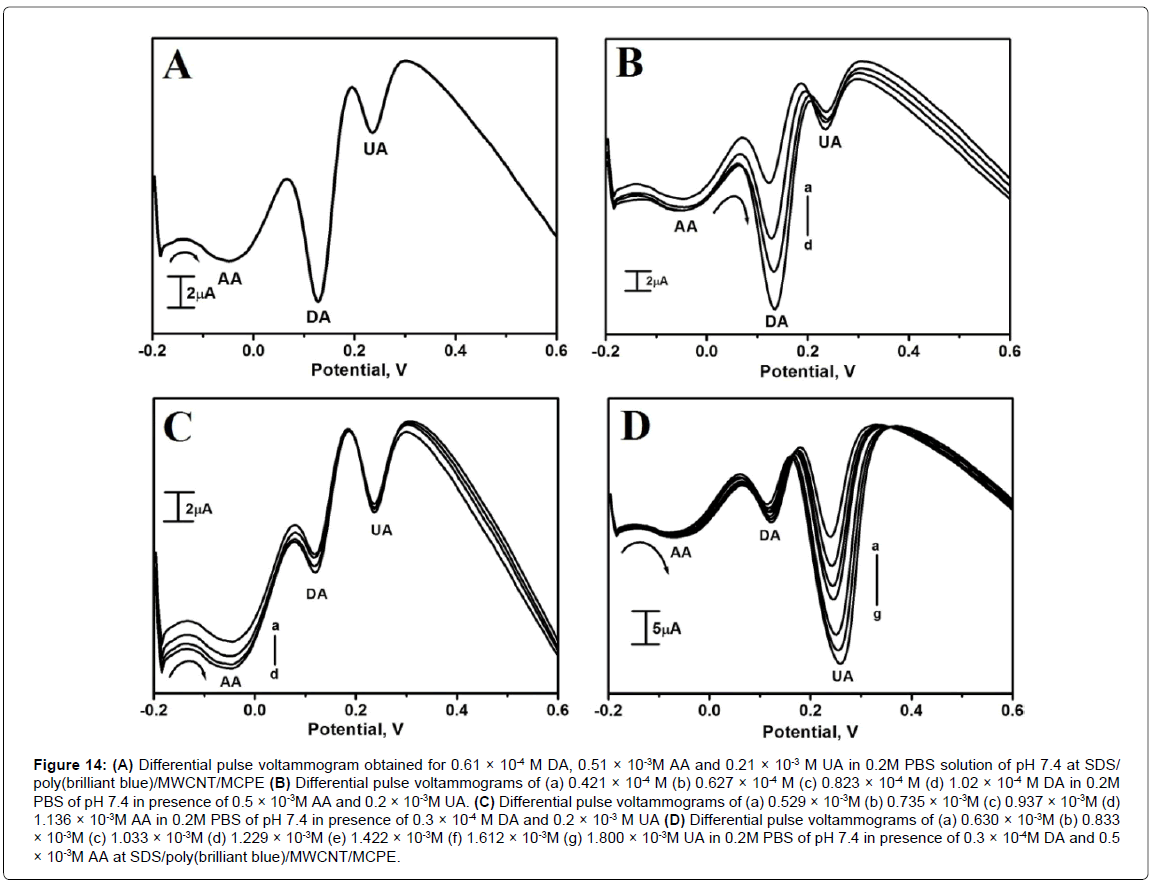
Differential pulse voltammetry (DPV) was used for the determination of DA, AA and UA at SDS/poly(brilliant blue)/ MWCNT/MCPEdue to its higher current sensitivity and absence of background current. The simultaneous study was carried out in the potential range from -0.2 to 0.6 V versus SCEthe Figure 14(A) shows the simultaneous determination of 0.61 × 10-4M DA, 0.51 × 10-3M AA, 0.21 × 10-3M UA in 0.2M PBS of pH 7.4 with well separated voltammetric signals corresponding to their oxidation at SDS/poly(brilliant blue)/ MWCNT/MCPE. The oxidation potential of DA, AA and UA was located at 0.126 V, -0.048 V and 0.234 V respectively. The peak to peak separation between DA-AA was 0.174 V and that of DA-UA was 0.108 V.
Figure 14: (A) Differential pulse voltammogram obtained for 0.61 × 10-4 M DA, 0.51 × 10-3M AA and 0.21 × 10-3 M UA in 0.2M PBS solution of pH 7.4 at SDS/ poly(brilliant blue)/MWCNT/MCPE (B) Differential pulse voltammograms of (a) 0.421 × 10-4 M (b) 0.627 × 10-4 M (c) 0.823 × 10-4 M (d) 1.02 × 10-4 M DA in 0.2M PBS of pH 7.4 in presence of 0.5 × 10-3M AA and 0.2 × 10-3M UA. (C) Differential pulse voltammograms of (a) 0.529 × 10-3M (b) 0.735 × 10-3M (c) 0.937 × 10-3M (d) 1.136 × 10-3M AA in 0.2M PBS of pH 7.4 in presence of 0.3 × 10-4 M DA and 0.2 × 10-3 M UA (D) Differential pulse voltammograms of (a) 0.630 × 10-3M (b) 0.833 × 10-3M (c) 1.033 × 10-3M (d) 1.229 × 10-3M (e) 1.422 × 10-3M (f) 1.612 × 10-3M (g) 1.800 × 10-3M UA in 0.2M PBS of pH 7.4 in presence of 0.3 × 10-4M DA and 0.5 × 10-3M AA at SDS/poly(brilliant blue)/MWCNT/MCPE.
Interference study
The interference study was carried out in themixture of samplesin an electrochemical cell containing DA, AA and UA. In their mixtures DPV was performed at the SDS/poly(brilliant blue)/ MWCNT/MCPEwhen the concentration of one species is changed, whereas the concentration of the other two species was maintained constant. From the Figure 14(B) it can be noticed that the peak current of DA was increased from 0.421 × 10-4M to 1.02 × 10-4M by constant keeping of the AA and UA concentration to 0.5 × 10-3M and 0.2 × 10-3M respectively. From the Figure 14(C) and Figure 14(D) it is seen that by keeping the concentration of other two analytes constant the anodic peak current of AA or UA increased upto a certain concentration range.
Conclusion
A simple and convenient method for the modification of the BCPE was proposed. The preparedSDS/poly(brilliant blue)/ MWCNT/MCPE shows excellent sensitivity, reproducibility, antifouling property and electrocatalytic activity towards the electrochemical oxidation of DA in the mixture of solutions contains large excess of AA and UA at physiological pH of 7.4 by using both CV and DPV techniques. Because of the distinguished voltammetric response obtained at SDS/poly(brilliant blue)/ MWCNT/MCPE the peak to peak separation of DA-AA was 0.161 V and that of DA-UA was 0.126 V by CV technique. This result was more enough for the electroanalysis of DA in presence of common interferences AA and UA. The proposed method can be used for other neurotransmitters. The modified electrode acts as very good sensor for the detection of dopamine.
References
- Yao H, Sun Y, Lin X, Tang Y, Liu A, et al. (2007) Selective determination of epinephrine in the presence of ascorbic acid and uric acid by electrocatalytic oxidation at poly(eriochrome black T) film-modified glassy carbon electrode. Anal Sci 23: 677-682.
- Jin GP, Lin XQ (2004) The electrochemical behavior and amperometric determination of tyrosine and tryptophan at a glassy carbon electrode modified with butyrylcholine. Electrochem Comm 6: 454-460.
- Razmi H, Agazadeh M, Habibi-A B (2003) Electrocatalytic oxidation of dopamine at aluminum electrode modified with nickel pentacyanonitrosylferrate films, synthesized by electroless procedure. J Electroanal Chem 547: 25-33.
- Zhang Y, Cai Y, Su S (2006) Determination of dopamine in the presence of ascorbic acid by poly(styrene sulfonic acid) sodium salt/single-wall carbon nanotube film modified glassy carbon electrode. Anal Biochem 350: 285-291.
- Xu F, Gao M, Wang L, Shi G, Zhang W, et al. (2001) Sensitive determination of dopamine on poly(aminobenzoic acid) modified electrode and the application toward an experimental Parkinsonian animal model. Talanta 55: 329-336.
- Ganesh PS, Swamy BEK (2015) Simultaneous electroanalysis of norepinephrine, ascorbic acid and uric acid using poly(glutamic acid) modified carbon paste electrode. J Electroanal Chem 752: 17-24.
- Wightman RM, May LJ, Michael AC (1988) Detection of dopamine dynamics in the brain. Anal Chem 60: 769A-779A.
- Raoof JB, Kiani A, Ojani R, Valiollahi R, Nadimi SR (2010) Simultaneous voltammetric determination of ascorbic acid and dopamine at the surface of electrodes modified with self-assembled gold nanoparticle films. J Solid State Electrochem 14: 1171-1176.
- Zhao GH, Li MF, Li ML (2007) Differential Pulse Voltammetric Determination of Dopamine with Coexistence of Ascorbic Acid on Boron-Doped Diamond Surface. Central European Journal of Chemistry 5: 1114-1123.
- Damier P, Hirsch EC, Agid Y, Graybiel AM (1999) The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson's disease. Brain 122: 1437-1448.
- Premkumar J, Khoo SB (2005) Electrocatalytic oxidations of biological molecules (ascorbic acid and uric acids) at highly oxidized electrodes. J Electroanal Chem 576: 105-112.
- Fox IH (1981) Metabolic basis for disorders of purine nucleotide degradation. Metabolism 30: 616-634.
- Raj CR, Kitamura F, Ohsaka T (2002) Square wave voltammetric sensing of uric acid using the self-assembly of mercaptobenzimidazole. Analyst 127: 1155-1158.
- Miland E, Miranda Ordieres AJ, Tuñón Blanco P, Smyth MR, Fágáin CO (1996) Poly(o-aminophenol)-modified bienzyme carbon paste electrode for the detection of uric acid. Talanta 43: 785-796.
- Dursun Z, Pelit L, Taniguchi I (2009) Voltammetric Determination of Ascorbic Acid and Dopamine Simultaneously at a Single Crystal Au(111) Electrode. Turk J Chem 33: 223-231.
- Wang Z, Liu J, Liang Q, Wang Y, Luo G (2002) Carbon nanotube-modified electrodes for the simultaneous determination of dopamine and ascorbic acid. Analyst 127: 653-658.
- Pacios M, Valle MD, Bartroli J, Esplandiu MJ (2008) Electrochemical behavior of rigid carbon nanotube composite electrodes. J Electroanal Chem 619: 117-124.
- Torres DS, Huerta F, Montillaa F, Morallon E (2011) Study on electroactive and electrocatalytic surfaces of single walled carbonnanotube-modified electrodes. Electrochim Acta 56: 2464-2470.
- Zare HR, Nasirizadeh N, Ardakani MM (2005) Electrochemical properties of a tetrabromo-p-benzoquinone modified carbon paste electrode. Application to the simultaneous determination of ascorbic acid, dopamine and uric acid. J Electroanal Chem 577: 25-33.
- Ganesh PS, Swamy BEK (2014) Electrochemical Determination of Dopamine in Presenceof Ascorbic Acid at Brilliant Blue Modified Carbon Paste Electrode: A Voltammetric Study. Journal of Chemical Engineering and Research 2: 113-120.
- Vasantha VS, Chen SM (2006) Electrocatalysis and simultaneous detection of dopamineand ascorbic acid using poly(3,4-ethylenedioxy)thiophenefilm modified electrodes. J Electroanal Chem 592: 77-87.
- Nugent JM, Santhanam KSV, Rubio A, Ajayan PM (2001) Fast Electron Transfer Kinetics on Multiwalled Carbon Nanotube Microbundle Electrodes. Nano Lett 1: 87-91.
- Gong K, Yan Y, Zhang M, Su L, Xiong S, et al. (2005) Electrochemistry and electroanalytical applications of carbon nanotubes: a review. Anal Sci 21: 1383-1393.
- Wang J (2005) Carbon-Nanotube Based Electrochemical Biosensors: A Review. Electroanal 17: 7-14.
- Patil RH, Hegde RN, Nandibewoor ST (2011) Electro-oxidation and determination of antihistamine drug, cetirizine dihydrochloride at glassy carbon electrode modified with multi-walled carbon nanotubes. Colloids Surf B Biointerfaces 83: 133-138.
- Umasankar Y, Thiagarajan S, Chen SM (2007) Nanocomposite of functionalized multiwall carbon nanotubes with nafion, nano platinum, and nano gold biosensing film for simultaneous determination of ascorbic acid, epinephrine, and uric acid. Anal Biochem 365: 122-131.
- Tsai YC, Chiu CC (2007) Amperometric biosensors based on multiwalled carbon nanotube-Nafion-tyrosinasenano biocomposites for the determination of phenolic compounds. Sens Actuat B Chem 125: 10-16.
- Zeng J, Gao X, Wei WZ, Zhai X, Yin J, et al. (2007) Fabrication of carbon nanotubes/poly(,2-diaminobenzene) nanoporous composite via multipulse chronoamperometric electropolymerization process and its electrocatalytic property toward oxidation of NADH. Sens Actuat B Chem 120: 595-602.
- Xu JM, Wang YP, Xian YZ, Jin LT, Tanaka K (2003) Preparation of multiwall carbon nanotubes film modified electrode and its application to simultaneous determination of oxidizable amino acids in ion chromatography. Talanta 60: 1123-1130.
- Rusling JF (1991) Controlling electrochemical catalysis with surfactant microstructures. Accounts of Chemical Research 24: 75-81.
- Gao JX, Rusling JF (1998) Electron transfer and electrochemical catalysis using cobalt-reconstituted myoglobin in a surfactant film. J Electroanal Chem 449: 1-4.
- Chowdappa N, Swamy BEK, Niranjana E, Sherigara BS (2009) Cyclic Voltammetric Studies of Serotonin at Sodium Dodecyl Sulfate Modified Carbon Paste Electrode. Int J Electrochem Sci 4: 425-434.
- Zhang SH, Wu KB (2004) Square Wave Voltammetric Determination of Indole-3-acetic Acid Based on the Enhancement Effect of Anionic Surfactant at the Carbon Paste Electrode. Bull Korean Chem Soc 25: 1321-1325.
- Ganesh PS, Swamy BEK (2015) Voltammetric Resolution of Dopamine in Presence of Ascorbic Acid and Uric Acid at Poly (Brilliant Blue) Modified Carbon Paste Electrode. J Anal Bioanal Tech 5: 229
- Sathisha TV, Swamy BEK, Chandrashekar BN, Thomas N, Eswarappa B (2012) Selective determination of dopamine in presence of ascorbic acid and uric acid at hydroxy double salt/surfactant film modified carbon paste electrode. J Electroanal Chem 674: 57-64.
- Sathisha TV, Swamy BEK, Reddy S, Chandrashekar BN, Eswarappa B (2012) Clay modified carbon paste electrode for the voltammetric detection of dopamine in presence of ascorbic acid. J Mol Liq 172: 53-58.
- Mahanthesha KR, Swamy BEK, Chandra U, Reddy S, Pai KV (2014) Sodium dodecyl sulphate/polyglycine/phthalamide/carbon paste electrode based voltammetric sensors for detection of dopamine in the presence of ascorbic acid and uric acid. Chemical Sensors 4: 10.
- Gosser Jr DK (1993) Cyclic Voltammetry Simulation and Analysis of Reaction Mechanisms (VCH, Weinheim).
- Gilbert O, Swamy BEK, Chandra U, Sherigara BS (2009) Electrocatalytic Oxidation of Dopamine and Ascorbic Acid at Poly (Eriochrome Black-T) Modified Carbon Paste. Electrode Int J Electrochem Sci 4: 582-591.
- Ganesh PS, Swamy BEK (2015) Simultaneous electroanalysis of hydroquinone and catechol at poly (brilliant blue) modified carbon paste electrode: A voltammetric study. J Electroanal Chem 756: 193-200.
- Mahanthesha KR, Swamy BEK (2013) Pretreated/Carbon paste electrode based voltammetric sensors for the detection of dopamine in presence of ascorbic acid and uric acid. J Electroanal Chem 703: 1-8.
- Zhu Z, Qu L, Guo Y, Zeng Y, Sun W, et al. (2010) Electrochemical detection of dopamine on a Ni/Al layered double hydroxide modified carbon ionic liquid electrode. Sens Actu B 151: 146-152.
- Wang M, Xu X, Gao J (2007) Voltammetric studies of a novel bicopper complex modifiedglassy carbon electrode for the simultaneous determination of dopamine and ascorbic acid. J Appl Electrochem 37: 705-710.
- Orozco EC, Silva MTR, Avendano SC, Romo MR, Pardave MP (2012) Electrochemical quantification of dopamine in the presence of ascorbic acid and uric acid using a simple carbon paste electrode modified with SDS micelles at pH 7. Electrochim Acta 85: 307-313.
- Sun W, Yang M, Jiao K (2007) Electrocatalytic oxidation of dopamine at an ionic liquid modified carbon paste electrode and its analytical application. Anal Bioanal Chem 389: 1283-1291.
- Avendano SC, Silva MTR, Pardave MP, Martinez LH, Romo MR, et al. (2010) Influence of CTAB on the electrochemical behavior of dopamine and on its analytic determination in the presence of ascorbic acid. J Appl Electrochem 40: 463-474.
- Zhao Y, Bai J, Wang L, Xu E, Hong P, et al. (2006) Simultaneous Electrochemical Determination of Uric Acid and Ascorbic Acid Using L-Cysteine Self-Assembled Gold Electrode. Int J Electrochem Sci 1: 363-371.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 15345
- [From(publication date):
December-2015 - Aug 23, 2025] - Breakdown by view type
- HTML page views : 10641
- PDF downloads : 4704