Spirulina platensis (Arthrospira spp.): A Potential Novel Feed Source For Pasture-Based Dairy Cows
Received: 05-Jul-2017 / Accepted Date: 20-Jul-2017 / Published Date: 20-Sep-2017 DOI: 10.4172/2332-2608.1000253
Abstract
The Australian dairy industry is heavily reliant on pasture as the main feed base for milk production. However, dietary energy intake is limited in pasture-based dairy systems and lactating cows tend to suffer from negative energy balance due to insufficient dry matter intake. As a result, low milk production and reproduction performances occur later on in the cow’s life-cycle. Spirulina is a highly nutritious feed source rich in proteins, lipids, minerals and vitamins, thus meeting the criteria as a potential and novel alternative feed source for the dairy industry. To our current knowledge, experimental and anecdotal information on the productive response of pasture-based dairy cows to the inclusion of Spirulina to lactating diets is lacking and warrants further elucidation. There is the need to understand the influence of Spirulina inclusion in a lactating cow’s diet on lactation and Live-weight parameters. This paper reviews the past and present research results on the effect of supplementation with Spirulina on animal production outcomes, summarises identified knowledge gaps and highlights future directions for research in the dairy industry.
Keywords: Spirulina; Dairy cows; Lactation; Live-weight; Milk fatty acid
Introduction
Dairy cows are globally emerging as the most economically important species of domesticated livestock for producing milk and milk products for human consumption [1]. The world demand for milk and milk products is on the rise because of the constant increasing quest for animal protein and the expanding market for milk products [2]. Traditionally, Australian dairy farmers rely on pasture as the main feed source for sustaining peak milk production. A major drawback is that pasture has limited and insufficient energy to sustain lactation length and consistent milk yield [3]. Also, milk fat content of solely pasturebased cows lacks desirable nutritive qualities. Therefore, alternative nutrient-rich dietary supplements that complement the traditional pasture feed base should be explored. Ideally, a rich feed source should be energy dense, highly digestible, nutritious and cost effective, and has no known negative impact on livestock and the environment [4]. In this context, Spirulina platensis fits the bill as a viable option for a novel, future feed source for dairy cows [5].
Spirulina platensis (Arthrospira sp.) is an edible cyanobacterial alga with a nutrient-rich chemical composition. It contains between 66-70% protein with an enviable profile of essential amino acids, polyunsaturated fatty acids, vitamins, minerals and carotenoids [6-10]. Spirulina has been evaluated as a feed additive for sheep, poultry, rabbits, and fish [7,11,12]. However, its application in cow diets within the dairy industry has neither been previously reported nor comprehensively studied, and represents a major knowledge gap.
Therefore, the main objective of this review is to provide an overview of previous studies on the effects of Spirulina supplementation on lactation and Live-weight parameters in important agricultural animals with an emphasis on dairy cattle. This review also summarises the identified knowledge gaps and highlights fruitful directions for future research aimed at unravelling the potential utilisation of Spirulina platensis as a dietary supplement in the dairy industry.
Spirulina platensis (Arthrospira spp.): Nutrient Composition, Production, Biological Processes and Utilisation
Nutrient composition of Spirulina
Spirulina contains between 7-11% lipids with 83% saponifiable fraction and 17% non-saponiofiable fraction [13]. In addition, Spirulina also have rich source of oleic, linoleic, linolenic acid [14]. Spirulina platensis (Arthrospira spp.) is a novel feed source that can provide adequate proteins, minerals, vitamins and essential fatty acids to lactating cows. It contains docosahexaenoic acid (DHA, 22:6) a long chain ω-3 polyunsaturated fatty acid (PUFA), and γ-linolenic acid (GLA) [15]. There is a range of 60-70% protein concentration in Spirulina with high bioavailability [15,16]. Adequate proportions of vitamins A and B12 have also been reported [12] along with macronutrients (Na, K, Ca and Mg) and micronutrients (Fe, Zn, Mn and Cu) [17]. Currently, there is limited production of Spirulina in Australia due to the associated high cost of production and a general lack of understanding on its potential use in the dairy industry and other livestock farming sectors. There is currently a high demand for Spirulina products for human consumption, and this has caused resurgence in the price of Spirulina [18]. However, animals can consume the same product in a powdered form, but it has an unpleasant odour which makes it undesirable to animals [16] (Table 1).
| Nutrient | % Composition | Details |
|---|---|---|
| Proteins | 65 | All 8 essential amino acids: isoleucine, leucine, methionine, phenylalanine, threonine, tryptophan and valine. |
| Carbohydrates | 15 | 10 non-essential amino acids: alanine, arginine, aspartic acid, cystine, glutamic acid, glycine, histidine, proline, serine and tyrosine. |
| Lipids | 6 | Gamma-linolenic acid (GLA), alpha-linolenic acid (ALA), linoleic acid (LA), stearidonic acid (SDA), eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and arachidonic acid (AA). |
| Vitamins | 0.75 | thiamine (B1), riboflavin (B2), niacin (B3), pyridoxine (B6), folic acid (B9), cyanocobalamin (B12), biotin (B7), vitamin D, pantothenic acid (B5), vitamin E (tocopherol), inositol. |
| Minerals | 8 | Potassium, calcium, chromium, copper, iron, magnesium, manganese, phosphorus, selenium, sodium, and zinc |
| Carotenoids | 346 mg/100 g (variation noticed according to the processing methods) | Alpha-carotene, beta-carotene, xanthophylis, cryptoxanthin, echinenone, zeaxanthin and lutein. |
Table 1: The major nutrients and compositions of Spirulinaplatensis.
Production of Spirulina platensis
Originally, Spirulina was found mainly in tropical regions of Central/South America and Africa, characterised by high bicarbonate, minerals and pH [19]. Spirulina was rediscovered in the 1960s by Leonard and Compere when they found that African tribes from Chad consumed Spirulina as food [20]. Since its rediscovery, mass production has occurred to fulfil the demands for human consumption and the aquaculture industry [20]. Previous reports have shown that Spirulina species can out-produce barley, wheat and corn, which are the traditional dairy feed supplements [21]. The promising attributes of Spirulina platensis indicate a need to increase its production in Australia. Peiretti and Meineri [11], Raoof et al. [22] and Shimamatsu [23] have suggested that increasing the nutrient 1use efficiency and growth rate in a low-cost growing medium should increase the production of Spirulina.
The biological processes of growing Spirulina platensis
Spirulina platensis grows by the biological process of simple cell division [20]. Spirulina platensis like many other photoautotrophs, depends on light for growth and photosynthesis. The stimulation of Spirulina growth has been reported to be affected by axenic culture inculcated with media containing saline, bicarbonates, peptones, minerals and glucose [24]. Spirulina platensis is a cyanobacterium that depends on the metabolic process of photosynthesis to obtain its chemical energy as shown below [19].
6CO2+12H2O →C6H12O6+6O2+6H2O
Spirulina platensis contains pigments such as chlorophyll, carotenoids and phycocyanobilin which are used to harvest radiations at different light spectra for photosynthesis [20]. Spirulina also contains another specialised pigmented protein called phycobilisome [25,26]. Phycobilisome combines with the other pigments to absorb light energy (550 nm-650 nm) where Chlorophyll-a cannot [19]. Most importantly, the harvested energy is directly channelled to the reaction centre in the thylakoid membrane where photosynthesis occurs. The presence of phycobilisome in combination with the other chromophores makes Spirulina an ordered and structured photosynthetic machinery which enables an efficient photosynthetic process that allows for faster growth and development of Spirulina platensis [19].
Spirulina platensis as a lipid source
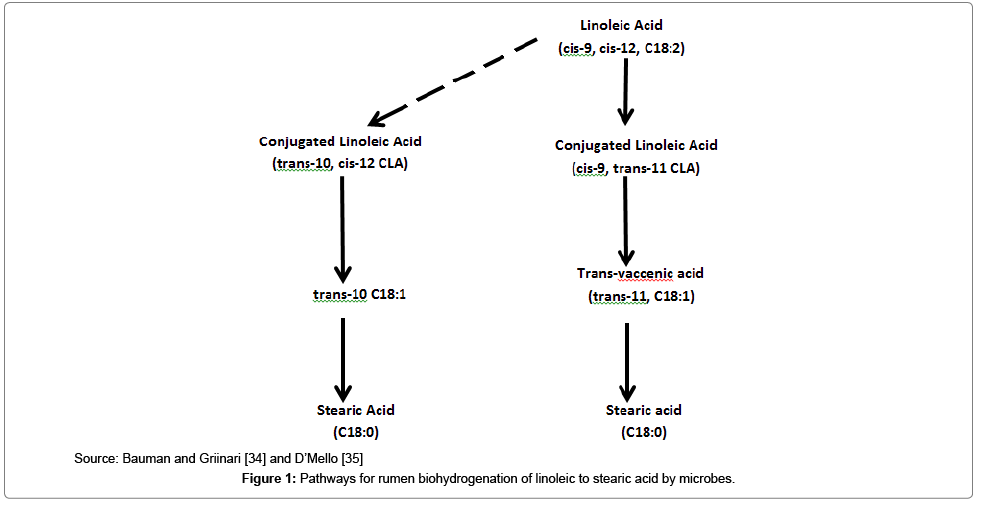
High producing and early-lactating cows are supplemented with lipids to increase the energy density of the ration [27]. Spirulina is a rich source of fat (7-11%) which can be utilised to increase energy intake of lactating cows. Lipids contain 2.5 times more energy that carbohydrates and proteins, and this could be essential for meeting the energy requirements of early lactating cows for milk production [28]. However, the downside to feeding fat to dairy cows is the unfavourable effect on rumen fermentation. The inclusion of fat in ruminant diets affects rumen fermentation and reduces digestibility. It is estimated that up-to 50% of structural carbohydrates is not digested when 10% fat is included in the diet of cows [29]. As a result, methane, hydrogen, volatile fatty acids production is reduced [29]. Reduced methane and hydrogen could be a good outcome because of climate change, but the same effect on volatile fatty acids would impact negatively on milk production. In addition, shortly after consumption, lipids are extensively hydrolysed and bio hydrogenated by lipase bacteria into different fatty acid intermediates in ruminants [30]. To combat the effect of bio hydrogenation, research on developing encapsulated rumen by-pass fat has been on-going [31]. Encapsulated fats can avoid bio hydrogenation and facilitate post-ruminal deposition of essential fatty acids [32]. The negative effect of bio hydrogenation is that an intermediate fatty acid, trans-10 cis-12 conjugated linoleic acid is produced and causes a coordinated reduction of mRNA in the mammary gland that affects fat synthesis [33-35]. This in return, causes reduced milk fat. Another effect of rumen biohydrogenation is the production of trans-fatty acid intermediates which function as saturated fats.
Research on lactating cows on the impact of supplementary fat on milk fat and milk protein in dairy cows abounds in the published literature. For instance, Lunsin et al. [27], Moghadam et al. [36], Lee et al. [37] and Resende et al. [38] found that dietary fat in lactating cow rations decreased milk fat and protein yields. However, encapsulation of dietary fat is an effective method of avoiding the impact of rumen biohydrogenation on milk composition and to improve the efficacy of fat supplementation within the dairy industry. Severe effects of rumen biohydrogenation on milk fat depression may be economically undesirable to dairy farmers because of its contribution to total milk solids upon which milk prices are based. Therefore, further studies in different dairy production systems are required to enable informed choices and tailored decisions when feeding lactating cows with specific dietary fat supplements.
Research Findings
Live-weight and body condition score
Live-weight and body condition score of a herd are intrinsically dependent on the quantity and quality of nutrition, which in-turn affects productivity and profitability of a farm. Live-weight traits are regularly used in the dairy industry to estimate the energy status of a cow during gestation, lactation and dry-off periods [39,40]. Most of the previous Spirulina studies conducted on Live-weight traits and gene expression patterns have mostly focussed on meat producing animals [6,8-10,41,42] without deliberation on milk animals [7]. Most pasture-based dairy system around the world is heavily reliance on pasture/forage as the main feed source [40]. Pasture-based system is where energy is most limiting. Limitation of energy intake has direct effect on Live-weight traits [3,43]. These physiological changes will influence Live-weight and body condition score of the cow. Therefore, to maintain a cow in good condition, it is important that they consume abundant energy dense diet adequate in protein, minerals, vitamins and essential fatty acid. Poultry, pigs and rabbits have been extensively studied to assess the beneficial effect of Spirulina supplement on Liveweight traits [7,11,12]. Little attention has been paid to dairy cattle. However, previous studies conducted into livestock growth responses to dietary supplementation with Spirulina have been consistently positive (Table 2).
| Authors | Species | Result |
|---|---|---|
| Holman et al. [6] | Sheep | Lambs consuming 10% Spirulina were heavier (41.9 ± 0.7 kg) than the control group (40.6 ± 0.7 kg) after 9 weeks of the experiment |
| Peiretti and Meineri [11] | Rabbit | The mean slaughter weight of rabbits feeding on 100 g/kg Spirulina was 3184 ± 73 g compared to the control group only 2983 ± 138 g after 31 days |
| Grinstead et al. [44] | Pig | Pigs fed 20 g SP kg-1 had greater ADG than pigs fed the control diet |
| Stanley and Jones [47] | Fish (Bigmouth buffalo Ictiobus cyprinellus) | Bigmouth buffalo Ictiobuscyprinellus was fed 29 g dry weight/kg body weight for 28 days and their daily growth was 14 g/kg body weight, and had a feed conversion efficiency of 2.0 |
| Kulpys et al. [21] | Cattle | Cows receiving 200 g of Spirulinaplatensis (Arthrospira spp.) were 11% fatter than the control |
Table 2: Summary of the positive impact of Spirulinaplatensis (Arthrospirasp) on growth traits of sheep, rabbits, pigs, fish and cattle.
In pig growth, trial with inclusion of 20 gkg-1 of Spirulina platensis increased the average daily gain significantly compared to the control [44]. A feeding trial with rabbits reported that an inclusion level of 10% Spirulina in the diet increased feed intake but no differences in final weight, weight gain and feed conversion efficiency [45]. In a study by Holman et al. [46], it was found that lambs supplemented with Spirulina were heavier and in better condition than the control. Lactating cows became fatter (8.5-11%) when supplemented with 200 g of Spirulina platensis daily [21]. Spirulina supplementation trial in bigmouth buffalo (Ictiobus cyprinellus) yielded positive daily growth and feed conversion efficiency outcomes [47]. From the review of these previous studies, there is a general tendency for Spirulina platensis to improve Liveweight and body condition score of livestock with marked differences in dosage. Therefore, there is the need for further investigation into the effect of Spirulina platensis on Live-weight and body condition score of high merit cows with different dosages in a pasture-based production system to enable informed choices and tailored decisions.
Spirulina and milk composition
Milk is an aqueous substance that is synthesised by the secretory epithelial cells of the mammary gland of a cow postpartum [48]. Milk is composed of water (87.3%), fat (3.9%), protein (4%) and solids non-fat (8.8%) [49]. Milk fat and proteins are the most important milk solids required by most milk payment schemes in Australia. Milk fats and proteins are affected mainly by nutrition and stage of lactation of the cow. Low milk fat and proteins are usually prominent in seasonally calving herds where adequate dry matter and energy intakes are limiting [50]. Therefore, the incorporation of energy-dense, nutrientrich supplements into the lactating ration of a cow in a seasonal calving production system is essential (Figure 1).
Milk yield: The primary factors affecting milk yield and its constituents in grazing cows are nutrition and energy intake. Milk yield response to lipid and protein supplements is dependent on the energy density of the diet [51] and Spirulina being a rich source of energy with adequate nutrients, is a suitable and apt supplement in a cow’s ration to increase milk yield. Kulpys et al. [21] found that dairy cows supplemented with 200 g of Spirulina platensis produced 6 kg more milk than those cows grazing pasture only. The positive impact of Spirulina platensis on milk yield was also confirmed by the study conducted by Šimkus et al. [52]. Other studies conducted elsewhere however found no effect of feeding algal-based diet on milk yield [53-56] (Table 3).
| Authors | Result |
|---|---|
| Stamey et al. [56] | Consuming n-3 rich algal had no effect on milk yield but increased the milk fat (P<0.05) |
| Moates et al. [55] | Consuming 20% docosahexaenoic acid algal meal had no effect on milk yield but decreased milk fat |
| Boeckaert et al. [53] | Cows consuming algal rich diet had decreased dry matter intake and milk yield, but the conjugated linoleic acid concentration was increased |
| Glover et al. [54] | Milk yield of cows consuming algae was not significantly affected but milk fat was significantly lowered |
| Simkus et al. [52] | Cows consuming Spirulinaplatensis had increased milk yield and milk protein |
Table 3: Summary of the impact of Spirulinaplatensis (Arthrospirasp) on milk yield and milk composition.
Spirulina being a rich source of protein can also be used as a protein supplement to increase milk yield and milk protein [21]. It has been demonstrated that milk yield can increase by up to 1.5 L/kg of protein supplement and the response can be lower if energy is limiting [51]. Feeding protein supplements to cows in early lactation caused an increase of between 0.4-1.8 kg milk/kg supplement [57]. All these results were mainly due to increases in dry matter and metabolisable energy intakes rather than the effect of protein content in the diet. Therefore, the positive response of milk yield to protein supplementation indicates that when dry matter energy intake is not limiting, there is a corresponding increase in milk yield. However, the conflicting findings in previous studies indicate a need to further investigate the effects of Spirulina platensis diets on lactating parameters, to unravel and understand the intricate relationship.
Milk protein and fat: Dietary crude protein in animal diets is degraded in the rumen to peptides, amino acids and ammonia [35,58]. The rumen microbes use dietary crude protein to synthesis their own microbial protein [59]. The production of microbial protein is dependent on the energy provided by the feed [60]. The response to protein supplementation is greatest when crude protein is readily available for microbial protein production [51]. Microbial protein is digested and absorbed along with some undegradable proteins from the diet as metabolisable protein, which is utilised in vivo for milk protein synthesis [51,61]. A study by Panjaitan et al. [59] found that supplementing Spirulina platensis to cattle grazing forages with low crude protein can increase the efficiency of microbial protein production in the rumen, which then can be translated into milk products. However, previous studies conducted on lactating cows using marine algal diets found that milk fat [53,56] and milk protein [52,59] were increased while others found a decreased in milk fat [54,55]. The disparities between these studies are mainly due to the different types and sources of marine algae diet used. Spirulina platensis could be essential in improving milk yield and milk composition and yet limited attention has been paid on understanding the impact on lactation traits of pasture-based lactating cows. To our knowledge, the response of pasture-based dairy cows to supplementation with Spirulina platensis and the subsequent impact on milk composition is not well known. Examination of literature reveals that previous trials in dairy cows have been mainly conducted using other types of marine algae and without deliberate evaluation of the impact of Spirulina platensis on milk compositions.
Milk fatty acid profile: The possibility of enriched protein and omega-3 fatty acid rich milk could be a good news for the healthconscious consumer. Feeding diet containing rich sources of longchain MUFA and PUFA to lactating cows have been shown to modify the fatty acid profiles of milk in favour of the omega-3 and omega-6 [32,62]. Previous studies conducted using dietary algal supplements have reported an increase in conjugated linoleic acid (CLA) [53], whereas Glover et al. [54] found decreases in monounsaturated fatty acids in milk fat. Stamey et al. [56] found that supplementation of dairy cows with conjugated linoleic acid significantly increased the proportions of trans-18:1 and Trans-11 18:1 fatty acids. Moate et al. [55] reported that cows receiving algal meal high in docosahexaenoic acid (DHA) had significant increase in the proportions of DHA in milk fat. These studies indicate dietary supplements containing essential fatty acids could be effective nutritionally for reducing the concentrations of undesirable saturated fatty acids in milk fat, and increasing the proportions of healthier long chain PUFA. However, further studies are warranted to elucidate these findings with Spirulina in a solely pasture-based dairy system. Most previous studies have focussed their attention on improving the meat quality of sheep and cattle without deliberation on the fatty acid profiles of milk. Therefore, there is the need to fill this pending gap to help us understand the mechanism underpinning the impact of Spirulina platensis on lactation traits. Further studies in different dairy production systems are required to enable informed choices and tailored decisions when feeding lactating cows with Spirulina platensis supplements, to improve production and reproduction performances of high merit cows within the Australian dairy industry.
Knowledge Gaps
• A wide body of evidence exists that shows the effect of different sources of protein and fat supplementation on milk yield, milk composition, BCS and Live-weight in lactating dairy cows but published empirical evidence of the impact of Spirulina supplements on these lactation parameters is lacking for dairy cows in pasture-based systems.
• Many authors have reported the effect of supplementing dairy cows with various sources of protein and fat on milk fatty acid composition, but the effect of Spirulina supplementation on milk fatty acid profile of dairy cows in a pasture-based system has not yet been fully explored.
• Little attention has been given to investigating the relationships between Spirulina supplementation and the amino acid profiles of dairy herds in pasture-based systems. Filling in this significant knowledge gap will assist dairy farmers operating under pasture-based settings to improve the protein content of their milk to optimise profitability in the dairy industry.
• A negative correlation exists between non-esterified fatty acids and reproductive traits in most dairy herds. This relationship is exacerbated by negative energy balance and inadequate nutrition. Spirulina is a rich source of energy (7% lipid). There are lack of reports on the effect of Spirulina supplementation on non-esterified fatty acids, β-hydroxybutyrate and ketone bodies.
• Progesterone, oestrogen and prostaglandin, luteinising and follicle stimulating hormones have been identified as limiting factors in the reproduction and fertility successes of dairy cows. Limited reports on the effects Spirulina supplementation upon Progesterone, oestrogen and prostaglandin, luteinising and follicle stimulating hormones abound in published literature in pasture-based dairy systems.
Therefore, research is needed to: 1) Investigate the relationships between Spirulina supplementation and cow BCS and Live-weight traits in pasture-based dairy systems 2) Quantify the milk and fatty acid compositions of dairy cows supplemented with Spirulina 3) Analyse the amino acid profile of milk of lactating cows supplemented with Spirulina platensis 4) Examine the effect of Spirulina supplementation on plasma metabolites 5) Evaluate the influence of Spirulina supplements on reproductive hormones (progesterone, oestrogen, prostaglandin, insulin growth factor-1, luteinising and follicle stimulating hormones) in pasture-based dairy cows.
Conclusion
Spirulina is a rich source of proteins, fatty acids, minerals and vitamins, thus making it a potential nutrient-rich feed resource for the pasture-based dairy industry. Previous supplementation experiments with Spirulina had mostly focussed on poultry, rabbits, fish, sheep and beef cattle with very little attention paid to dairy cattle. Understanding lactating cows respond to supplementation with Spirulina would be vital in assessing the benefits of its inclusion in a dairy cow ration. Moreover, investigations into mechanisms and active ingredients responsible for productive performance in dairy cows would be critical in elucidating the benefits of Spirulina usage in the dairy industry into the foreseeable future.
References
- Faye B, Konuspayeva G (2012) The sustainability challenge to the dairy sector–The growing importance of non-cattle milk production worldwide. International Dairy Journal 24: 50-56.
- Dror DK, Allen LH (2011) The importance of milk and other animal-source foods for children in low-income countries. Food and Nutrition Bulletin 32: 227-243.
- Butler W (2003) Energy balance relationships with follicular development, ovulation and fertility in postpartum dairy cows. Livestock Production Science 83: 211-218.
- Muhammad N, Maigandi S, Hassan W, Daneji A (2008) Growth performance and economics of sheep production with varying levels of rice milling waste. Sokoto Journal of Veterinary Sciences 7: 59-64
- Asmare B, Melaku SPeters KJ (2010) Supplementation of Farta sheep fed hay with graded levels of concentrate mix consisting of noug seed meal and rice bran. Tropical Animal Health and Production 42: 1345-1352.
- Holman B, Kashani A, Malau-AduliA (2014) Effects of Spirulina (Arthrospiraplatensis) supplementation level and basal diet on liveweight, body conformation and growth traits in genetically divergent Australian dual-purpose lambs during simulated drought and typical pasture grazing.Small Ruminant Research 120: 6-14.
- Holman B, Malau-AduliA (2013) Spirulina as a livestock supplement and animal feed. Journal of Animal Physiology and Animal Nutrition 97: 615-623.
- Kashani A, Holman BWB, Nichols PD, Malau-Aduli AEO (2015) Effect of level of Spirulina supplementation on the fatty acid compositions of adipose, muscle, heart, kidney and liver tissues in Australian dual-purpose lambs. Annals of Animal Science 15: 945-960.
- Malau-Aduli AEO, Holman BWB, Kashani A, Nichols PD (2016) Sire breed and sex effects on the fatty acid composition and content of heart, kidney, liver, adipose and muscle tissues of purebred and first-cross prime lambs. Animal Production Science 56: 2122-2132
- Malau-Aduli AEO, Kashani A (2015) Molecular genetics-nutrition interactions in the expression of AANAT, ADRB3, BTG2 and FASN genes in the heart, kidney and liver of Australian lambs supplemented with Spirulina (Arthrospiraplatensis). Genes and Genomics 37: 633-644.
- Peiretti P, Meineri G (2011) Effects of diets with increasing levels of Spirulinaplatensis on the carcass characteristics, meat quality and fatty acid composition of growing rabbits. Livestock Science 140: 218-224.
- Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. Journal of Bioscience and Bioengineering 101: 87-96.
- Clement G, Giddey C, Menzi R (1967) Amino acid composition and nutritive value of the alga Spirulina maxima. Journal of the Science of Food and Agriculture 18: 497-501.
- Hudson BJ, Karis IG (1974) The lipids of the alga Spirulina. Journal of the Science of Food and Agriculture 25: 759-763.
- Lordan S, Ross RP, Stanton C (2011) Marine bioactives as functional food ingredients, potential to reduce the incidence of chronic diseases. Marine Drugs 9: 1056-1100.
- Becker E (2007) Micro-algae as a source of protein. Biotechnology Advances 25: 207-210.
- Falquet J (1997) The nutritional aspects of Spirulina. Antenna Technology 1-25.
- Australian Spirulina (2015) Product of Australia.Situation and Outlook.
- Vonshak A (1997) Spirulinaplatensisarthrospira: physiology, cell-biology and biotechnology.1st edn, Taylor & Francis, London.
- Koru A (2012) Earth food Spirulina (Arthrospira), production and quality standards, Food Additive.
- Kulpys J, Paulauskas E, Pilipavicius V, Stankevicius R (2009) Influence of cyanobacteria Arthrospira (Spirulina) platensis biomass additive towards the body condition of lactation cows and biochemical milk indexes. Agronomy Research 7: 823-835.
- Raoof B, Kaushik B, Prasanna R (2006) Formulation of a low-cost medium for mass production of Spirulina. Biomass and Bioenergy 30: 537-542.
- Shimamatsu H (2004) Mass production of Spirulina, an edible microalga. In: Ang PO editor. Asian Pacific Phycology in the 21st Century: Prospects and Challenges. Developments in Hydrobiology 173: 39-44.
- Ogawa T, Terui G (1970) Studies on the growth of Spirulinaplatensis, on the pure culture of Spirulinaplatensis. Journal of Fermentation Technology 48: 361.
- Bryant DA (1991) Cyanobacterial phycobilisomes: progress toward complete structural and functional analysis via molecular genetics. Cell Culture and Somatic Cell Genetics of Plants 7: 257-300.
- Glazer AN (1984) Phycobilisome a macromolecular complex optimized for light energy transfer. BiochimicaetBiophysicaActa (BBA)-Reviews on Bioenergetics 768: 29-51.
- Lunsin R, Wanapat M, Yuangklang C,Rowlinson P (2012) Effect of rice bran oil supplementation on rumen fermentation, milk yield and milk composition in lactating dairy cows. Livestock Science 145: 167-173.
- Doreau M, Chilliard Y (1997) Digestion and metabolism of dietary fat in farm animals. British Journal of Nutrition 78: 15-35.
- Ikwuegbu O, Sutton J (1982) The effect of varying the amount of linseed oil supplementation on rumen metabolism in sheep. British Journal of Nutrition 48: 365-375.
- Jenkins T (1993) Lipid metabolism in the rumen. Journal of Dairy Science 76: 3851-3863.
- Duske K, Hammon HM, Langhof AK, Bellmann O, Losand B, et al. (2009) Metabolism and lactation performance in dairy cows fed a diet containing rumen-protected fat during the last twelve weeks of gestation. Journal of Dairy Science 92: 1670-1684.
- Hutchinson I, Hennessy A, Dewhurst R, Evans A, Lonergan P, et al. (2012) The effect of strategic supplementation with trans-10, cis-12 conjugated linoleic acid on the milk production, estrous cycle characteristics, and reproductive performance of lactating dairy cattle. Journal of Dairy Science 95: 2442-2451.
- Baumgard L, Matitashvili E, Corl B, Dwyer D, Bauman D (2002) Trans-10, cis-12 conjugated linoleic acid decreases lipogenic rates and expression of genes involved in milk lipid synthesis in dairy cows. Journal of Dairy Science 85: 2155-2163.
- Bauman D, Griinari J (2001) Regulation and nutritional manipulation of milk fat: low-fat milk syndrome. Livestock Production Science 70: 15-29.
- D’Mello J (2000) Farm animal metabolism and nutrition. CABI Publishing, Wallingford
- Moghadam JM, Mahjoubi E, Dirandeh E (2015) Effect of linseed feeding on blood metabolites, incidence of cystic follicles, and productive and reproductive performance in fresh Holstein dairy cows. Journal of Dairy Science 98:1828-1835.
- Lee C, Hristov A, Heyler K, Cassidy T, Long M, et al.(2011) Effects of dietary protein concentration and coconut oil supplementation on nitrogen utilization and production in dairy cows. Journal of Dairy Science 94: 5544-5557.
- Resende T, Kraft J, Soder K, Pereira A, Woitschach D, et al.(2015) Incremental amounts of ground flaxseed decrease milk yield but increase n-3 fatty acids and conjugated linoleic acids in dairy cows fed high-forage diets. Journal of Dairy Science 98: 4785-4799.
- Malau-Aduli AEO, Abubakar BY (1992) Estimation of 305-day yield from total milk yields in Bunaji and Friesian-Bunaji crosses. Nigerian Journal of Animal Production 19: 141-145.
- Stockdale C (2001) Body condition at calving and the performance of dairy cows in early lactation under Australian conditions: a review.Australian Journal of Experimental Agriculture 41: 823-839.
- Kashani A, Holman BWB, Nichols PD,Malau-Aduli AEO (2015) Effect of dietary supplementation with Spirulina on the expressions of AANAT, ADRB3, BTG2 and FASN genes in the subcutaneous adipose and Longissimusdorsi muscle tissues of purebred and crossbred Australian sheep. Journal of Animal Science and Technology 57: 1-8.
- Malau-Aduli AEO, Holman BWB (2015) Effect of Spirulina supplementation on plasma metabolites in crossbred and purebred Australian Merino lambs. International Journal of Veterinary Science and Medicine 3: 13-20.
- Butler W (2000) Nutritional interactions with reproductive performance in dairy cattle. Animal Reproduction Science 60: 449-457.
- Grinstead G, Tokach M, Dritz S, Goodband R, Nelssen J (2000) Effects of spirulinaplatensis on growth performance of weanling pigs. Animal Feed Science Technology 83: 237-247.
- Peiretti P, Meineri G (2008) Effects of diets with increasing levels of Spirulinaplatensis on the performance and apparent digestibility in growing rabbits. Livestock Science 118: 173-177.
- Holman B, Kashani A, Malau-Aduli A (2012) Growth and body conformation responses of genetically divergent Australian sheep to Spirulina (Arthrospiraplatensis) supplementation. America Journal of Experimental Agriculture 2:160-173.
- Stanley JG, Jones JB (1976) Feeding algae to fish. Aquaculture 7: 219-223.
- Capuco A, Ellis S, Hale S, Long E, Erdman R, et al. (2003) Lactation persistency: insights from mammary cell proliferation studies. Journal of Animal Science 81: 18-31.
- Couvreur S, Hurtaud C, Marnet P, Faverdin P, Peyraud J (2007) Composition of milk fat from cows selected for milk fat globule size and offered either fresh pasture or a corn silage-based diet. Journal of Dairy Science 90: 392-403.
- Sutton JD (1990) Dietary composition of milk composition.In dairying in the 1990’s, Dairy Research Foundation Symposium, University of Sydney.
- Kellaway R, Harrington T (2004) Feeding concentrates, supplement for dairy cows. (Revised edn), Landlinks Press, Collinwood.
- Simkus A, Oberauskas V, Laugalis J, Zelvyt? R, Monkevi?ien? I, et al. (2007) The effect of weed SpirulinaPlatensis on the milk production in cows. VeterinarijairZootechnika-Lith 38:74-77.
- Boeckaert C, Vlaeminck B, Dijkstra J, Issa-Zacharia A, Van Nespen T, et al. (2008) Effect of dietary starch or micro algae supplementation on rumen fermentation and milk fatty acid composition of dairy cows. Journal of Dairy Science 91: 4714-4727.
- Glover KE, Budge S, Rose M, Rupasinghe HPV, MacLaren L, et al. (2012) Effect of feeding fresh forage and marine algae on the fatty acid composition and oxidation of milk and butter. Journal of Dairy Science 95: 2797-2809.
- Moate PJ, Williams SRO, Hannah MC, Eckard RJ, Auldist MJ, et al. (2013) Effects of feeding algal meal high in docosahexaenoic acid on feed intake, milk production, and methane emissions in dairy cows. Journal of Dairy Science 96: 3177-3188.
- Stamey JA, Shepherd DM, de Veth MJ, Corl BA (2012) Use of algae or algal oil rich in n-3 fatty acids as a feed supplement for dairy cattle. Journal of Dairy Science 95: 5269-5275.
- Hough G (1991) Marketing potential for lupins in dairy cattle production, efficient utilisation of lupins in dairy cattle production, the effect of heat treatment on the feeding value of lupins. Final Report to Grain Research Council, Western Australian Cattle Industry Compensation Fund and Grain Legumes Research Council, Perth.
- Lee C, Hristov A, Heyler K, Cassidy T, Lapierre H, et al. (2012) Effects of metabolizable protein supply and amino acid supplementation on nitrogen utilization, milk production, and ammonia emissions from manure in dairy cows. Journal of Dairy Science 95: 5253-5268.
- Panjaitan T, Quigley S, McLennan S, Swain A, Poppi D (2015) Spirulina (Spirulinaplatensis) algae supplementation increases microbial protein production and feed intake and decreases retention time of digesta in the rumen of cattle. Animal Production Science 55: 535-543.
- Zhou X, Zhang Y, Zhao M, Zhang T, Zhu D, et al.(2015) Effect of dietary energy source and level on nutrient digestibility, rumen microbial protein synthesis, and milk performance in lactating dairy cows. Journal of Dairy Science 98: 7209-7217.
- Lee C, Giallongo F, Hristov A, Lapierre H, Cassidy T, et al. (2015) Effect of dietary protein level and rumen-protected amino acid supplementation on amino acid utilization for milk protein in lactating dairy cows. Journal of Dairy Science 98: 1885-1902.
- Caroprese M, Marzano A, Marino R, Gliatta G, Muscio A, et al. (2010) Flaxseed supplementation improves fatty acid profile of cow milk. Journal ofDairy Science 93: 2580-2588.
Citation: Otto JR, Malau-Aduli AEO (2017) Spirulina platensis (Arthrospira spp.): A Potential Novel Feed Source For Pasture-Based Dairy Cows. J Fisheries Livest Prod 5: 253. DOI: 10.4172/2332-2608.1000253
Copyright: © 2017 Otto JR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 12581
- [From(publication date): 0-2017 - Sep 03, 2025]
- Breakdown by view type
- HTML page views: 11040
- PDF downloads: 1541