Stability and Concentrations of Humectants in Tobacco
Received: 19-Sep-2017 / Accepted Date: 14-Oct-2017 / Published Date: 19-Oct-2017 DOI: 10.4172/2155-9872.1000380
Abstract
Humectants, especially glycerol and propylene glycol, are tobacco additives that are used to facilitate several processes in the production of tobacco products and to maintain the moisture content. Humectants in tobacco are usually detected by gas chromatography with flame ionization detection after a pre-analytical conditioning procedure. During this conditioning step, significant decreases of especially propylene glycol levels were observed.
The goal of the present study is to find out the reason for this decrease, and to propose a method that minimizes this loss. Therefore, detailed studies were performed directed to this problem, which revealed that evaporation is the most likely source of this loss.
Based on our findings, we propose a method without a pre-analytical conditioning step. Using the present method, in 10 different brands of commercially available cigarettes it was checked whether the measured concentrations of the humectants correspond with the declared concentrations as supplied by the manufacturers. The analysis showed that the measured levels were in general much lower than the specified amounts, possibly due to evaporation during processing tobacco to cigarettes. Therefore, it was suggested that the manufacturers should also specify the final amounts of humectants in cigarettes after the manufacturing process.
Keywords: GC-FID; Humectants; Glycerol; Propylene glycol; Tobacco; Cigarettes
Introduction
Several humectants such as glycerol (GLY), and propylene glycol (PG), are commonly used as ingredients in manufactured cigarettes to maintain the moisture content of the cut tobacco filler and add flavor [1,2]. Appropriate moisture content in cigarettes also influences the shelf life of the final product [3]. While GLY occurs naturally in many varieties of plants, including tobacco, PG and tri-ethylene glycol (TEG) do not [4]. The humectant concentrations vary greatly among different cigarettes [5].
Both glycerol and propylene glycol are on the European Commission’s list of priority additives for which enhanced reporting obligations by industry apply. This list has been complied based on the frequency or amount of use, and on their reported effects on toxicity, addictiveness or flavor properties of tobacco products [6].
In the past, several methods have been published for the detection of humectants in tobacco by the tobacco industry [7-10]. These methods used gas chromatography with flame ionization detection (GC-FID) after a conditioning step in which the tobacco was stored for some time under controlled temperature and humidity. In several studies in literature and in our own experiences, it appeared that humectants in tobacco and cigarettes are not stable. Especially PG decreased in concentration upon analyses of humectants in cigarettes when the method described in the Standard Operating Procedure (SOP) of the Health Canada, Official Method T-304 was used [11].
To study the cause of this decrease in the PG concentrations, the present study was performed. In addition, the occurrence of humectant ingredients in commercially available cigarettes were determined and compared with the declared amounts.
Materials And Methods
Materials
Ten brands of commercially available cigarettes were bought in a local shop. All chemicals and solvents used were of analytical grade.
The GC equipment for the determination of humectants was a gas chromatograph (model 3900) equipped with an auto sampler (CP 8400) and a FID detector (Varian Assoc., Middelburg, the Netherlands). The GC column was a CP Wax 52 CB capillary column with a length of 25 m, an internal diameter of 0.25 mm and a particle size of 1.2 μm (Varian Assoc.).
The equipment for the headspace analysis was obtained from Agilent (Amstelveen, the Netherlands) and consisted of a GC (Agilent 6850) with an MS detector (Agilent 5975C) equipped with a thermo desorption unit (Markes Unity Series 2). The capillary column was an Rxi-624Sil MS with a length of 30 m, an internal diameter of 0.25 mm and a particle size of 1.4 μm.
Methods
The conditioning of cigarettes was performed at 22°C, at a relative humidity of 60% during 48 h. The determination of humectants in whole tobacco was performed as follows. GLY, PG, di-ethyl glycerol (DEG) and TEG were determined by GC-FID according to Health Canada, Official Method T-304: Method for the Determination of Humectants in Whole Tobacco [11]. Briefly, 2 g tobacco was extracted with 50 mL methanol after addition of the internal standard 1,3-butanediol for 60 min under continuous mechanical shaking. After leaving in the dark for 30 min, the clear supernatant was transferred into a glass injection vial and injected directly into the GC. The injection volume was 1 μL with a split level of 25:1. The lowest calibration standards were 2.50 mg/g for glycerol, 1.875 mg/g for PG and 0.938 mg/g for DEG and TEG.
The extraction of unground tobacco was performed as follows. To 2.0 g tobacco, 25 mL methanol was added containing the internal standard (1,3-butanediol). Then the mixture was shaken for 1 h.
The extraction of ground tobacco was performed as follows. In a mortar, about 4.5 g tobacco was ground. To 2.0 g tobacco, 25 mL methanol was added containing the IS. Then the mixture was shaken for 1 h.
The decay of PG was measured by placing 2 g tobacco in a 500 mL Scott flask. Then the concentration of PG in the air was determined by automatic sampling of the whole content (air) of the flask followed by head space injection on a GC. This was repeated every 44 min until the concentration of PG was decreased to a low level.
Results
Measurements of humectants
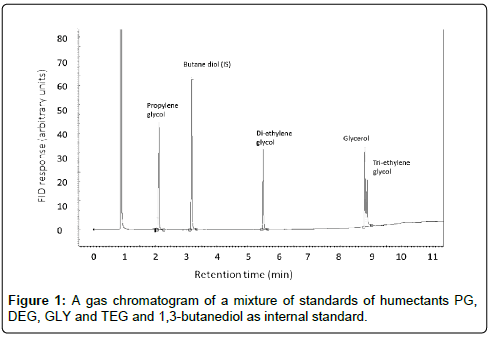
A gas chromatographic method was developed using detection with FID in which a mixture of standards of humectants, such as PG, DEG, GLY and TEG are separated and can be quantified using 1,3-butanediol as internal standard. An example of this separation is shown in the chromatogram in Figure 1.
This method for the detection of humectants was further defined by detection limits and reproducibility. The limit of quantification (LOQ) was 0.094 mg/g tobacco for PG, 0.30 mg/g for GLY, 0.24 mg/g for TEG and DEG. The average relative standard deviations (based on independent duplicates) were 2.6% for glycerol and 4.6% for PG. The linearity and recovery were similar as reported in the method of Health Canada [11]. The humectants DEG and TEG were not found in the cigarettes that we investigated; therefore, the partial overlap of GLY and TEG in the chromatogram will not hinder their quantification.
Stability of humectants
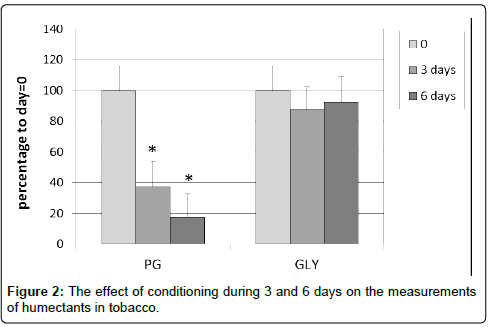
In the pre-analytical phase in the determination of humectants in tobacco, we observed a substantial decline in the concentrations of the humectants. Especially the concentration of PG seems to decrease after prolonged conditioning for 3 and 6 days to 38 and 18%, respectively, with p-values of 3.1*E-5 and 1.4*E-5. Also, the concentration of GLY appeared to be somewhat lower, but this decrease was not statistical significant (Figure 2).
To find the reason for this decline in humectant concentrations, a study was performed to check the following possibilities:
1. The influence of grinding,
2. A possible chemical or enzymatic conversion during the total process,
3. A possible evaporation during conditioning and drying during the pre-analytical phase.
The possible effect of grinding was investigated by a comparative study with ground and unground tobacco from cigarettes. We observed no difference in the concentrations of humectants between the both methods after extraction and GC-FID analysis (data not shown).
Then we did the same experiment of grinding tobacco in an atmosphere of normal ambient air and in the presence of 100% nitrogen gas. Again, there was not a significant difference between both conditions in the concentrations of PG and GLY with and without grinding.
In both studies, no other known or unknown peaks were visible, indicating that there were no other compounds formed during both processes.
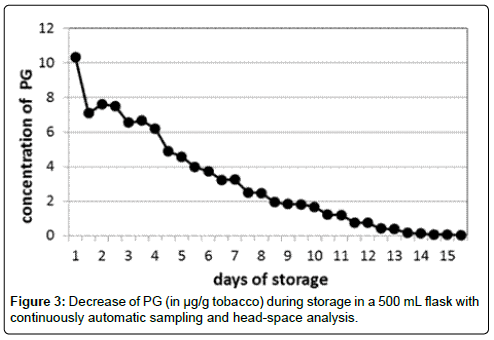
To obtain an additional proof for a possible evaporation, a continuous headspace study was performed. During several days, every 45 min an air sample was taken from a closed flask volume in which 2 g of tobacco was kept. After analysis, a decrease of the concentration of PG was observed which was finally ended in an almost zero concentration after 16 days (Figure 3).
In a separate experiment, the same procedure was repeated to determine the total recovery of both humectants after 5 and 7 days, for PG and GLY, respectively. It appeared that under these conditions both the concentrations of PG and GLY decreased to about 75 and 67%, respectively.
Amount of humectants in cigarettes
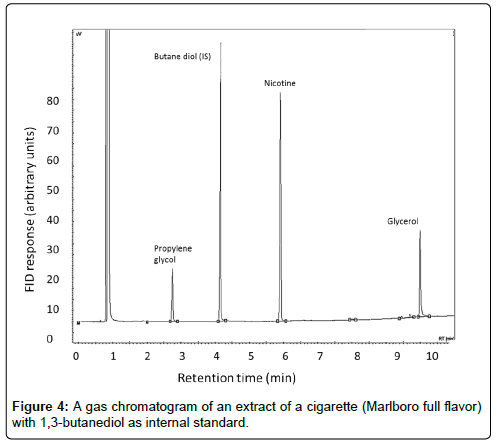
With the final method of analysis, without conditioning and without grinding and in the presence of an air atmosphere, in 10 different brands of cigarettes, the concentrations of humectants were quantified. A typical example of a gas chromatogram of humectants in cigarettes is shown in Figure 4, representing an extract of a ‘Marlboro full flavor’ cigarette with 1,3-butanediol as internal standard.
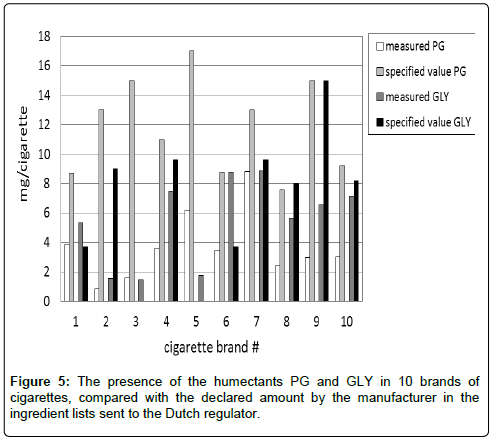
Humectants were measured in 10 brands of cigarettes with the described method. It appeared that in general a lower amount was measured in the cigarettes then the amount specified by the manufacturers in the ingredient lists (lists of all ingredients added during the manufacturing process) they sent to the Dutch regulator [12]. Especially for PG, the difference was rather substantial as can be seen from the individual concentrations in 10 brands of cigarettes (Figure 5).
Discussion
In this study we showed the development and characterization of a simple method for the determination of humectants in tobacco from cigarettes.
The method was rather similar to the methods described by Canada Health and the WHO [11,13]. During the development of the method, we experienced substantial losses of PG and GLY. Possible causes of this loss could be grinding, oxidation, enzymatic conversion or evaporation. From the additional experiments we performed, it is clear that the loss of humectants cannot be ascribed to grinding and oxidation to the air. Also, enzymatic conversion is not likely because we are not aware of enzymes that are capable to convert humectants. In addition, in all our studies we did not observe any new and unknown peaks in the GC-FID chromatograms. Thus, the only reason could be evaporation of humectants, especially PG. PG is a colorless viscous liquid with a density of 1.036 g/cm³, a boiling point of 188°C and its vapor pressure is 11 Pa. On conditioning, the total amount of PG decreased with time. The final proof we obtained in a continuous sampling study using headspace sampling and subsequent GC-FID analysis. The amount of PG that was present in the vapor phase in a closed flask with 2 g of tobacco decreased steadily to almost zero after a few weeks, which was in agreement with the decreased amount in the tobacco. GLY was not detectable in this system, but in a separate experiment, also GLY decreased in a similar way under these forced conditions.
In conclusion, these experiments show that PG and to a lower extend GLY will evaporate from tobacco and cigarettes. Evaporation of PG and, to a lower extent, also of GLY will occur after opening a package, when the cigarettes are conditioned or when forced evaporation conditions are applied. The difference between the evaporation of PG and GLY can possible be explained by the difference in vapor density, being 10.6 and 0.01 Pa for PG and GLY, respectively. Therefore, we propose to omit the conditioning step in the procedure and add a method to measure the water content before and after complete drying of the tobacco.
With the selected method of analysis, i.e., without conditioning and without grinding and in the presence of an air atmosphere, a number of cigarettes were checked for the amounts of humectants and compared with the specified amount which was indicated by the tobacco companies in the ingredient lists sent to the regulator. There was no agreement between the measured and the declared amount of both PG and GLY. This agreement was even worse for PG than for GLY. A possible cause can be the evaporation during processing tobacco to cigarettes. It is not expected that substantial humectant losses occur in a closed package, during shelf-life, as this would deteriorate flavor of the final product [1-3].
The actual amount present in the tobacco product that is consumed is more relevant for risk assessment and other purposes such as establishing content-emissions relationships than the added amount during manufacturing. In this light, it would be highly recommended that manufacturers not to only declare the added amounts of humectants in their ingredient list submission, but also the actual amount that is present in the cigarettes after production and before packaging. These levels need to be determined without a pre-analytical conditioning step, by the present method or a similar one as proposed by WHO/TobLabNet [13]. We recommend the same procedure for other volatile additives in tobacco products, such as flavors. In this case, the added amounts will also be higher than the final content in tobacco products.
Acknowledgements
This study was conducted with financial support of the Netherlands Food and Consumer Product Safety Authority (NVWA) and the Tobacco Free Initiative of the World Health Organization (WHO HQ/TFI).
Declaration of Interest
The authors declare no conflict of interest. All authors agreed with the content of this publication.
References
- Hoffmann D, Hoffmann I (1997) The changing cigarette, 1950-1995. J Toxicol Environ Health 50: 307-364.
- Klus H, Scherer G, Müller L (2012) Influence of Additives on Cigarette Related Health Risks in Beiträge zur Tabakforschung. Contributions to Tobacco Research 25: 411-493.
- Carmines EL, Gaworski CL (2005) Toxicological evaluation of glycerin as a cigarette ingredient. Food Chem Toxicol 43: 1521-1539.
- Rodgman A (2002) Some Studies of the Effects of Additives on Cigarette Mainstream Smoke Properties. II. Casing Materials and Humectants in Beiträge zur Tabakforschung. Contributions to Tobacco Research 20: 279-299.
- Rainey CL, Shifflett JR, Goodpaste JV, Bezabeh DZ (2013) Quantitative Analysis of Humectants in Tobacco Products Using Gas Chromatography (GC) with Simultaneous Mass Spectrometry (MSD) and Flame Ionization Detection (FID) in Beiträge zur Tabakforschung. Contributions to Tobacco Research 25: 576-585.
- https://ec.europa.eu/health/sites/health/files/scientific_committees/scheer/docs/scheer_o_001.pdf
- Friedman RL, Raab WJ (1963) Determination of Tobacco Humectants by Gas Liquid Chromatography. Analytical Chemistry 35: 67-69.
- Giles J (1970) Collaborative Study on the Determination of Propylene Glycol Glycerine and Triethylene Glycol in Tobacco. J Ass Offic Anal Chem 53: 655-658.
- Williams J (1971) Collaborative Study of the Determination of Propylene Glycol Glycerol and Triethylene Glycol in Tobacco. J Ass Offic Anal Chem 54: 560-564.
- CORESTA Recommended Method No. 61 (2011) Determination of 12-Propylene Glycol Glycerol and Sorbitol in Tobacco and Tobacco Products by High Performance Liquid Chromatography (HPLC).
- Health Canada Determination of Humectants in Whole Tobacco (1999) Health Canada - Official Method T-304.
- World Health Organization (2016) Standard operating procedure for determination of humectants in cigarette tobacco filler. WHO TobLabNet Official Method SOP 06.
Citation: Jansen E, Ramlal R, Cremers H, Talhout R (2017) Stability and Concentrations of Humectants in Tobacco. J Anal Bioanal Tech 8: 380. DOI: 10.4172/2155-9872.1000380
Copyright: © 2017 Jansen E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 11934
- [From(publication date): 0-2017 - Nov 23, 2025]
- Breakdown by view type
- HTML page views: 10798
- PDF downloads: 1136