Stromal Derived Factor 1 alpha (SDF-1a) in a Dual Therapy with the DPP-4 Inhibitor 1 Vildagliptin does not Enhance Beta Cell Function but Improves Glucose Clearance after Oral Glucose in Streptozotocin Diabetic Mice
Received: 19-Jul-2016 / Accepted Date: 19-Sep-2016 / Published Date: 23-Sep-2016 DOI: 10.4172/2572-0406.1000110
Abstract
Pancreatic alpha cells secrete glucagon-like peptide 1 (GLP-1), the production of which is enhanced by the chemokine stromal derived factor 1 (SDF-1). Since SDF-1, like GLP-1, is a substrate of dipeptidyl peptidase-4 (DPP-4), we examined whether dual therapy with SDF-1 and a DPP-4 inhibitor would preserve beta cell function and mass in diabetes. Diabetes was induced by daily injections of streptozotocin for 5 days. One diabetic group was treated with daily injections of SDF-1 alpha or vehicle for 5 days. These mice were concurrently treated with either the DPP-4 inhibitor vildagliptin or vehicle and they remained on vildagliptin or vehicle for an additional 3 weeks. Glucose clearance and beta cell function was evaluated from glucose and insulin data during OGTTs performed after 0, 2 and 4 weeks of treatment. Beta cell mass was determined histologically. Body weight did not differ between diabetic groups during the study. OGTT data showed that diabetic mice treated with SDF-1 alpha in combination with vildagliptin had increased glucose clearance which was not observed with vildagliptin alone. In contrast, vildagliptin alone enhanced beta cell function with no further enhancement by the combination with SDF-1 alpha. Beta cell mass and islet GLP-1 content were not altered by the treatments. In conclusion, SDF-1 alpha and vildagliptin combination therapy improves glucose clearance in streptozotocin induced diabetes in the mouse by an effect which seems independent from enhanced beta cell function.
Keywords: Glucose; Insulin; GLP-1; SDF-1; DPP-4; Diabetes
9774Introduction
Beta cell function and mass decline in diabetes, resulting in circulating insulin insufficiency and hyperglycemia [1]. Preventing beta cell decline is therefore an attractive pharmacological strategy for the treatment of the disease. Glucagon like peptide 1 (GLP-1) has repeatedly been demonstrated to prevent beta cell apoptosis and promote beta cell proliferation in animal models [2], although these observations have yet to be demonstrated in humans. Recently it was reported that pancreatic islet alpha cells of humans and rodents produce intact GLP-1 [3-5]. We have also recently demonstrated that dipeptidyl peptidase 4 (DPP-4), the enzyme that inactivate GLP-1, is present in pancreatic islets with distinct cellular distributions in different species [6,7]. Also the chemokine stromal derived factor 1 (SDF-1) is a chemokine that is produced in islets and has been shown to increase both prohormone convertase 1 expression and proglucagon expression as well as secretion of GLP-1 from clonal alpha cells and isolated rodent islets in vitro [8]. A combination of SDF-1 activation, which increases GLP-1 production and secretion, and DPP-4 inhibition, which prevents inactivation of both SDF-1 and the produced GLP-1, would be a potential target to increase islet GLP-1 thereby promote beta cell function. Such a combination has been examined in acute myocardial infarction, in which DPP-4 inhibition reduces infarct size in an SDF-1 dependent manner [9]. Whether the combination of SDF-1 activation and DPP-4 inhibition would be beneficial in diabetes is not known, however. In the present study, we therefore treated streptozotocin diabetic mice with a combination of recombinant SDF-1 alpha and the DPP-4 inhibitor vildagliptin.
Materials and Methods
Experimental animals
Four month old female C57BL6/JBomTac mice were purchased from Taconic Europe (Skensved, Denmark). All mice were caged in groups of 8 in a controlled climate room with a 12 h light/dark cycle, 18 ± 1°C temperature and 40-50% humidity. C57BL6 mice were used since they are more sensitive to streptozotocin than most mouse strains, while females are slightly resistant. Mice were made diabetic by multiple subcutaneous injections of streptozotocin (injection of 40 mg/kg per day for 5 days; volume load 20 μL per injection) (Sigma Aldrich, St. Louis MO, USA). This protocol produces a milder and more gradual form of diabetes than high dose single injection. A control group was injected with vehicle (10 mmol/l sodium citrate solution pH 4.5; injection of 20 μL per day for 5 days). All experimental protocols were approved by the regional animal ethical committee for Malmö/Lund.
Experimental procedure
Diabetic animals were randomized to one of three treatment groups and caged according to group. Two of the groups received drinking water supplemented with the DPP-4 inhibitor vildagliptin (3 μmol/ mouse per day); kindly provided by Novartis Pharmaceuticals, East Hanover, NJ, U.S.A. while the control group received plain water with no supplementation as previously described [10]. The dose of vildagliptin was 3 μmol/mouse per day. This dose was predicted to result in continuous blocking of GLP-1 inactivation by DPP-4 [10]. One of the vildagliptin treated groups received daily intraperitoneal injections of recombinant mouse SDF-1a (R&D Systems, Minneapolis MN, USA) (50 μg/mouse) for the first five days of the treatment period and then only vildagliptin for the remainder of the study. Consumption of water was monitored throughout the study to ensure that exposure to vildagliptin did not differ between groups. The active treatment period was four weeks in total. A diagrammatic representation of the study timeline is presented in Figure 1.
Oral glucose tolerance tests
Oral glucose tolerance tests were performed 8 days after the induction of diabetes with STZ, immediately prior to the start of drug treatment, two weeks after drug treatment and at study termination four weeks after the start of drug treatment. Mice were anesthetized by intraperitoneal injection of a fixed dose combination of fentanyl (0.02 mg/mouse)-fluanisone (0.5 mg/mouse) [Vet pharma, Leeds U.K] and midozalam (0.125 mg/mouse) (Roche, Basel Switzerland) 15 min prior to oral administration of 75 mg D-glucose, via gavage. Blood samples were taken from the intraorbital retrobulbar sinus plexus at 0, 15, 30, 60 and 90 min post gavage. Plasma was separated from whole blood by centrifugation at 4°C. Samples were frozen at -20°C until being assayed for glucose and insulin.
Biochemical analyses
Plasma samples were assayed for insulin by a mouse insulin ELISA (Mercodia, Uppsala Sweden). Plasma glucose was determined by the glucose oxidase method with ABTS as the substrate.
Immunohistochemistry
Pancreata were removed, weighed and then fixed in neutral buffered formalin prior to embedding in paraffin. 5 μm thick sections were mounted onto superfrost plus glass slides. Briefly, after deparaffinization, sections were blocked in 3% H2O2/10% methanol and again in 10% normal rabbit serum prior to overnight incubation with a polyclonal guinea pig anti- insulin/proinsulin antibody (EuroProxima, Arnhem, The Netherlands). Sections were washed in PBS and incubated with a horseradish peroxidase conjugated rabbit anti-guinea pig secondary antibody (DAKO, Glostrup Denmark), washed again and developed with 3,3´-diaminobenzidine chromogen reagent (DAKO, Glostrup Denmark). Sections were cross- stained with hematoxylin, dehydrated and mounted. For GLP-1 immunodetection the same procedure was applied with minor modifications. After deparaffinization, slides were incubated in 10 mM sodium citrate (pH 6.0) with 0.05% Tween 20 for 20 min at 95°C prior to blocking in 3% H2O2/10% methanol. The primary antibody, mouse monoclonal anti- GLP-1 (HYB 147-06 8G9, Enzo Life Sciences, Farmingdale, NY, USA), which does not cross-react with glucagon, was diluted 1:250 in PBS containing 0.1% Triton-X and 0.25% BSA. The secondary antibody was anti-mouse IgG HRP conjugated SignalStain Boost Detection Reagent (Cell Signaling Technologies, Danvers, MA, USA. Slides were scanned in a ScanScope digital slide scanner (Aperio, Vista California) and GLP-1 was quantified using the Aperio Image Scope program and positive pixel count algorithm version 9.0. Insulin positive and total pancreatic areas of each scanned slide were determined using the CellSens Dimensions software package (Olympus). Four slides per mouse and four mice per group were utilized for beta cell mass calculation.
Statistical analysis
Area under the curve was calculated using the trapezoidal rule. The insulinogenic index was calculated as the incremental area under the curve for insulin divided by the incremental area under the curve for glucose for the periods 0-30 min. Glucose clearance was determined by the glucose elimination rate constant KG calculated as the difference between the log transformed, baseline subtracted, 15 and 60 min glucose concentrations divided by the time interval. Data are presented as mean ± SEM unless otherwise indicated. Statistically significant differences were determined by Student’s t-test using the Prism 6.0 software package (Graphpad, San Diego, CA).
Result
Body weight, glucose and insulin
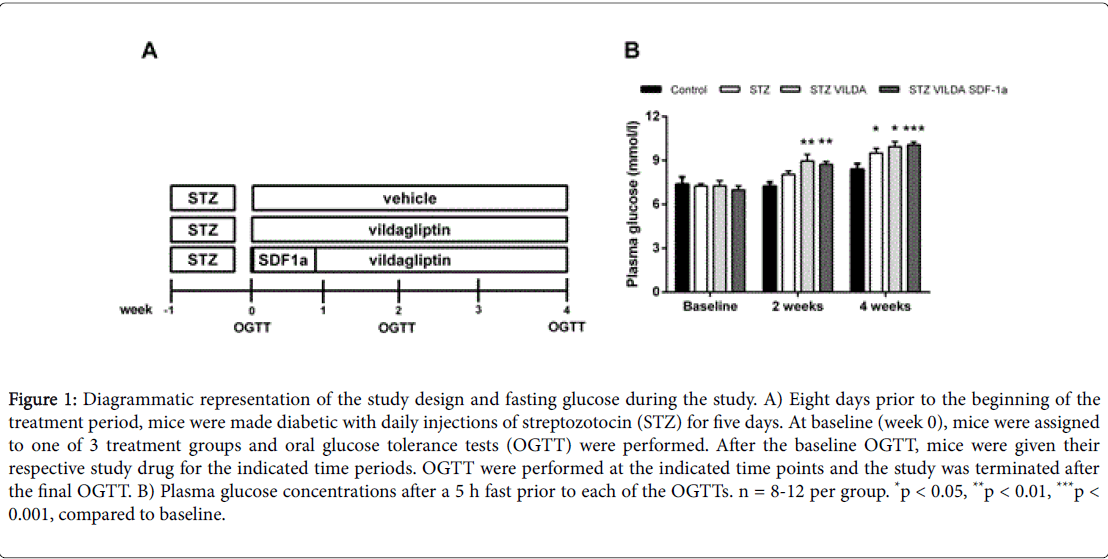
Body weight and water consumption did not differ between groups throughout the study (data not shown). An OGTT was performed eight days after the first streptozotocin injection, immediately prior to initiating the drug dosing regimen (Figure 1A). At this time point, there was no significant fasting or postprandial hyperglycemia (Figures 1B and 2A). However each of the streptozotocin injected groups showed near ablation of the insulin response to oral glucose (Figure 2B). Two weeks after the initiation of drug treatment, all streptozotocin diabetic groups had significant fasting and postprandial hyperglycemia in addition to a nearly absent insulin response during the OGTT (Figures 1B, 2C and 2D). After 4 weeks of drug treatment, all diabetic groups still showed fasting and postprandial hyperglycemia (Figures 1B and 2E). However the vildagliptin alone and the vildagliptin plus SDF-1a combination treatment groups showed significant enhancement of insulin levels (Figure 2F).
Figure 1: Diagrammatic representation of the study design and fasting glucose during the study. A) Eight days prior to the beginning of the treatment period, mice were made diabetic with daily injections of streptozotocin (STZ) for five days. At baseline (week 0), mice were assigned to one of 3 treatment groups and oral glucose tolerance tests (OGTT) were performed. After the baseline OGTT, mice were given their respective study drug for the indicated time periods. OGTT were performed at the indicated time points and the study was terminated after the final OGTT. B) Plasma glucose concentrations after a 5 h fast prior to each of the OGTTs. n = 8-12 per group. *p < 0.05, **p < 0.01, ***p < 0.001, compared to baseline.
Beta cell function and glucose tolerance
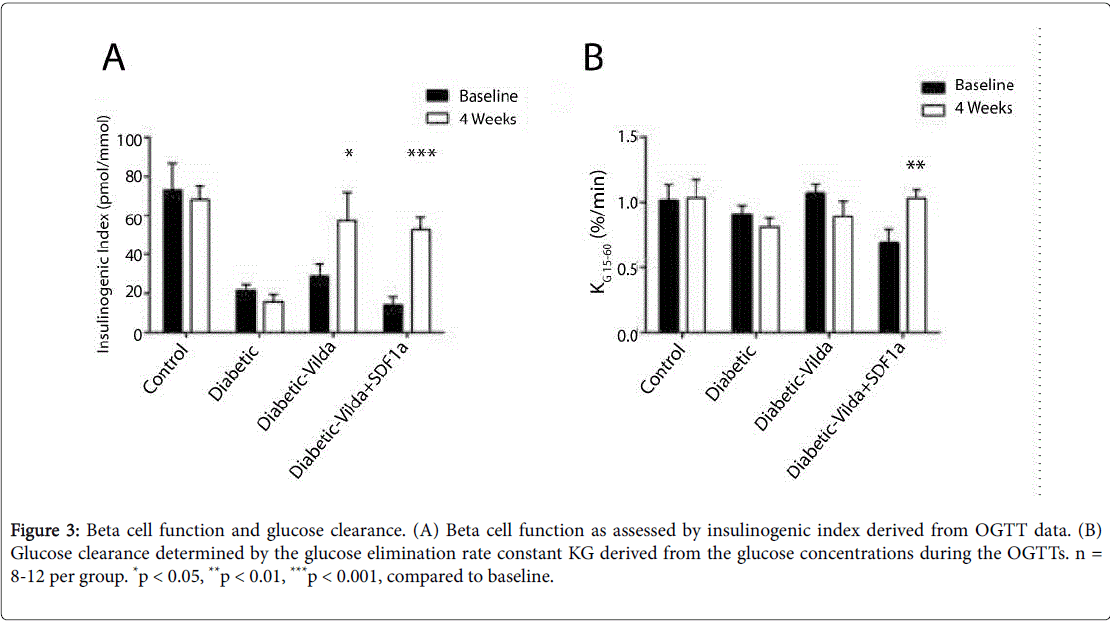
Beta cell function, as determined by the insulinogenic index during the OGTT, was improved by vildagliptin alone and vildagliptin-SDF-1a treatment after four weeks with no difference between the groups (Figure 3A). The combination of vildagliptin and SDF-1a showed a significant improvement in glucose clearance as determined by the glucose elimination rate constant KG, whereas the vildagliptin alone group had no increase in glucose clearance (Figure 3B).
Figure 3: Beta cell function and glucose clearance. (A) Beta cell function as assessed by insulinogenic index derived from OGTT data. (B) Glucose clearance determined by the glucose elimination rate constant KG derived from the glucose concentrations during the OGTTs. n = 8-12 per group. *p < 0.05, **p < 0.01, ***p < 0.001, compared to baseline.
Beta cell mass and islet GLP-1
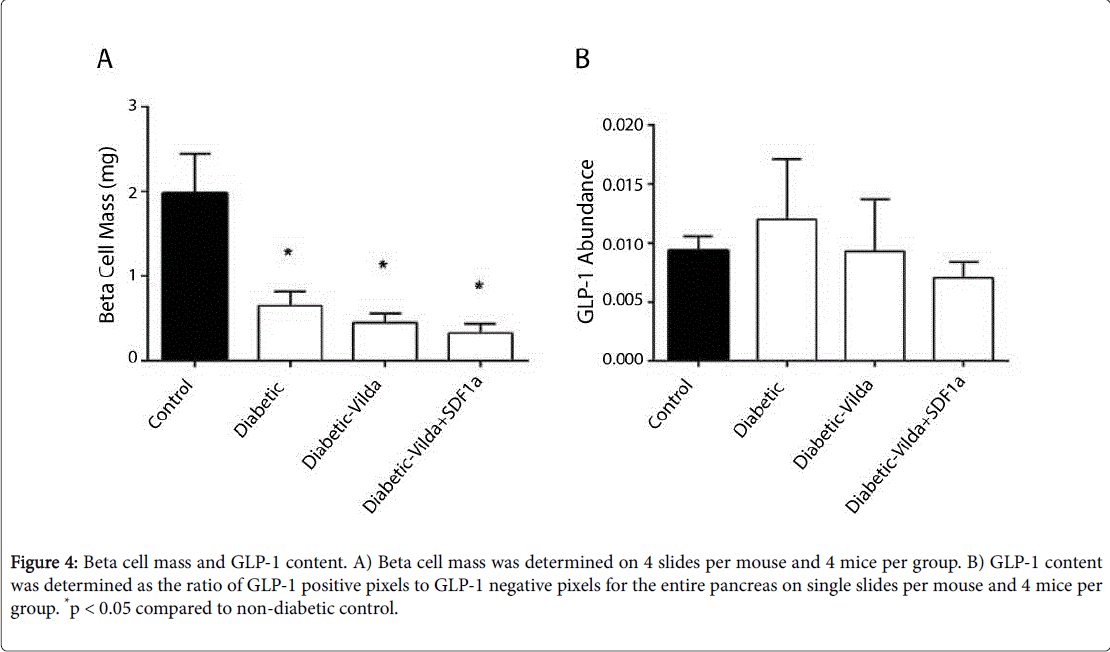
There was a greater than 50% reduction in beta cell mass in streptozotocin-diabetic mice without treatment (Figure 4A). After 4 weeks of pharmacological treatment with vildagliptin, alone or with combination of vildagliptin with SDF-1a, there were, however, no differences in beta cell mass compared to untreated diabetic control animals (Figure 4A). Similarly, GLP-1 content, determined by immunohistochemistry, was not significantly different between any of the groups studied (Figure 4B).
Figure 4: Beta cell mass and GLP-1 content. A) Beta cell mass was determined on 4 slides per mouse and 4 mice per group. B) GLP-1 content was determined as the ratio of GLP-1 positive pixels to GLP-1 negative pixels for the entire pancreas on single slides per mouse and 4 mice per group. *p < 0.05 compared to non-diabetic control..
Discussion
Our study showed that DPP-4 inhibition with vildagliptin alone and the combination of SDF-1 and vildagliptin improve beta cell function in streptozotocin-diabetic mice. The enhancement of beta cell function by vildagliptin is well established [11] and shown here also when beta cell mass is severely depleted. Habener and colleagues have demonstrated that beta cell damage or stress up-regulates islet SDF-1 mRNA expression and secretion of SDF-1 from beta cells (8). The natural induction of SDF-1 and GLP-1 within the islet could thus be an endogenous mechanism to prevent further beta cell damage and restore normal beta cell function. Both SDF-1 and GLP-1 are validated substrates for DPP-4, both in vitro and in vivo [12]. DPP-4 inhibition would thus prevent N-terminal degradation of the active hormones and prolong their biological effects and our hypothesis was that the combination of a DPP-4 inhibitor and SDF-1 alpha would enhance beta cell function through increase in local islet GLP-1. However, a main finding of this study is that there is no additive effect on beta cell function by combining SDF-1 alpha and vildagliptin. This was consistent with our findings that there was no difference in beta cell mass and islet GLP-1 between the groups. Therefore, besides that DPP-4 inhibition seems robust also in a model of severe beta cell deficiency, our results do not support that adding SDF-1 alpha would further enhance beta cell function. The treatment concept may, however, be further tested in longer term studies to exclude that a longer period of the combination would increase islet GLP-1.
In contrast to the failure of the combination to enhance beta cell function more than what was achieved by vildagliptin alone, the glucose clearance after the oral glucose was enhanced by the combination of vildagliptin and SDF-1 alpha in the streptozotocindiabetic mice. This would suggest that actions of vildagliptin are dependent on augmentation of extra islet effects [11], which, however, requires further mechanistic studies.
The significance of the finding of this study is that the dual pharmacological treatment with SDF-1 alpha and DPP-4 inhibition improves glucose clearance and also beta cell function even when beta cell mass is severely depleted. This would suggest that further studies are required to test this in other models. However, the combination did not further increase beta cell function when compared to vildagliptin alone and, therefore, the increase in beta cell function is mainly driven by vildagliptin, whereas the increase in glucose clearance seems to be driven by the combination in a beta cell independent manner.
Acknowledgement
The authors would like to acknowledge Kristina Andersson for her excellent technical assistance. The work was supported by grants from the Crafoord Foundation, The Royal Physiographic Society of Lund, The Swedish Research Council, The Lund University Medical Faculty and Region Skåne.
References
- Meigs JB, Muller DC, Nathan DM, Blake DR, Andres R (2003) The natural history of progression from normal glucose tolerance to type 2 diabetes in the Baltimore longitudinal study of aging. Diabetes 52: 1475-1484.
- Garber AJ (2011) Incretin effects on beta-cell function, replication, and mass: the human perspective. Diabetes Care 34: S258-S263.
- Hansen AM, Bodvarsdottir TB, Nordestgaard DN, Heller RS, Gotfredsen CF, et al. (2011) Upregulation of alpha cell glucagon-like peptide 1 (GLP-1) in Psammomysobesus: an adaptive response to hyperglycaemia? Diabetologia 54: 1379-1387.
- Heller RS, Aponte GW (1995) Intra-islet regulation of hormone secretion by glucagon- like peptide-1-(7-36) amide. Am J Physiol 269: G852-G860.
- Marchetti P, Lupi R, Bugliani M, Kirkpatrick CL, Sebastiani G, et al. (2012) A local glucagon-like peptide 1 (GLP-1) system in human pancreatic islets. Diabetologia 55: 3262-3272.
- Liu L, Omar B, Marchetti P, Ahrén B (2014) Dipeptidyl peptidase-4 (DPP-4):Localization and activity in human and rodent islets. BiochemBiophys Res Commun 453: 398-404.
- Omar B, Liehua L, Yamada Y, Seino Y, Marchetti P, et al. (2014) Dipeptidyl peptidase 4 (DPP-4) is expressed in mouse and human islets and its activity is decreased in human islets from type 2 diabetic individuals. Diabetologia 57: 1876-1883.
- Liu Z, Stanojevic V, Avadhani S, Yano T, Habener JF (2011) Stromal cell-derived factor-1 (SDF-1)/chemokine (C-X-C motif) receptor 4 (CXCR4) axis activation induces intra-islet glucagon-like peptide-1 (GLP-1) production and enhances beta cell survival. Diabetologia 54: 2067-2076.
- Zhong J, Rajagopalan S (2015) Dipeptidyl peptidase-4 regulation of SDF-1/CXCR4 axis: implications for cardiovascular disease. Front Immunol 6: 477.
- Omar BA, Vikman J, Winzell MS, Voss U, Ekblad E, et al. (2013) Enhanced beta cell function and anti-inflammatory effect after chronic treatment with the dipeptidyl peptidase-4 inhibitor vildagliptin in an advanced-aged diet-induced obesity mouse model. Diabetologia 56: 1752-1760.
- Ahrén B, Foley JE (2016) Improved glucose regulation in type 2 diabetic patients with DPP-4 inhibitors: focus on alpha and beta cell function and lipid metabolism. Diabetologia 59: 907-917.
- Mentlein R (1999) Dipeptidyl-peptidase IV (CD26)--role in the inactivation of regulatory peptides. Regul Pept 85:Â 9-24.
Citation: Omara B, Liehuaa L, Ahrén B (2016) Stromal Derived Factor 1 alpha (SDF-1a) in a Dual Therapy with the DPP-4 Inhibitor 1 Vildagliptin does not Enhance Beta Cell Function but Improves Glucose Clearance after Oral Glucose in Streptozotocin Diabetic Mice. J Chem Biol Ther 2: 110. DOI: 10.4172/2572-0406.1000110
Copyright: © 2016 Omara B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 12500
- [From(publication date): 11-2016 - Jul 17, 2025]
- Breakdown by view type
- HTML page views: 11562
- PDF downloads: 938