Research Article Open Access
Study of the Ratio between Morphine-3-�?²-D-Glucuronide and Morphine-6-�?²-DGlucuronide in Blood Samples from Heroin Fatalities
Rino Froldi1, Katia Guerrini2, Antonella Argo3, Marta Cippitelli1, Lucia Dell'Acqua2, F iorenza Farè2, Paolo Procaccianti3, Gabriella Roda2*, Chiara Rusconi2, Giacomo Luca Visconti2, Luigi Ferrante4 and Veniero Gambaro2
1Institute of Legal Medicine and Insurance, Via Don Minzoni 9, 62100 Macerata, Italy
2Department of Pharmaceutical Sciences, University of Milan, Via Mangiagalli 25, 20133 Milan, Italy
3Institute of Legal Medicine and Insurance, University Hospital, Via del Vespro 129, 90127 Palermo, Italy
4Department of Biomedical Science and Public Health, Faculty of Medicine, Polytechnic University of Marche, Ancona, Italy
- *Corresponding Author:
- Gabriella Roda
Department of Pharmaceutical Sciences
University of Milan, Via Mangiagalli
25, 20133 Milan, Italy
Tel: 0250319328
E-mail: gabriella.roda@unimi.it
Received Date: February 12, 2015, Accepted Date: April 07, 2015, Published Date: April 14, 2015
Citation: Froldi R, Guerrini K, Argo K, Cippitelli M, Fare F, et al. (2015) Study of the Ratio between Morphine-3-β-D-Glucuronide and Morphine-6-β-D-Glucuronide in Blood Samples from Heroin Fatalities. J Anal Bioanal Tech 6:240. doi: 10.4172/2155-9872.1000240
Copyright: © 2015 Froldi R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
In heroin fatalities the diagnosis of the cause of death, based only on chemical and toxicological data, may be particularly difficult; a complete knowledge of the case history is fundamental. Thus, for each case analytical results should be interpreted taking into account also autopsy findings, information from the scene and relevant medical history. In fact wide variability is present in post-mortem blood concentration of morphine (MOR), the main metabolite of heroin, which is usually the most important analytical result for the interpretation of the cause of death. Recently, increasing interest has grown towards the role of the metabolites morphine-3-β-D-glucuronide (M3G) and morphine-6-β-D-glucuronide (M6G) in mediating heroin effects. To this purpose SPE technique was employed to extract MOR, M3G and M6G from autopsy blood samples. Subsequently a LC/MS-MS method for the determination of these analytes was developed, using a gradient elution with a binary mobile phase, the analytes being revealed by means of an ESI-QqQ Mass Spectrometer operating in positive ionization and MRM mode. After validation, the method was applied to twenty-one blood specimens collected from cases of suspected acute narcotism which previously underwent a Systematic Toxicological Analysis (STA) to highlight the presence of ethanol and of the main drugs of abuse and/or their metabolites. The concentration ratios of MOR, M3G and M6G were investigated. The influence of some risk factors, such as the contemporary use of alcohol, methadone or cocaine, was also studied. Some important indications emerged: the ratio M3G/M6G, being quite constant, should be a valid reference value to assess toxicity. Furthermore the value of M3G/M6G ratio detected in heroin addict, in this study, is lower than that found in people who assume morphine for therapeutic purposes. This ratio could be of help to overcome the difficult interpretation of chemical and toxicological data.
Abstract
In heroin fatalities the diagnosis of the cause of death, based only on chemical and toxicological data, may be particularly difficult; a complete knowledge of the case history is fundamental. Thus, for each case analytical results should be interpreted taking into account also autopsy findings, information from the scene and relevant medical history. In fact wide variability is present in post-mortem blood concentration of morphine (MOR), the main metabolite of heroin, which is usually the most important analytical result for the interpretation of the cause of death. Recently, increasing interest has grown towards the role of the metabolites morphine-3-β-D-glucuronide (M3G) and morphine-6-β-D-glucuronide (M6G) in mediating heroin effects. To this purpose SPE technique was employed to extract MOR, M3G and M6G from autopsy blood samples. Subsequently a LC/MS-MS method for the determination of these analytes was developed, using a gradient elution with a binary mobile phase, the analytes being revealed by means of an ESI-QqQ Mass Spectrometer operating in positive ionization and MRM mode. After validation, the method was applied to twenty-one blood specimens collected from cases of suspected acute narcotism which previously underwent a Systematic Toxicological Analysis (STA) to highlight the presence of ethanol and of the main drugs of abuse and/or their metabolites. The concentration ratios of MOR, M3G and M6G were investigated. The influence of some risk factors, such as the contemporary use of alcohol, methadone or cocaine, was also studied. Some important indications emerged: the ratio M3G/M6G, being quite constant, should be a valid reference value to assess toxicity. Furthermore the value of M3G/M6G ratio detected in heroin addict, in this study, is lower than that found in people who assume morphine for therapeutic purposes. This ratio could be of help to overcome the difficult interpretation of chemical and toxicological data.
Keywords
Morphine; Morphine-3-β-D-glucuronide; Morphine-6-β-D-glucuronide; Heroin fatalities; LC-MS; Autopsy blood
Introduction
Heroin fatal overdose is still a public health problem in most countries, as described by the Annual Report of the OEDT [1]. Several studies have therefore focused on this topic, but the exact mechanism of fatal heroin overdose is still unclear. Typically death derives from the administration of a dose beyond the current tolerance of the person. This amount of drug induces respiratory depression till coma and cardio-pulmonary arrest [2]. The cause of death is established by the coroner, using the criteriology of the legal medicine, anamnestic and circumstantial data. In heroin related deaths, chemical and toxicological data are not as decisive as in the case of poisoning determined by other drugs of abuse. Most deaths occur among experienced users (around 35 years old, with 5-10 years of drug addiction), who should be skilled in evaluating safe doses and have high levels of tolerance, although this last aspect is hard to predict. Nonetheless, these heroin addicts often suffer from pathologies, which may influence drug metabolism and consequently increase toxicity [3]. The intake of a previously tolerated dose after loss/reduction of tolerance (e.g. after heroin detoxification or after imprisonment) could also be responsible for heroin intoxication. The contemporary use of therapeutic or illicit drugs, especially methadone, benzodiazepines, ethanol and cocaine, must also be considered. Several authors have investigated the influence of these substances, in heroin fatalities [2-9]. Besides, heroin metabolism is quite complex. This drug can’t be usually found in biological fluids because of its short half-life [2-4] minutes after intravenous injection). In the body, heroin is indeed rapidly hydrolyzed to 6-monoacetylmorphine (6-MAM), which in turn is converted to morphine (MOR). This metabolite is conjugated with glucuronic acid to mainly give morphine-3-β-D-glucuronide (M3G) and morphine-6-β-D-glucuronide (M6G) [10].
The concentration of morphine in blood has frequently been regarded as a measure of heroin action and toxicity. However, a number of overdose fatalities show relatively low blood concentrations of morphine, i.e. below or similar to those of living consumers or intoxicated heroin users [3,7]. Some authors have studied the ratio of “free” to “total” morphine, in order to correlate this value with the time of death or the influence of other misused drugs [11-13]. “Free” morphine stands for un-conjugated morphine, while “total” morphine refers to the amount of morphine which is determined after application of a hydrolysis step to the sample. In this way, morphine glucuronides, but also 6-MAM and other minor metabolites, are converted to morphine and analyzed by GC/MS. Indeed, this technique doesn’t allow the direct determination of M3G and M6G. In the last years, however, increasing interest has grown towards the role of these metabolites in mediating heroin or morphine action [14-29].
This paper describes a SPE extraction procedure and a validated LC/MS-MS method for the simultaneous determination of heroin metabolites in post-mortem blood samples. The development of techniques able to simultaneously detect heroin metabolites is very useful to try to elucidate the cause of death when dealing with potential heroin related deaths.
The aim of the work was the investigation of the role of heroin metabolites, so that the method was applied to blood specimens belonging to cases of suspected heroin acute narcotism. Moreover, as suggested by some authors [23-25], the concentration ratios of the analytes were calculated and related to some risk factors, such as the contemporary use of alcohol, methadone or cocaine. In particular the ratio M3G/M6G was studied and compared to that found in people assuming morphine for therapeutic purposes to understand if this ratio could be an important reference to evaluate the changes which occur in the glucuronidation mechanism in drug addicts.
Material and Methods
Reagents and standards
Morphine base (MOR) and morphine-D3 (MOR-D3) were supplied by S.A.L.A.R.S. (Italy). Morphine-3-β-D-glucuronide (M3G), morphine-6-β-D-glucuronide (M6G), morphine-3-β-D-glucuronide-D3 (M3G-D3), morphine-6-β-D-glucuronide-D3 (M6G-D3) and 6-monoacetylmorphine (6-MAM) were purchased from Cerilliant (USA).
Water (18,2 mΩ/cm) was prepared by a Milli-Q System (Millipore, France). Methanol was obtained from Sigma Aldrich (Germany) and acetonitrile was supplied from Carlo Erba (Italy). Sodium tetraborate/hydrochloric acid pH 9 buffer solution, formic acid and ammonium formate were purchased from Fluka (Germany), while hexane and ethyl acetate from Prolabo (Italy). All reagents were of analytical grade and were stored as required by the manufacturer.
MOR, M3G, M6G and 6-MAM reference standards were used to prepare calibration standard mixtures at 2000, 1000, 500, 250, 100 ng/mL in methanol. The internal standard mixture (ISM-D3) 1000 ng/mL in methanol was prepared by mixing MOR-D3, M3G-D3 and M6G-D3 standards.
Post-mortem blood samples
Central blood samples (n=21) belonging to male subjects were classified into three classes on the basis of Systematic Toxicological Analysis (STA) results [11]. In detail the five classes and the corresponding cases are:
I=Heroin: case No.1-10.
II=Heroin and ethanol (BAC ≥ 0.5 g/L): case No.11-16;
III=Heroin, methadone and ethanol (BAC ≥ 0.5 g/L): case No.17; Heroin and cocaine: case No.18-19; Heroin, cocaine and ethanol (BAC ≥ 0.5 g/L): case No.20-21.
The available data concerning each case are given in Table 1.
| Class | Case No | AGE | BAC (g/L) | MOR | M3G | M6G | M3G/M6G | Relevant circumstances |
|---|---|---|---|---|---|---|---|---|
| I | 1 | 45 | 0.1 | 358 | 1710 | 285 | 6.00 | First injection?! Family denies a history of drug dependence |
| 2 | 22 | n.p. | 294 | 1148 | 317 | 3.62 | led to the hospital; reported a double dose of heroin | |
| 3 | 45 | n.p. | 166 | 250 | 60 | 4.17 | Found dead in a toilet with a syringe nearby | |
| 4 | 44 | 0.3 | 254 | 266 | 92 | 2.89 | Found dead at home with a syringe in the arm | |
| 5 | 31 | n.p. | 371 | 633 | 196 | 3.23 | Found dead in a toilet with a syringe in the arm | |
| 6 | 34 | 0.4 | 60 | 788 | 165 | 4.78 | Found dead in his car; injection sites at autopsy | |
| 7 | 27 | n.p. | 231 | 188 | 42 | 4.48 | uk | |
| 8 | 28 | n.p. | 139 | 788 | 130 | 6.06 | uk | |
| 9 | 30 | n.p. | 266 | 833 | 225 | 3.70 | uk | |
| 10 | 40 | n.p. | 153 | 457 | 85 | 5.38 | uk | |
| II | 11 | 39 | 0.7 | 187 | 427 | 92 | 4.64 | Found dead at home with a syringe nearby |
| 12 | 22 | 0.5 | 646 | 398 | 90 | 4.42 | Found dead at home with suspected “brown-sugar” | |
| 13 | 39 | 0.7 | 649 | 2307 | 721 | 3.20 | History of drug dependence; injection sites at autopsy | |
| 14 | 42 | 2.2 | 117 | 46 | 14 | 3.29 | History of drug dependence; found dead at his home | |
| 15 | 35 | 1.3 | 152 | 322 | 88 | 3.66 | Found dead in a toilet with a syringe nearby | |
| 16 | 32 | 1.0 | 156 | 97 | 50 | 1.94 | Found dead with a syringe nearby | |
| III | 17 | 35 | 0.5 | 108 | 474 | 63 | 7.52 | In treatment for heroin dependence |
| 18 | 24 | 0.2 | 334 | 569 | 185 | 3.08 | Found in his bed; no signs of injection; white foam and blood at the mouth and at the nose | |
| 19 | 30 | 0.1 | 515 | 2138 | 821 | 2.60 | Found in his bed; no signs of injection; white foam and blood at the mouth and at the nose | |
| 20 | 45 | 0.6 | 1349 | 11 | 10 | 1.10 | Found dead in a street with a syringe nearby | |
| 21 | 43 | 0.5 | 145 | 394 | 82 | 4.80 | Found dead with a syringe nearby | |
| Mean | 316.68 | 678.29 | 181.57 | 4.03 | ||||
| ± SD | 289.21 | 646.04 | 213.25 | 1.47 | ||||
| Min | 60 | 11 | 10 | 1.10 | ||||
| MAX | 1349 | 2307 | 821 | 7.52 | ||||
Table 1: Classification, age, gender and blood ethanol concentration of the twenty-one cases analyzed by LC/MS-MS (BAC=Blood Alcohol Concentration; n.p.: not present, i.e. <0.5 g/L; M: male; uk: unknown). Concentrations (ng/mL) of morphine (MOR), morphine-3-β-Dglucuronide (M3G), morphine-6-β-D-glucuronide (M6G) concentration ratio of the analytes (n.d.: not detected; /not calculated). Mean, Standard Deviation (± SD) and range (min, MAX) of each analyte and of the ratios of analytes concentrations.
LC/MS-MS analysis
The analyses were performed on a Varian LC-320 triple quadrupole mass spectrometer, equipped with two Varian LC-212 chromatographic pumps and a Varian 410 tray cooled autosampler. The system is managed by Varian Workstation software (Version 6.9.2).
Chromatographic separation was performed at 40°C on a Kinetex® 2.6 μm x 50 mm (Phenomenex) protected by a Security Guard column C18 4 x 2.0 mm (Phenomenex). The mobile phase consisted of 3 mM ammonium formate buffer pH=3.0 (phase A) and acetonitrile with 0.1% formic acid (phase B). Flow rate: 0.2 mL/min. Gradient: from 5% phase B to 10% from 0.30 min to 5.0 min and then to 65.0% over the next nine min, before returning to the initial conditions within 1 min and equilibrating for 6 min. Total run time: 21 min. Injection volume: 10 μL. Manifold temperature: 42°C, housing temperature: 50°C. Ionization was achieved using electrospray in the positive mode (ESI+); needle voltage: +5000 V; shield voltage: +600 V; nebulizing gas (N2) pressure: 0.276 KPa; drying gas (N2) pressure: 0.276 KPa; drying gas (N2) temperature: 200°C. Multiple Reaction Monitoring (MRM) mode was chosen for the detection, using Argon at a pressure of 267 Pa as CID gas.
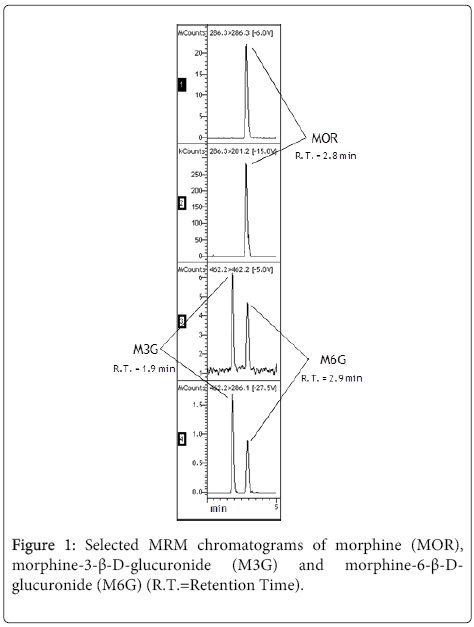
Among the three analytes, M3G was first eluted, while the separation between MOR and M6G was found to be particularly challenging. MRM mode assured distinct identification, and moreover a fairly good separation was achieved thanks to the application of a slight gradient and a constant column temperature (Figure 1).
The MRM transitions selected for each analyte and its corresponding deuterated internal standard (IS) are shown in Table 2.
| Compound | Q1 first mass | Q3 first mass | Capillary (V) | Collision Energy (V) |
|---|---|---|---|---|
| MOR | 286.3 | 286.3 | 70.9 | 6.0 |
| 286.3 | 201.2 | 70.9 | 15.0 | |
| M3G, M6G | 462.2 | 462.2 | 32.9 | 5.0 |
| 462.2 | 286.1 | 32.9 | 27.5 | |
| MOR-D3 | 289.1 | 289.1 | 80.0 | 5.5 |
| 289.1 | 200.9 | 80.0 | 17.0 | |
| M3G-D3, M6G-D3 | 465.5 | 465.5 | 32.9 | 5.0 |
| 465.5 | 289.1 | 32.9 | 25.5 | |
| 6-MAM | 328.4 | 328.4 | 75.0 | 5.0 |
| 328.4 | 165.1 | 75.0 | 31.5 |
Table 2: MRM transitions of each analyte and corresponding IS.
Both the pseudotransition from pseudomolecular ion to pseudomolecular ion and true transitions resulting from fragmentation were taken into account. In the case of morphine the pseudotransition was used for quantification [14] because this transition gave best results in terms of precision and linearity. In the case of the glucuronides the transition corresponding to the true fragmentation was used for quantitative determinations.
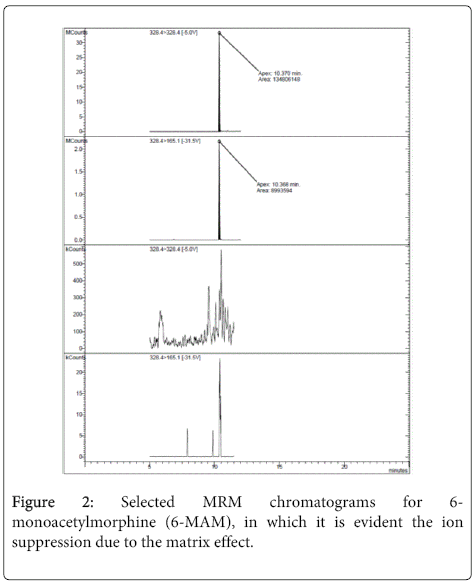
As regard as the detection of 6-MAM, MOR-D3 was used as IS; it has to be noticed that in the experimental conditions adopted for M3G and M6G, either chromatographic or extraction parameters, the large ion suppression due to matrix effects (see Method validation section, Figure 2) prevented us the contemporary detection of 6-MAM and morphine metabolites.
Sample preparation
For each working day, the following samples were prepared and analyzed: a “blank” solvent Working sample, a “blank” blood Working sample; Working Standard samples (WSs) 10, 25, 50, 100 and 200 ng; unknown Working samples.
Sample were composed of 0.5 mL of water or blood, 2.0 mL of water, 2.0 mL of pH 9 buffer and 50 μL of ISM-D3.
0.5 mL of water, of “blank” blood and of each unknown blood sample was transferred into a tube. 2 mL of water, 50 μL of ISM-D3 and 2.0 mL of pH 9 buffer solution were added. After vortex mixing and centrifugation (3500 gm, 10 min), the supernatant was extracted as described below.
The five WSs of MOR, M3G and M6G, were prepared by transferring 100 μL of the calibration standard mixtures at 100, 250, 500, 1000 and 2000 ng/mL in five tubes and evaporating the solvent to dryness under a stream of nitrogen at 40°C. 0.5 mL of “blank” blood was then added to each tube and, after vortex mixing, the WSs were handled as the unknown and “blank” specimens.
Solid-phase Extraction method
Samples were extracted using Bond Elut Certify® Varian cartridges, conditioned with 2 mL of methanol and 2 mL of pH 9 buffer solutions and not allowed to dry during this phase. After loading the sample, keeping the flow at around 1 mL/min, the cartridge was washed with 4.0 mL of pH 9 buffer solutions, and subsequently dried under air flow for around ten minutes.
A final wash with 3.0 mL of a mixture hexane:ethyl acetate (8:2) was applied and the cartridge was dried again under air flow for one minute. The compounds of interest were finally eluted with 2 mL of methanol, the extract was dried under a nitrogen stream at 40°C and the residue was reconstituted with 250 µL of mobile phase A and analyzed. If the concentration of the analytes was above the range of linearity, the samples were diluted, in order to obtain a concentration following within the calibration range.
Statistical analysis
The mean of the concentration ratio M3G/M6G was compared to the means of M3G/M6G of some reported by clinical trials using Wilcoxon signed rank test.
Results
Method validation
Because of the complexity of “blank” matrices, a simplified validation procedure and a higher variability and tolerance towards the results were applied.
Specificity of the LC/MS-MS was evaluated by repeatedly extracting and analyzing 0.5 mL of “blank” solvent and 0.5 mL of 10 “blank” bloods from different sources spiked with 50 µL of the ISM-D3, checking the presence of interfering peaks for each MRM at the R.T. of the investigated compounds.
For the study of linearity, calibration curves were performed by spiking 0.5 mL “blank” blood samples with five increasing concentration levels (WSs 10, 25, 50, 100, 200 ng respectively) of MOR, M3G and M6G. R2 values above 0.9940 were found for all the analytes, indicating a good linearity of the method (Table 3).
| Compound | Range (ng) | Slope | Intercept | R2 | LLOQ (ng) | LOD (ng) | RSD% | REC% (± SD) | %ME |
|---|---|---|---|---|---|---|---|---|---|
| MOR | 10-200 | 0.045 | 0.029 | 0.9992 | 10 | 1 | 7.0 | 100.0 (± 7.0) | 69.0 |
| M3G | 10-200 | 0.017 | 0.003 | 0.9995 | 10 | 1 | 11.0 | 100.0 (± 11.1) | 40.4 |
| M6G | 10-200 | 0.015 | 0.055 | 0.9940 | 10 | 1 | 14.8 | 100.3 (± 14.3) | 72.2 |
Table 3: Results of the validation study: range, slope, intercept and correlation coefficient (R2) of the linearity study; Lower Limit of Quantitation (LLOQ); Limit of Detection (LOD); average percent Relative Standard Deviation (%RSD) and average percent Recovery (% REC) ± Standard Deviation (SD) as a measure of the intermediate precision and of the accuracy of the method, respectively. Matrix effects (% M.E.) of WSs 50 ng (n=5).
The calculation of the concentration of the analytes and repeatability were assessed determining the Response Ratio RR as follows.
Five working standards solutions (Wws) were prepared and analysed each day to determine RAstd defined as:
RAstd=Astd/AIS
Where Astd is the area of the peak of the quantifier MRM transition of the standard solution; AIS is the area of the peak of the quantifier MRM transition of the IS.
Then for each solution the response ratio RR was calculated as:
RR=RAstd / Cstd
Where Cstd is the concentration of the standard solution expressed as total ng of analyte. This value was finally divided by the unknown sample volume (0.5 mL) to get the concentration as ng/mL (of blood).
The RRmean was then determined as the mean of the five RR obtained for the single standard solutions.
The concentration of analytes was determined applying the following formula:
Cx=RAsample/RRmean
Where Cx=analyte concentration in the unknown specimen or the standard concentration expressed as total ng of analyte. This value was finally divided by the unknown sample volume (0.5 mL) to get the concentration as ng/mL (of blood).
RAsample=ratio between the area of the peak of the quantifier MRM transition of the analyte in the sample and the area of the peak of the quantifier MRM transition of the IS; RRmean=mean response ratio calculated from the analysis of five working standards.
Repeatability was evaluated by calculating the ± SD (Standard Deviation) and the percent RSD (% Relative Standard Deviation) of the Response Ratio (RR) of the five WSs prepared on three different days for the study of linearity (Table 3).
For the study of the accuracy, each WSs of the linearity study was quantified using the RRmediumTOT calculated for the study of intermediate precision. The results (ngexp) were then compared to the theoretical values (ngtheor) in order to calculate the corresponding percent Recovery (% REC) of the method, by applying the following formula: % REC=(ngexp/ngtheor) x100 (Table 3).
The LOD was set by analyzing a series of decreasing concentrations of drug-fortified “blank” blood samples. On the basis of the results, the LOD of the three analytes was set at 1 ng (Table 3). The LLOQ was evaluated on the basis of the % RSD and % REC values of the lowest WSs (10 ng) of the three regression lines calculated for the assessment of linearity. Good results were obtained on each working day, so that the LLOQ was set at 10 ng for all the molecules. A lower LLOQ could be evaluated for morphine, but a 10 ng value was enough for the purposes of our method (Table 3).
The following procedure [9] was applied to evaluate matrix effects. Five WSs (50 ng) were prepared from “blank” blood and extracted by SPE together with five “blank” blood samples, which were fortified after SPE to become post-extraction WSs (50 ng). On the same day, five unextracted WSs (50 ng) were prepared in 3 mM ammonium formate buffer.
Matrix effects for each analyte were expressed by a percent value, calculated in the following way:
Matrix effect (%)=B/A x 100
where A is the average peak area of the unextracted standards and B is the average peak area of the post-extraction WSbs.
As shown in Table 3 all the analytes, especially M3G, showed large ion suppression (% ME). Nonetheless, the accuracy of the method was not affected, as indicated by the validation study (% REC). In the case of 6-MAM ion suppression was responsible for the fact that this analyte, present in blood in low concentration, always resulted below LOD and for this reason it was not possible to determine it.
Discussion
M3G is an inactive metabolite [17-18], the activity of M6G on µ-opioid receptors has been demonstrated in a number of studies [19-21], and this molecule is under investigation as therapeutic agent for the treatment of post-operative pain [22-25]. From LC/MS-MS results, the concentration ratio M3G/M6G was evaluated (Table 1).
As mentioned in the introduction, the interpretation of analytical results is the most challenging task in post-mortem toxicology. As far as the cause of death is concerned, a complete knowledge of the case history is fundamental. Thus, for each case analytical results should be interpreted taking into account also autopsy findings, information from the scene and relevant medical history. Nonetheless, some considerations are possible. A high variability in MOR blood concentrations can be noticed, in accordance with literature. A wide distribution of M3G and M6G concentrations is also present (Table 1).
If only the ten cases belonging to the class I are considered, this variability is still present. This class includes cases in which only heroin metabolites were found by the systematic toxicological analysis (STA, 11), being therefore no evident risk factors present; for classification, see also paragraph “Post-mortem blood samples”.
As suggested by some authors [24,25], the concentration ratio M3G/M6G, was also evaluated (Table 1). Thus, the relationship between two concentrations may be investigated. Looking at the ratios of all the other cases, it can be noticed that variability is still present, but within a narrower range (Table 1).
The relationship between analytical results and time of death was also considered, as suggested by some authors [9,13,25]. Unfortunately, no great information about time of death was available. Nonetheless, in case No.4 and No.5 people were found with a syringe still in the arm, indicating that a quite immediate death had occurred. The ratio of M3G to M6G is however quite close to the mean and to the other ones in both cases, 2.89 and 3.23, respectively.
As described by several authors [3,4], heroin metabolism can be affected by several factors, for example the contemporary use of other psychoactive drugs. These substances may change blood concentrations of heroin metabolites and/or contributing to pharmacological and toxic effects. The twenty-one cases were therefore divided into three classes according to the presence of other drugs of abuse, as described in paragraph “Post-mortem blood samples”. Mean values, ± SD and range were calculated for each class, but no significant differences both in absolute concentrations and ratios can be found, except for ethanol. As far as ethanol is concerned, the cases were further classified into three groups according to BAC (Blood Alcohol Concentration, g/L): the first group included case No.1-10 (i.e. class I), where BAC was <0.5 g/L; in the second group, BAC was between 0.5 and 1.0 and case No.11, 12 and 13 were included; the third group was formed by cases No. 14, 15 and 16, which had a BAC ≥ 1.0 g/L. Cases No. 17-21 were not considered because also other “risk factors” were present. We found that no significant differences among the three groups were present.
In cases 18-21, STA revealed the contemporary use of cocaine; cases No.18, 19 and 21 showed a ratio of M3G to M6G close to the mean one, 3.08, 2.60 and 4.80 respectively. In case 20 a particularly low M3G/M6G ratio was evidenced (1.10).
Finally, it must be underlined that a wide variability of the ratios of morphine metabolites has been described also in the clinical field, when morphine treatment is involved. In this regard, several authors have demonstrated a lack of relationship between this value and long-term or post-operative therapy outcomes [28,29]. In this work, a significantly lower mean M3G/M6G (4.03 ± 1.47) compared to some reported by clinical trials was found (p<0.0001) (Table 4). Other authors confirm our data suggesting a mean M3G/M6G less than that of morphine glucuronides detected in patient receiving morphine therapy. (Table 5) [24] This finding is in accordance with other published data [25] and seems to confirm that morphine glucuronidation may change in drug addicts, as demonstrated by in vitro results and outcomes from animals studies [29]. Certainly, differences between morphine therapy and “street heroin” use, as well as the overall health of patients and addicts, and also all the difficulties of post-mortem toxicology, must be considered. Nonetheless, in heroin fatalities M3G/M6G should be further investigated, beside other concentrations and/or ratios, in order to study the role of some risk factors, such as the contemporary use of ethanol and cocaine or the loss of tolerance. To this purpose, a larger number of blood samples will be analyzed, when available.
| Drug | Application route |
Case n. | M3G/M6G | Reference |
|---|---|---|---|---|
| Morphine | oral | 34 | 6.5 | 30 |
| Morphine | oral | 17 | 8.4 ± 3.3 | 31 |
| Morphine | intravenous | 19 | 8.2 ± 3.3 | 31 |
| Morphine | oral | 16 | 6.7 ± 1.0 | 32 |
Table 4: Morphine Glucuronides in serum or plasma of patients after morphine therapy: mean ± standard deviation.
| Drug | Case n. | M3G/M6G | Reference |
|---|---|---|---|
| Heroin | 21 | 3.30 ± 1.7 | 24 |
| Heroin | 21 | 4.03 ± 1.4 | current study |
Table 5: Concentration Ratio of M3G/M6G in blood of deceased heroin addicts: mean ± standard deviation.
Conclusions
The optimized method for the determination of morphine and its metabolites M3G and M6G in blood can lead to a better understanding of the mechanisms which are responsible for heroin overdose fatalities. Form the preliminary analysis of the twenty-one cases some important indications emerged which should be further investigated: the ratio M3G/M6G, being quite constant, should be a valid reference value to assess toxicity. Furthermore the value of M3G/M6G ratio detected in heroin addict, in this study, is lower than that found in people who assume morphine for therapeutic purposes. The present study could be an important reference also to evaluate the changes which occur in the glucuronidation mechanism in drug addicts.
References
- Annual Report( 2011) EMCDDA (European Monitoring Centre for Drugs and Drug Addiction.
- White JM, Irvine RJ (1999) Mechanisms of fatal opioid overdose. Addiction 94: 961-972.
- Warner-Smith M, Darke S, Lynskey M, Hall W (2001) Heroin overdose: causes and consequences. Addiction 96: 1113-1125.
- Bertol E, Mari F, Baglini G, Boccardo F (1997) Non-lethal acute heroin intoxications in combination with alcohol and benzodiazepine assumption. Bollettino per le farmacodipendenze e l’alcolismo 4.
- Polettini A, Groppi A, Montagna M (1999) The role of alcohol abuse in the etiology of heroin-related deaths. Evidence for pharmacokinetic interactions between heroin and alcohol. J Anal Toxicol 23: 570-576.
- Sporer KA (1999) Acute heroin overdose. Ann Intern Med 130: 584-590.
- Fugelstad A, Ahlner J, Brandt L, Ceder G, Eksborg S, et al. (2003) Use of morphine and 6-monoacetylmorphine in blood for the evaluation of possible risk factors for sudden death in 192 heroin users. Addiction 98: 463-470.
- Polettini A, Poloni V, Groppi A, Stramesi C, Vignali C, et al. (2005) The role of cocaine in heroin-related deaths. Hypothesis on the interaction between heroin and cocaine. Forensic SciInt 153: 23-28.
- Al-Asmari AI, Anderson RA (2007) Method for quantification of opioids and their metabolites in autopsy blood by liquid chromatography-tandem mass spectrometry. J Anal Toxicol 31: 394-408.
- Rook EJ, Huitema AD, van den Brink W, van Ree JM, Beijnen JH (2006) Pharmacokinetics and pharmacokinetic variability of heroin and its metabolites: review of the literature. CurrClinPharmacol 1: 109-118.
- Gambaro V, Argo A, Cippitelli M, Dell'Acqua L, Farè F, et al. (2014) Unexpected variation of the codeine/morphine ratio following fatal heroin overdose. J Anal Toxicol 38: 289-294.
- Spiehler V, Brown R (1987) Unconjugated morphine in blood by radioimmunoassay and gas chromatography/mass spectrometry. J Forensic Sci 32: 906-916.
- Goldberger BA, Cone EJ, Grant TM, Caplan YH, Levine BS, et al. (1994) Disposition of heroin and its metabolites in heroin-related deaths. J Anal Toxicol 18: 22-28.
- Taylor K, Elliott S (2009) A validated hybrid quadrupole linear ion-trap LC-MS method for the analysis of morphine and morphine glucuronides applied to opiate deaths. Forensic SciInt 187: 34-41.
- Dienes-Nagy A, Rivier L, Giroud C, Augsburger M, Mangin P (1999) Method for quantification of morphine and its 3- and 6- glucuronides, codeine, codeine glucuronide and 6-monoacetylmorphine in human blood by liquid chromatography-electrospray mass spectrometry for routine analysis in forensic toxicology. J Chromatogr A 854: 109-118.
- Skopp G, Pötsch L, Klingmann A, Mattern R (2001) Stability of morphine, morphine-3-glucuronide, and morphine-6-glucuronide in fresh blood and plasma and postmortem blood samples. J Anal Toxicol 25: 2-7.
- Penson RT, Joel SP, Bakhshi K, Clark SJ, Langford RM, et al. (2000) Randomized placebo-controlled trial of the activity of the morphine glucuronides. ClinPharmacolTher 68: 667-676.
- Lötsch J (2005) Opioid Metabolites. J Pain Symptom Manag 29:10-24.
- Rossi GC, Brown GP, Leventhal L, Yang K, Pasternak GW (1996) Novel receptor mechanisms for heroin and morphine-6 beta-glucuronideanalgesia. NeurosciLett 216: 1-4.
- Pasternak GW (2001) Insights into mu opioid pharmacology the role of mu opioid receptor subtypes. Life Sci 68: 2213-2219.
- Ulens C, Baker L, Ratka A, Waumans D, Tytgat J (2001) Morphine-6beta-glucuronide and morphine-3-glucuronide, opioid receptor agonists with different potencies. BiochemPharmacol 62: 1273-1282.
- Lötsch J (2004) Morphine metabolites as novel analgesic drugs? CurrOpinAnaesthesiol 17: 449-453.
- Binning AR, Przesmycki K, Sowinski P, Morrison LMM, Smith TW (2010) A randomised controlled trial on the efficacy and side-effect profile (nausea/vomiting/sedation) of morphine-6-glucuronide versus morphine for post-operative pain relief after major abdominal surgery. Eur J Pain15: 402-408.
- Aderjan R, Hofmann S, Schmitt G, Skopp G (1995) Morphine and morphine glucuronides in serum of heroin consumers and in heroin-related deaths determined by HPLC with native fluorescence detection. J Anal Toxicol 19: 163-168.
- Bogusz MJ, Maier RD, Driessen S (1997) Morphine, morphine-3-glucuronide, morphine-6-glucuronide, and 6-monoacetylmorphine determined by means of atmospheric pressure chemical ionisation-mass spectrometry-liquid chromatography in body fluids of heroin victims. J Anal Toxicol 21:346-355.
- Lawrence AJ, Michalkiewicz A, Morley JS, MacKinnon K, Billington D (1992) Differential inhibition of hepatic morphine UDP-glucuronosyltransferases by metal ions. BiochemPharmacol 43: 2335-2340.
- Antonilli L, Suriano C, Paolone G, Badiani A, Nencini P (2003) Repeated exposures to heroin and/or cadmium alter the rate of formation of morphine glucuronides in the rat. J PharmacolExpTher 307: 651-660.
- Verplaetse R, Tytgat J (2011) Liquid chromatography tandem mass spectrometry in forensic toxicology: what about matrix effects? TIAFT Bulletin 41: 8-16.
- Andersen G, Christrup LL, Sjøgren P, Hansen SH, Jensen NH (2002) Changing M3G/M6G ratios and pharmacodynamics in a cancer patient during long-term morphine treatment. J Pain Symptom Manage 23: 161-164.
- Wolff T, Samuelsson H, Hedner T (1995) Morphine and morphine metabolite concentrations in cerebrospinal fluid and plasma in cancer pain patients after slow-release oral morphine administration. Pain 62: 147-154.
- Ashby M, Fleming B, Wood M, Somogyi A (1997) Plasma Morphine and Glucuronide (M3G and M6G) Concentrations in Hospice Inpatients. J Pain SymptManag 14: 157-167.
- van Dongen RT, Crul BJ, Koopman-Kimenai PM, Vree TB (1994) Morphine and morphine-glucuronide concentrations in plasma and CSF during long-term administration of oral morphine. Br J ClinPharmacol 38: 271-273.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 15524
- [From(publication date):
April-2015 - Aug 16, 2025] - Breakdown by view type
- HTML page views : 10811
- PDF downloads : 4713