Superior Parietal Volume in Adolescents with a History of Trauma
Received: 29-May-2016 / Accepted Date: 04-Jul-2016 / Published Date: 08-Jul-2016 DOI: 10.4172/2375-4494.1000303
Abstract
Objective: Early trauma exposure has been demonstrated to significantly impact brain volume. Childhood trauma also contributes to onset of psychopathology, particularly depression. We sought to identify gray matter volume changes unique to trauma exposure in adolescent depression and hypothesized that adolescents with diagnosis of major depressive disorder (MDD) and childhood trauma would have smaller gray matter volume and thickness in areas previously identified as reduced with childhood trauma exposure relative to non-traumatized depressed adolescents and healthy controls. Methods: We obtained structural MRI scans for 120 adolescents with a history of past trauma exposure and a current diagnosis major depressive disorder (MDD, n=29), a diagnosis of MDD, but no trauma exposure (n=49), and healthy controls (n=42). Adolescents with a diagnosis of MDD and trauma exposure compared with adolescents with MDD and no history of trauma exposure showed increased gray matter volume in the right superior parietal lobe (p=0.001), a cortical region important for processing of visuospatial cues and implicated in traumatic memory. Positive trauma history status included sexual or physical abuse, or trauma with risk of death or bodily harm. Results: Our findings indicate increased right superior parietal volume in depressed adolescents with history of trauma exposure that is distinct from findings related to depression or suicidal behavior. Conclusion: Our finding of increased superior parietal volume in adolescents exposed to past trauma compared with adolescents not exposed to past trauma may indicate differences in information processing, particularly visuospatial processing and working memory, in trauma exposed, depressed adolescents. The absence of any significant relationships between superior parietal lobe volume abnormality and measures of present symptom severity, suicidal ideation, past suicide attempt and medication in adolescents with history of depression and past trauma suggests that increased right superior parietal lobe volume may be related to trauma exposure in adolescents with depression.
Keywords: Trauma; Adolescents; Depression; Superior parietal lobe; Brain volume; Cortical thickness; Gray matter
218780Objective
Exposure to childhood trauma has been shown to have profound impact on brain development and brain volume [1]. Childhood trauma also contributes to onset of psychopathology, particularly depression [2,3]. Maltreated children show an earlier onset of depression, more severe symptoms, poorer treatment response, and greater risk for suicide [3]. Recognition of neurobiological markers of maltreatment in depressed adolescents may aid in identifying patients in need of trauma-focused treatments and help understand the profound effect of childhood trauma on psychopathology.
There is evidence that childhood trauma exposure may affect brain structure differently at various stages of development, both in terms of time of exposure and period of brain development [4]. Specifically, diminished corpus callosum volume is described in both children and adults with early childhood trauma exposure [5], while reduced hippocampal volume compared with healthy controls has been found in adults with a history of childhood trauma who had post-traumatic stress disorder [6], and in adolescents with depression and early life adversity [7], but not in traumatized children [5]. Bilateral lateral occipital gyrus volume reduction was shown in young adults (age M=21.8, SD=2.4) who witnessed domestic violence in childhood compared with healthy controls [8]. Evoked neuromagnetic hyperactivation of the superior parietal lobe in response to aversive stimuli was observed in participants with PTSD after torture and war exposure [9], and patients with PTSD showed increased regional blood flow to the right superior parietal lobe during resting state compared with healthy controls [10].
We sought to identify gray matter volume changes unique to trauma exposure in adolescent depression. Based on previous developmental traumatology studies, we hypothesized that adolescents with diagnosis of major depressive disorder (MDD) and childhood trauma, defined as physical or sexual abuse or exposure to trauma with risk of death or bodily harm, would have smaller gray matter volume and thickness in areas previously identified as reduced with childhood trauma exposure, including corpus callosum, amygdala, anterior cingulate gyrus, bilateral lateral occipital gyrus, bilateral superior parietal volume and hippocampus relative to non-traumatized depressed adolescents and healthy controls.
Methods
Participants
120 adolescents were scanned including adolescents with:(1) history of trauma exposure (physical abuse, sexual abuse, or traumatic event with risk of death or bodily injury) and MDD (Tr, n=29); (2) MDD diagnosis, but no history of trauma (NTr, n=49); and (3) no personal or family history of psychiatric disorder, suicide attempt, or trauma exposure (healthy controls (HC), n=42). Exclusions included neurological disorders, head injury, Wechsler verbal score<80 [11], pregnancy, MRI ineligibility, bipolar disorder, psychosis, substance abuse or positive drug screen, and left-handedness due to concurrent functional imaging studies.
Participants provided informed consent prior to the start of any study procedures. University of Pittsburgh IRB approved the protocol. Patients were recruited from the Services for Teens at Risk Clinic Registry and HC were recruited by advertisement. DSM-IV criteria for MDD were assessed using the KSADS-PL [12] at clinic registry entry. Depression, anxiety, suicidal ideation and pubertal status were assessed with the Beck Depression Inventory (BDI) [13], Screen for Child Anxiety Related Disorders(SCARED) [14], Suicidal Ideation Questionnaire (SIQ) [15] and Petersen Pubertal Development Scale [16], respectively (Table 1) at time of the scan. Past trauma was determined by psychiatrist assessment and chart review in all participants upon enrollment in the study. Positive trauma history status included sexual or physical abuse, or trauma with risk of death or bodily harm.
| Region | Side | Post Hoc test | Volume | t | df | P |
|---|---|---|---|---|---|---|
| Superior Parietal Lobe (BA7) | R | Tr>NTr | Volume | 3.5 | 77 | 0.001 |
| coordinates | ||||||
| F x y z cluster | ||||||
| Size mm2 | ||||||
| 12.21 19 -81 36 939.54 | ||||||
| In Tr compared with NTr (BA 7, x=19 y=-81, z=36, vertex=34, cluster size=939.54 mm2; p<.05 corrected) with cortical volume post-hoc t-tests, group(Tr, NTr), covarying for age, gender, scanner, and total brain volume. | ||||||
Table 1: Cortical Volume in the Right Superior Parietal Lobe.
Two MRI scanners were utilized: 49 scans were acquired on a 3T Siemens Allegra, and 51 scans on a 3T Siemens Trio. T1-weighted magnetization prepared rapid gradient echo (MPRAGE) structural images of 240 0.8-mm slices were acquired (repetition time: 1630ms; echo time: 2.48ms; inversion time: 800ms; field of view: 200mm; flip angle: 8x; matrix: 256r256). Brain cortical thickness and gray and white matter volumes were measured using FreeSurfer5.1. Smoothing FWHM kernel size 10. Topographical defects were automatically corrected and images were normalized. Cortical thickness measures were computed as distances between the gray/white matter boundary and the pial surface [17]. Cortical volumes were calculated from the surface mask [18]. Two whole-brain surface-based analyses of covariance (ANCOVA) were completed in Qdec1.4 (FreeSurfer) to examine main effect of group on cortical thickness and volume, respectively, with age, gender, total brain volume, and scanner as covariates. Both hemispheres were analyzed individually, with correction performed for both hemispheres. Monte Carlo simulation analyses were performed to correct for multiple voxel-wise comparisons in Qdec, with cluster-wise significance threshold of p<0.05. Correction was by Monte Carlo z simulation: 1.3(.05) threshold, initial cluster forming threshold: 0.05 uncorrected.
Monte Carlo simulation was performed on all groups with both hemispheres done individually, and performed on both thickness and volume analyses. 3 group (HC vs. Tr, HC vs. NTr, Tr vs. NTr) x 2 hemispheres x 2 brain analyses (thickness and volume)=12 total iterations of Monte Carlo simulation were performed for each of 12 different analyses.
To examine between-group differences in cortical volume and thickness arising from the above ANCOVAs, cortical volume and thickness values were extracted from all identified cortical regions. Post-hoc pair-wise, between-group independent t-tests were conducted on these extracted values. Significance thresholds were p(2- tailed)<0.05 and Bonferroni corrected for post-hoc comparisons. Because only post-hoc volume values were significant, exploratory correlational analyses were completed on volume only. Exploratory correlational analyses were completed in SPSS 20.0 to examine relationships between volume abnormalities in and clinical variables in both depressed groups.
Results
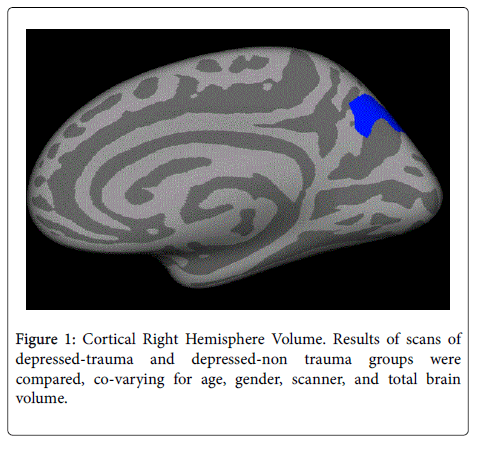
Groups did not differ significantly in gender. HC (age M=14.54, SD=1.86) were significantly younger than either Tr (age M=15.79, SD=1.52) and NTr (age M=15.87, SD=1.23), but with no significant difference in pubertal status. Tr and NTr did not differ significantly on BDI, SCARED, SIQ, suicide history, or medication status (Figure 1). Experiences of maltreatment during childhood were determined by psychiatric assessment and chart review for all participants.
One-way ANCOVA results revealed a significant main effect of group for volume in the right superior parietal lobe (BA 7, x=19 y=-81, z=36, vertex=34, cluster size=939.54 mm2; p<0.05 corrected) and left lateral occipital gyrus cluster (BA18, x=-13, y=-91, z=4, vertex=34, cluster size=3188.79 mm2; p<0.05 corrected) from the initial whole brain analyses. There was a main effect of group for cortical thickness in the left lateral occipital gyrus (BA18, x=-13, y=-91, z=4, vertex=34, cluster size=2654.49; p<0.05 corrected). Post-hoc pair-wise comparisons did not indicate a significant difference in left lateral occipital gyrus volume (Tr<NTr, F(2,118)=1.098, p=0.298) or cortical thickness (Tr<NTr, F(2,118)=0.346, p=0.558). Only right superior parietal volume was significant in post-hoc pair-wise comparisons, revealing that Tr had significantly greater right superior parietal volume than NTr (Tr>NTr, F(2,118)=12.21, p=0.001) (Figure 1).
Exploratory analyses showed no significant relationships between BDI, SCARED, SIQ, past suicide attempt, medication, and right superior parietal volume in Tr, using a statistical threshold of p=0.05/5(p=0.01).
Conclusion
We report greater right superior parietal volume in Tr compared with NTr adolescents with depression. Volume was not significantly different between Tr and HC. The right superior parietal lobe is critical for sensorimotor integration [19] and processing contextual clues related to visuospatial perception of self in the environment [20]. Human brain lesion studies indicate that the superior parietal lobe is essential for manipulation of information in working memory [21], and transcranial magnetic stimulation of the bilateral superior parietal lobe disrupted abstract and incongruent reasoning in healthy participants [22].
Aberrant neural activity in the superior parietal lobe is also implicated in adults with post-traumatic stress disorder (PTSD). A finding of increased volume in Tr versus NTr adolescents with depression is unexpected. In a study of women with borderline personality disorder who had a history of childhood physical and sexual abuse, larger right parietal volume was associated with greater symptoms of depersonalization. However, in this study, women with borderline personality disorder had smaller right parietal volumes than healthy controls [23]. Evoked neuromagnetic hyperactivation of the superior parietal lobe in response to aversive stimuli was observed in participants with PTSD after torture and war exposure [9]. An EEG resting state study showed increased theta activity in parietal areas including BA 7 in 17 patients with PTSD compared with healthy patients [24]. Finally, 19 participants with PTSD showed increased regional blood flow to the right superior parietal lobe during resting state compared with healthy controls [10].
Abnormalities of the structure of right superior parietal lobe are reported in traumatized adults. Twelve adults with depression and history of physical or sexual abuse had right superior parietal lobe cortical thickness that varied inversely with child trauma questionnaire score [25]. Adult disaster survivors with PTSD, but not survivors without PTSD, showed cortical thinning in the right superior parietal lobe compared with healthy controls [26].
Our finding of increased superior parietal volume in Tr compared with NTr adolescents may indicate differences in information processing, particularly visuospatial processing and working memory, in trauma exposed, depressed adolescents. The absence of any significant relationships between superior parietal lobe volume abnormality and measures of present symptom severity, suicidal ideation, past suicide attempt and medication in adolescents with history of depression and past trauma suggests that increased right superior parietal lobe volume may be related to trauma exposure in adolescents with depression.
Limitations of the present study include use of two scanners, a covariate in analyses. Another limitation was analysis of the relationship between trauma, MDD, and brain volume in a sample initially collected to study suicidal behavior. While there are unique findings related to brain volume in suicide attempt [27], suicide attempt status did not show a relationship with superior parietal volume in this analysis. There were no significant findings between affected groups and healthy controls. This could be related to modest sample size or younger age of healthy controls despite matching for pubertal status. An additional limitation was exclusion of emotional abuse and neglect because of inability to reliably quantify emotional abuse and neglect in one psychiatric assessment and chart review. Finally, time of first trauma and duration of trauma were not well quantified. Future volumetric studies should include emotional abuse and neglect and onset and duration of abuse [28].
In summary, our findings indicate increased right superior parietal volume in depressed adolescents with history of trauma exposure that is distinct from findings related to depression or suicidal behavior. The extent to which this precedes or is a consequence of trauma exposure remains to be clarified in future neuroimaging studies involving traumatized adolescents.
References
- De Bellis MD, Zisk A (2014) The biological effects of childhood trauma. Child AdolescPsychiatrClin N Am 23: 185-222, vii.
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, et al. (1998) Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med 14: 245-258.
- Teicher MH, Samson JA (2013) Childhood maltreatment and psychopathology: A case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. Am J Psychiatry 170: 1114-1133.
- Baker LM, Williams LM, Korgaonkar MS, Cohen RA, Heaps JM, et al. (2013) Impact of early vs. late childhood early life stress on brain morphometrics. Brain Imaging Behav 7: 196-203.
- De Bellis MD, Keshavan MS, Shifflett H, Iyengar S, Beers SR, et al. (2002) Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biol Psychiatry 52: 1066-1078.
- Teicher MH, Anderson CM, Polcari A (2012) Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. ProcNatlAcadSci U S A 109: E563-572.
- Rao U, Chen LA, Bidesi AS, Shad MU, Thomas MA, et al. (2010) Hippocampal changes associated with early-life adversity and vulnerability to depression. Biol Psychiatry 67: 357-364.
- Tomoda A, Polcari A, Anderson CM, Teicher MH (2012) Reduced visual cortex gray matter volume and thickness in young adults who witnessed domestic violence during childhood. PLoS One 7: e52528.
- Catani C, Adenauer H, Keil J, Aichinger H, Neuner F (2009) Pattern of cortical activation during processing of aversive stimuli in traumatized survivors of war and torture. Eur Arch Psychiatry ClinNeurosci259: 340-351.
- Kim SJ, Lyoo IK, Lee YS, Kim J, Sim ME, et al. (2007) Decreased cerebral blood flow of thalamus in PTSD patients as a strategy to reduce re-experience symptoms. ActaPsychiatrScand 116: 145-153.
- Pitcher TM, Piek JP, Barrett NC (2002) Timing and force control in boys with attention deficit hyperactivity disorder: subtype differences and the effect of comorbid developmental coordination disorder. Hum MovSci 21: 919-945.
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, et al. (1997) Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36: 980-988.
- Beck AT, Ward CH, Mendelson M, Mock J,Erbaugh J (1961) An inventory for measuring depression. Arch Gen Psychiatry 4: 561-571.
- Birmaher B, Khetarpal S, Cully M, Brent DA, McKenzie S (2003) Screen for Child Anxiety Related Disorders (SCARED). Innovations in Clinical Practice: Focus on Children & Adolescents. Professional Resource Press, Inc. Sarasota, FL.
- Reynolds WM (1987) Suicidal Ideation Questionnaire (SIQ). Psychological Assessment Resources.
- Petersen AC, Crockett L, Richards M, Boxer A (1988) A self-report measure of pubertal status: Reliability, validity, and initial norms. J Youth Adolesc 17: 117-133.
- Fischl B, Dale AM (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. ProcNatlAcadSci U S A 97: 11050-11055.
- Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, et al. (2009) High consistency of regional cortical thinning in aging across multiple samples. Cereb Cortex 19: 2001-2012.
- Wolpert DM, Goodbody SJ, Husain N (1998) Maintaining internal representations: the role of the human superior parietal lobe. Nat Neurosci 1: 529-533.
- Lester BD, Dassonville P (2014) The role of the right superior lobule in processing visual context for the establishment of the egocentric reference frame. J CognNeurosc26: 2201-2209.
- Koenigs M, Barbey AK, Postle BR, Grafman J (2009) Superior parietal cortex is critical for the manipulation of information in working memory. J Neurosci 29: 14980-14986.
- Tsujii T, Sakatani K, Masuda S, Akiyama T, Watanabe S (2011) Evaluating the roles of the inferior frontal gyrus and superior parietal lobule in deductive reasoning: an rTMS study. Neuroimage 58: 640-646.
- Irle E, Lang C, Weniger G, Sachsse U (2007) Size abnormalities of superior parietal cortices are related to dissociation in borderline personality disorder. Psychiatry Res 156: 139-149.
- Imperatori C, Farina B, Quintiliani MI, Onofri A, Castelli-Gattinara P, et al. (2014) Aberrant EEG functional connectivity and EEG power spectra in resting state post-traumatic stress disorder:asLORETA study. BiolPsychol102: 10-17.
- Jaworska N, MacMaster FP2, Gaxiola I3, Cortese F3, Goodyear B4, et al. (2014) A preliminary study of the influence of age of onset and childhood trauma on cortical thickness in major depressive disorder. Biomed Res Int 2014: 410472.
- Qi S, Mu Y, Liu K, Zhang J, Huan Y, et al. (2013) Cortical inhibition deficits in recent onset PTSD after a single prolonged trauma exposure. NeuroimageClin 3: 226-233.
- Pan LA, Ramos L, Segreti A, Brent DA, Phillips ML (2014) Right superior temporal gyrus volume in adolescents with a history of suicide attempt. Br J Psychiatry, pii:bjp.bp.114.151316.
- Mueller-Pfeiffer C, Schick M, Schulte-Vels T, O’Gorman R, Michels L, et al. (2013) Atypical visual processing in posttraumatic stress disorder. NeuroImage Clinical 3: 531-538.
Citation: Selioutski D, Zimmer TM, Segreti AM, Martin PC, Klawson EK, et al. (2016) Superior Parietal Volume in Adolescents with a History of Trauma. J Child Adolesc Behav 4: 303. DOI: 10.4172/2375-4494.1000303
Copyright: © 2016 Pan L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 13296
- [From(publication date): 8-2016 - Aug 17, 2025]
- Breakdown by view type
- HTML page views: 12263
- PDF downloads: 1033