Synergistic Inhibition of Multiple Myeloma Growth by Anti-CD138-Interferon-alpha14 Fusion Protein and Lenalidomide
Received: 24-Feb-2018 / Accepted Date: 27-Feb-2018 / Published Date: 06-Mar-2018
Abstract
Although recent advances have improved the management of multiple myeloma, it remains an incurable malignancy. We now demonstrate that anti-CD138 genetically fused to interferon alpha14 (IFNα14) synergizes with the approved therapeutic lenalidomide in arresting the proliferation of the human multiple myeloma cell line NCIH929 both in vitro and in vivo. This synergism is the consequence of the combined effects of multiple, complementary anti-tumor activities including potentiated activation of STAT1 and downregulation of c-Myc, interferon regulatory factor 4 (IRF4) and poly(ADP-ribose) polymerase 1 (PARP-1). Caspase activation and glucose utilization also play a role in the induction of apoptosis by lenalidomide+anti-CD138-IFNα14 as inhibition of caspase activation, glycolysis or oxidative phosphorylation (OXPHOS) decreased but did not eliminate the apoptosis seen following treatment. Treatment with lenalidomide+anti-CD138-IFNα14 results in replicative stress resulting from increased accumulation of reactive oxygen species (ROS). Unexpectedly, we observed that the cellular stress elicited by treatment with lenalidomide or lenalidomide+anti-CD138-IFNα14 results in the degradation of Chk1, the Ser/Thr kinase central to the genome surveillance pathways of the DNA damage response and cell cycle checkpoints. Using an in vivo xenograft model we found that treatment with anti-CD138-IFNα14+lenalidomide was much more effective than either treatment alone with approximately 40% (9/24) of the animals with established tumors cured. Based on our findings, clinical testing of combination therapy with lenalidomide and anti-CD138-IFN fusion proteins for the treatment of multiple myeloma may be merited.
Keywords: Antibody immunotherapy; Interferon-α; Lenalidomide; Multiple myeloma
Introduction
Multiple myeloma (MM) is a cancer characterized by a clonal proliferation of malignant plasma cells (PCs). MM is the second most frequent hematological malignancy after non-Hodgkin lymphoma. It is estimated that there will be 30,280 new cases of MM in the U.S. in 2016, with 12,590 deaths and approximately 118,600 people living with MM (NCI SEER Fact Sheets: Myeloma). Although novel drugs have done much to improve survival, MM remains a treatable but incurable malignancy.
Type I interferons (IFNα and IFNβ) are pleiotropic cytokines with a broad spectrum of anti-cancer activities including direct antiproliferative and pro-apoptotic effects as well as activation of the immune response. IFNs were the first recombinant proteins to be used in the therapy of cancer [1]. When used to treat malignancies, IFNs have proven most effective at higher doses [2], but to date the side effects associated with high dose IFN treatment have limited their effectiveness. In an effort to achieve therapeutically effective doses of IFN at the site of the tumor in the absence of toxicity, we have produced antibody-based fusion proteins in which IFN is genetically joined to an antibody that recognizes a tumor-associated antigen. The goal is for the antibody to target the fusion protein (FP) to the tumor resulting in the accumulation of therapeutically effective doses without systemic toxicity. This approach has been used effectively to target IFN to various tumors [3-7] and an anti-CD20-IFNα is currently undergoing phase I clinical testing (ClinicalTrials.gov Identifier NCT02519270). A similar approach has been successfully used to target an attenuated IFNα to myeloma cells using anti-CD38 [8].
To target IFN to MM, we have fused it to the B-B4 antibody that specifically binds CD138 [7,9], also known as syndecan-1, which is highly expressed on mature plasma cells. Indeed, initial studies have demonstrated that this fusion protein has effective anti-tumor activity [5,7]. In humans there is only one form of IFNβ, but multiple species of IFNα [10]. Although IFNα2 has been the most broadly assessed clinically and was used to produce our initial fusion protein, a recent study showed that among the 12 human IFNα subtypes, α14 has the strongest anti-proliferative activity against cancer cells [11]. Therefore, for these studies we have used an anti-CD138 FP containing IFNα14.
Thalidomide and its derivatives lenalidomide and pomalidomide are immune modulatory drugs (IMiDs) used in the treatment of hematologic malignancies including MM. ImiDs bind cereblon (CRBN) [12], the substrate-recognition component of a cullindependent ubiquitin ligase, and cause selective ubiquitination and degradation of two lymphoid transcription factors, Ikaros (IKZF1) and Aiolos (IKZF3) [13]. Although this selective degradation may be responsible for the therapeutic effects of lenalidomide in the treatment of myeloma [14], recent studies indicate a role for Ikaros in a pathway that regulates cytoplasmic calcium [15,16] and degrading IKZF1 results in augmentation of cytosolic calcium levels and activation of the calcium-dependent calpain, CAPN1, an inducer of apoptosis. In addition, it has been shown that CRBN functions as a chaperon protein for the MCT1-CD147 complex and is essential for its proper cell surface expression and for MCT1 function in exporting lactate from the cell [15,17]. By binding CRBN, ImiDs disable lactate export by MCT1 resulting in an accumulation of intracellular lactate, a depletion of glutathione, an increase in hydrogen peroxide, mitochondrial damage, and ultimately, cell death.
We now demonstrate that treatment with anti-CD138-IFNα14 plus lenalidomide results in synergistic anti-tumor efficacy against some myeloma cells lines with potent synergy seen with the human myeloma NCI-H929 both in vitro and in vivo . This synergism is the consequence of the combined effects of the two different treatments, through modulation of STAT1 signaling, down regulation of cell survival factors, glucose metabolism, reactive oxygen species, and cellular stress pathways, notably with the degradation of the cell cycle regulator Chk1. The observed multifaceted synergy between anti-CD138- IFNα14 and lenalidomide suggests a novel strategy for the treatment of multiple myeloma.
Materials and Methods
Cells and chemicals
Cell lines were the generous gift of Dr. W. Michael Kuehl with ANBL-6 provided with the kind permission of Dr. Diane Jelinek. Cells were cultured as described previously [7] with CD138 expression confirmed by flow cytometry. All cell lines were tested for mycoplasma using the Agilent Technologies mycoplasma PCR primer set (Cat. # 302008) prior to the production of frozen stocks which were used for all subsequent experiments. Lenalidomide was purchased from Selleck Chemicals (S1029). rIFNα14 (11145-1) was purchased from PBL Assay Science. Q-VD-OPh (A1901) was purchased from Apexbio. ROSIDTM Total ROS detection kit (ENZ-51011) and DiOC6(3) were purchased from Enzo. MitoTracker®Red CMRos (M7512) was purchased from ThermoFisher. 2-DG (D8375) was purchased from Sigma-Aldrich. Tofacitinib (CP-690,550) and metformin were purchased from AdooQ Bioscience.
Construction of vectors and protein production
Construction of anti-CD138 and anti-CD138-IFNα2 has been described [7]. Anti-CD138-IFNα14 was produced by replacing the IFNα2 sequence with that of IFNα14 (NCBI reference sequence: NP_002163.2). All antibodies are IgG1κ and were produced and purified from Chinese hamster ovary cells following transfection of H and L chain expression vectors using protein A as described previously [7].
Determination of cellular metabolic activity
NCI-H929 cells were treated with varying concentrations of fusion protein, lenalidomide or both at 37°C for 3 days. Metabolic activity was determined using MTS solution (Promega, G3581) by measuring absorbance at 490 nm using a Synergy HT Multi-Detection Microplate Reader (BioTek Instruments). GraphPad Prism (GraphPad Software) was used to analyze data as described previously [7]. Data are expressed as percent proliferation with untreated cells being 100%. The t-test was used to calculate p values using GraphPad Prism.Alternatively, NCI-H929 cells were incubated with fixed concentrations of anti-CD138, anti-CD138-IFNα14 or rIFNα14 in the presence or absence of lenalidomide at 37°C for 3 days. Metabolic activity was determined in triplicate using MTS as described above.
Inhibition of caspases
NCI-H929 cells were incubated untreated, or with 4 pM of anti- CD138-IFNα14, 0.8 μM of lenalidomide, or 4 pM of anti-CD138- IFNα14+0.8 μM of lenalidomide in the presence or absence of caspase inhibitor Q-VD-OPh (25 to 100 μM), a cell permeable, irreversible inhibitor of caspases 1, 3, 8 and 9 (IC50=50 nM, 25 nM, 100 nM and 430 nM respectively). Metabolic activity was determined by MTS assay in triplicate as described above. The percent proliferation was calculated relative to cells grown in the absence of treatment. The experiment was repeated 3 times and the data are expressed as the average of the three experiments. The t-test was used to calculate p values using GraphPad Prism and combining the data from the 3 independent experiments.
Western blots
Cells were treated for 24, 48 or 72 h with 5 pM of anti-CD138- IFNα14, 1 μM of lenalidomide or both. Cells were lysed using 50 mM Tris-HCl pH 8, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate containing a protease inhibitor cocktail (Roche Applied Science, 05892791001) and PhosSTOP (Roche Applied Science, 04906845001). The lysates were reduced with β-mercaptoethanol and separated by SDS-PAGE. Following transfer to 0.45 μm PVDF membranes (Millipore, IPVH09120) and blocking with 3% bovine serum albumin (BSA) in PBS+0.1% Tween 20, membranes were incubated with rabbit anti-c-Myc (mAb D84C12, Cell Signaling Technology), anti-IRF4 (Epitomics, aab1333298), anti-PARP-1 (Cell Signaling Technology, 9532S), anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Sigma-Aldrich, G9545), or anti-phospho-Chk1 (Ser 345; Cell Signaling Technology, 2341). Secondary anti-rabbit IgG-HRP (Cell Signaling Technology, 7074S) was used, and the blots were developed using enhanced chemiluminescence (Thermo Fisher Scientific, 34078). Alternatively mouse mAb 2G1D5 to Chk1 (Cell Signaling Technology, 2360) and anti-mouse IgG-HRP (Cell Signaling Technology, 70765) was used. In some experiments rabbit anti-β-actin (Cell signaling Technology, Y970S) and anti-rabbit-HRP (Cell Signaling Technology, 7074S) were used to quantitate gel loading.
To quantitate protein levels, films were scanned and analyzed. Briefly, films were scanned using an EPSON Perfection 4490 scanner with data collected as a TIFF file. The TIFF file was opened in Adobe Photoshop and converted into a gray scale JPEG file that was then analyzed using NIH ImageJ (http://www.yorku.ca/yisheng/Internal/ Protocols/ImageJ.pdf). For each gel, the region of interest (ROI) was defined, and the pixel density of each ROI and corresponding negative region determined. Similar analysis was done for the loading control for each gel. After appropriate backgrounds were subtracted, the relative signal was determined by normalizing to the loading controls. For each time point, the normalized value obtained for the untreated sample was set as 100%, and all values for the treated samples were expressed as percentage of the untreated sample. Data from 3 or 4 membranes from 2 independent experiments were pooled and the SEM calculated.
Measurement of ΔΨm
Cells were incubated with 10 nM of 3,3′-dihexyloxacarbocyanine iodide (DiOC6(3); Enzo, EN2-52303) for 20 min at 37°C and analyzed using flow cytometry.
Apoptosis assay
After treatment, cells were stained with Alexa Fluor 488-labeled Annexin V and propidium iodide (PI) using the Vybrant Apoptosis Kit #2 (Molecular Probes, V13241) and analyzed by flow cytometry.
Murine tumor model
Six-eight week old female NOD-scid IL2rγnull (NSG) mice were used to establish NCI-H929 tumors. Mice were inoculated subcutaneously with 1 × 107 NCI-H929 cells in matrigel (Corning, 354248) on their backs. Mice were treated with PBS, 100 μg of anti- CD138-IFNα14, 500 μg of lenalidomide or 100 μg of anti-CD138- IFNα14+500 μg of lenalidomide on days 14, 16, and 18 post tumor challenge. Treatment was intraperitoneal (i.p.) with lenalidomide and anti-CD138-IFNα14 administered separately. Each group consisted of eight mice. Bidirectional tumor growth was measured twice weekly, and mice were sacrificed when tumors reached 1.4 cm as per institutional guidelines. Data were analyzed in GraphPad Prism. All animal studies were performed in compliance with the US Department of Health and Human Services Guide for the Care and Use of Laboratory Animals and were approved by the UCLA Animal Research Committee.
Results
Anti-CD138-IFNα14 synergizes with lenalidomide in inhibiting growth and causing apoptosis of the NCI-H929 myeloma.
For initial studies to determine if there were synergistic antitumor effects when anti-CD138-IFNα14 was used in combination with lenalidomide, we incubated the myeloma cell lines U266, H929, RPMI8226, MM1-144 and ANBL-6 with 5 fold serial dilutions of 100 pM anti-CD138-IFNα14, 20 μM lenalidomide and the combination for 72 h and determined the inhibition of proliferation using the MTS assay (Suppl. Figure 1). Calculation of the combination index (CI)- isobologram equation allows quantitative determination of drug interactions with CI<1, CI=1 and CI>1 indicating synergism, additive effect, and antagonism, respectively [18]. For NCI-H929, RPMI 8226, and MM1-144 we found the CI to be <1 for all concentrations examined, indicating synergistic inhibition of cell proliferation. For U226 the CI was <1 for all concentrations except the highest. In contrast, for ANBL-6 the CI was >1 at all concentrations indicating that anti-CD138-IFNα14 treatment interfered with the antiproliferative effects of lenalidomide. Thus synergistic inhibition of proliferation can be seen with simultaneous treatment of some but not all myeloma cell lines with anti-CD138-IFNα14 and lenalidomide.
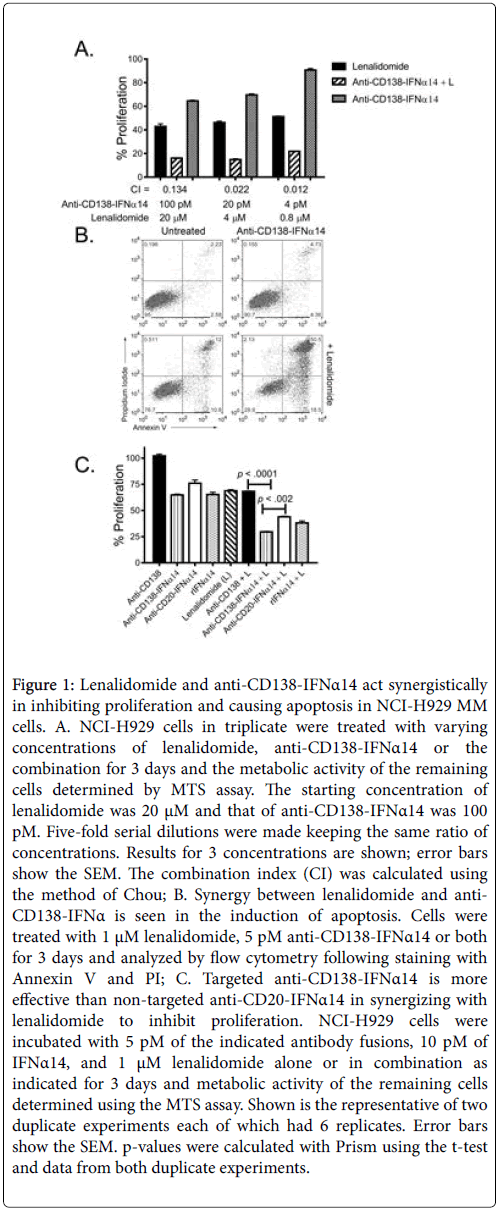
For this study we focused on the human myeloma cell line NCIH929 which showed the most clearly evident synergistic inhibition of proliferation by the combination treatment (Suppl. Figure 1 and Figure 1A). This cell line was established from the malignant effusion in a 62 year-old female and expresses CD138. The cells are negative for Epstein-Barr virus nuclear antigen (EBNA) and have an activated RAS allele (www.ATCC.org). NCI-H929 cells are also near tetraploid with a rearrangement of the c-myc proto-oncogene and expression of cmyc RNA making them representative of the patient subgroup who derives the most benefit from treatment with IMiDs [15]. To determine if the synergistic inhibition of cell growth resulted at least in part from increased apoptosis, NCI-H929 cells incubated with anti-CD138- IFNα14, lenalidomide or anti-CD138-IFNα14+lenalidomide for 5 days were stained with Annexin V-FITC and propidium iodide (PI) and analyzed by flow cytometry. Greater apoptosis was seen following combination treatment than with either single agent (Figure 1B). To confirm these results and to determine if targeted IFNα14 was more effective than non-targeted IFNα14 in inhibiting cell proliferation, NCI-H929 cells were incubated with a fixed concentration of anti- CD138, anti-CD138-IFNα14 or anti-CD20-IFNα14 (5 pM) with or without 1 μM of lenalidomide for 3 days and the metabolic activity of the remaining cells assayed using the MTS assay (Figure 1C). For comparison cells were also incubated with 10 pM of rIFNα14 with or without 1 μM of lenalidomide. Anti-CD138 did not inhibit proliferation and the inhibition of proliferation seen following treatment with the combination of anti-CD138+lenalidomide resembled that seen with lenalidomide alone. The combination of anti- CD138-IFNα14+lenalidomide was significantly more effective in inhibiting cell proliferation than either treatment alone p<.0001 (Figure 1C). Comparison of anti-CD138-IFNα14+lenalidomide with anti-CD20-IFNα14+lenalidomide showed that inhibition of proliferation by anti-CD138-IFNα14+lenalidomide was more effective than was inhibition by non-targeted anti-CD20-IFNα14+lenalidomide (p<.002) showing that targeting plays an important role in determining efficacy.
Figure 1: Lenalidomide and anti-CD138-IFNα14 act synergistically in inhibiting proliferation and causing apoptosis in NCI-H929 MM cells. A. NCI-H929 cells in triplicate were treated with varying concentrations of lenalidomide, anti-CD138-IFNα14 or the combination for 3 days and the metabolic activity of the remaining cells determined by MTS assay. The starting concentration of lenalidomide was 20 μM and that of anti-CD138-IFNα14 was 100 pM. Five-fold serial dilutions were made keeping the same ratio of concentrations. Results for 3 concentrations are shown; error bars show the SEM. The combination index (CI) was calculated using the method of Chou; B. Synergy between lenalidomide and anti- CD138-IFNα is seen in the induction of apoptosis. Cells were treated with 1 μM lenalidomide, 5 pM anti-CD138-IFNα14 or both for 3 days and analyzed by flow cytometry following staining with Annexin V and PI; C. Targeted anti-CD138-IFNα14 is more effective than non-targeted anti-CD20-IFNα14 in synergizing with lenalidomide to inhibit proliferation. NCI-H929 cells were incubated with 5 pM of the indicated antibody fusions, 10 pM of IFNα14, and 1 μM lenalidomide alone or in combination as indicated for 3 days and metabolic activity of the remaining cells determined using the MTS assay. Shown is the representative of two duplicate experiments each of which had 6 replicates. Error bars show the SEM. p-values were calculated with Prism using the t-test and data from both duplicate experiments.
Anti-CD138-IFNα14 and anti-CD138-IFNα14+lenalidomide activate the STAT1 and STAT3 pathways.
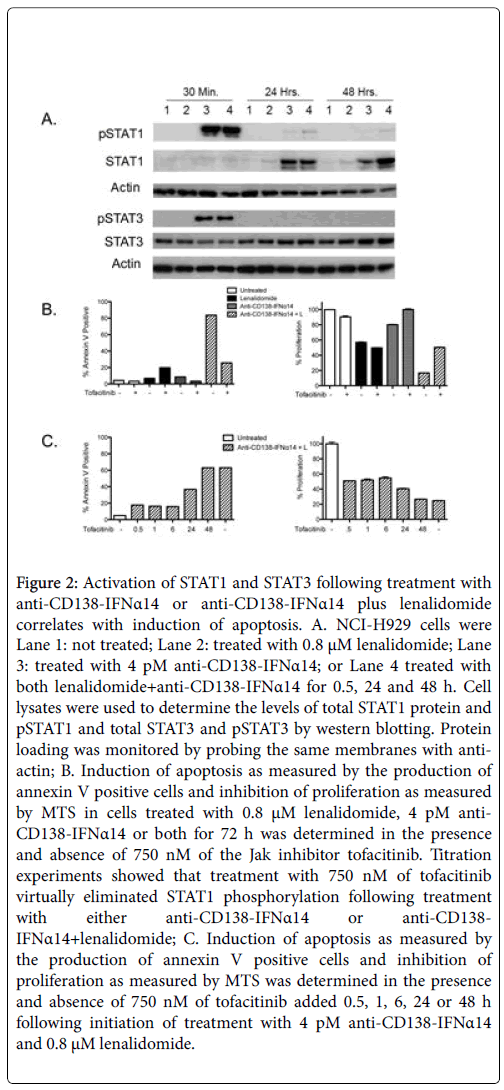
STAT proteins play a major role in the IFNα signaling pathway with activation of STAT1 required for IFNα-mediated cell death [19]. In contrast, STAT3 activation counteracts inflammation and promotes cell survival/proliferation [20]. To determine if STAT activation played a role in cell death and if there was any synergistic activation of the STATs following combination treatment, cells were either not treated (Figure 2A, Lane 1) or treated for 0.5, 24 or 48 h with 0.8 μM of lenalidomide (Figure 2A, Lane 2), 4 pM of anti-CD138-IFNα14 (Figure 2A, Lane 3) or anti-CD138-IFNα14+lenalidomide (Figure 2A, Lane 4), and cell lysates examined by western blotting for levels of total and phosphorylated STAT1 and STAT3 protein. At 30 min, pSTAT1 was observed in both anti-CD138-IFNα14 and anti-CD138- IFNα14+lenalidomide treated cells. By 48 h pSTAT1 was observed only in the cells treated with the combination of anti-CD138- IFNα14+lenalidomide. An increase in STAT1 protein was seen 24 and 48 h after treatment with anti-CD138-IFNα14 or anti-CD138- IFNα14+lenalidomide, but not with cells treated with only lenalidomide. Somewhat greater accumulation of STAT1 protein was seen at 48 h in cells treated with anti-CD138-IFNα14+lenalidomide (Figure 2A). pSTAT3 was seen at 30 min in cells treated with both anti- CD138-IFNα14 and anti-CD138-IFNα14+lenalidomide but not in cells treated with only lenalidomide. By 24 h pSTAT3 was not detected (Figure 2A). Consistent with a requirement for STAT1 activation for eliciting IFN-mediated cell death, cells treated with anti-CD138- IFNα14 and anti-CD138-IFNα14+lenalidomide for 72 h in the presence of the STAT1 activation inhibitor tofacitinib [21] showed greatly reduced apoptosis and inhibition of proliferation (Figure 2B). Inhibition of STAT1 phosphorylation during the first 6 h greatly reduced but did not completely inhibit the induction of apoptosis and inhibition of proliferation seen following treatment with lenalidomide +anti-CD138-IFNα14. Inhibition of STAT1 phosphorylation at 24 h was somewhat less effective in preventing the inhibition of proliferation and apoptosis and by 48 h the treated cells appeared completely committed to stop proliferating and undergo apoptosis (Figure 2C).
Figure 2: Activation of STAT1 and STAT3 following treatment with anti-CD138-IFNα14 or anti-CD138-IFNα14 plus lenalidomide correlates with induction of apoptosis. A. NCI-H929 cells were Lane 1: not treated; Lane 2: treated with 0.8 μM lenalidomide; Lane 3: treated with 4 pM anti-CD138-IFNα14; or Lane 4 treated with both lenalidomide+anti-CD138-IFNα14 for 0.5, 24 and 48 h. Cell lysates were used to determine the levels of total STAT1 protein and pSTAT1 and total STAT3 and pSTAT3 by western blotting. Protein loading was monitored by probing the same membranes with antiactin; B. Induction of apoptosis as measured by the production of annexin V positive cells and inhibition of proliferation as measured by MTS in cells treated with 0.8 μM lenalidomide, 4 pM anti- CD138-IFNα14 or both for 72 h was determined in the presence and absence of 750 nM of the Jak inhibitor tofacitinib. Titration experiments showed that treatment with 750 nM of tofacitinib virtually eliminated STAT1 phosphorylation following treatment with either anti-CD138-IFNα14 or anti-CD138- IFNα14+lenalidomide; C. Induction of apoptosis as measured by the production of annexin V positive cells and inhibition of proliferation as measured by MTS was determined in the presence and absence of 750 nM of tofacitinib added 0.5, 1, 6, 24 or 48 h following initiation of treatment with 4 pM anti-CD138-IFNα14 and 0.8 μM lenalidomide.
c-Myc, IRF4, and PARP are more effectively down regulated by the combination of anti-CD138-IFNα14 and lenalidomide than by either agent alone.
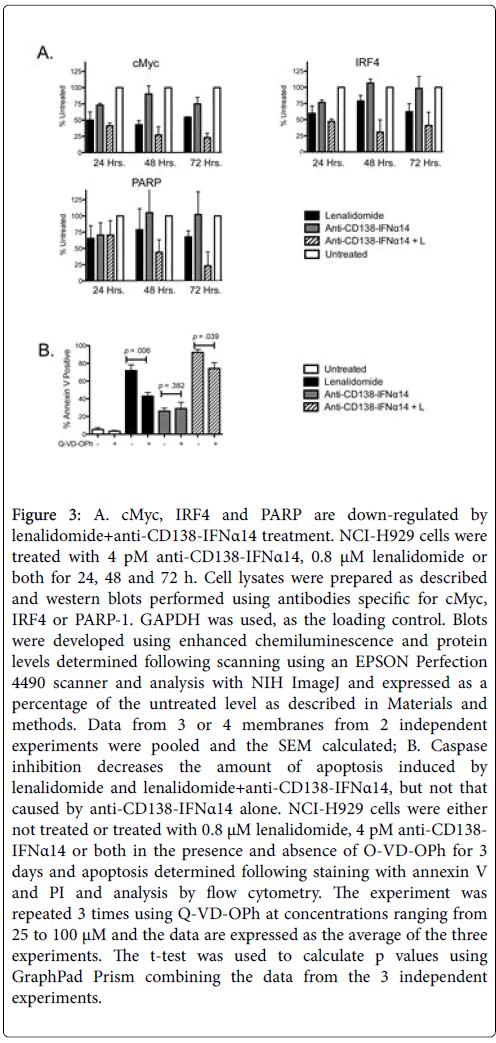
Deregulated expression of MYC is a consistent feature of plasma cell neoplasms [22] and progression from MGUS to MM is associated with activation of the MYC gene pathway [23]. Western blot analysis showed that the combination of anti-CD138-IFNα14 with lenalidomide was more effective than either agent alone in causing downregulation of c-Myc (Figure 3A). Thus down-regulation of c-Myc expression may contribute to or reflect the synergistic activity of anti- CD138-IFNα14+lenalidomide.
Figure 3: A. cMyc, IRF4 and PARP are down-regulated by lenalidomide+anti-CD138-IFNα14 treatment. NCI-H929 cells were treated with 4 pM anti-CD138-IFNα14, 0.8 μM lenalidomide or both for 24, 48 and 72 h. Cell lysates were prepared as described and western blots performed using antibodies specific for cMyc, IRF4 or PARP-1. GAPDH was used, as the loading control. Blots were developed using enhanced chemiluminescence and protein levels determined following scanning using an EPSON Perfection 4490 scanner and analysis with NIH ImageJ and expressed as a percentage of the untreated level as described in Materials and methods. Data from 3 or 4 membranes from 2 independent experiments were pooled and the SEM calculated; B. Caspase inhibition decreases the amount of apoptosis induced by lenalidomide and lenalidomide+anti-CD138-IFNα14, but not that caused by anti-CD138-IFNα14 alone. NCI-H929 cells were either not treated or treated with 0.8 μM lenalidomide, 4 pM anti-CD138- IFNα14 or both in the presence and absence of O-VD-OPh for 3 days and apoptosis determined following staining with annexin V and PI and analysis by flow cytometry. The experiment was repeated 3 times using Q-VD-OPh at concentrations ranging from 25 to 100 μM and the data are expressed as the average of the three experiments. The t-test was used to calculate p values using GraphPad Prism combining the data from the 3 independent experiments.
IRF4, a transcription factor whose expression is critical for MM survival, has been shown to be a master regulator targeting many genes involved in the survival and proliferation of MM [24]. To determine if changes in IRF4 expression could play a role in the observed synergistic cell death, cells were treated for 24, 48, or 72 h with lenalidomide, anti-CD138-IFNα14, or both and the expression of IRF4 detected by western blotting (Figure 3A). As was observed with c-Myc, combination treatment with lenalidomide and anti-CD138-IFNα14 resulted in the greatest decrease in IRF4 expression in NCI-H929.
PARP plays a key role in triggering mitochondria to release their proapoptotic, granules and enhanced PARP-1 cleavage was seen in NCI-H929 cells treated with anti-CD138-IFNα14+lenalidomide compared to cells treated with either agent alone (Figure 3A). This was especially evident by 72 h of treatment. The monoclonal antibody (mAb) used to detect PARP-1 recognizes a peptide centered around Gly623 and although there was a decrease in intact PARP-1, a cleavage fragment reacting with that mAb was not observed.
The effects mediated through lenalidomide but not anti- CD138-IFNα14 involve caspase activation.
Although caspase activation is often considered to play an essential role in the induction of apoptosis, caspase-independent cell death has been described [25] Therefore the pan-caspase inhibitor, Q-VD-OPh, was used to assess the role of caspase activation in the induction of apoptosis following treatment with lenalidomide, anti-CD138-IFNα14 or both. Caspase activation appeared to not play a role in the induction of apoptosis seen following treatment with anti-CD138-IFNα14 as similar levels of apoptosis were seen in the presence and absence of QVD- OPh. However, Q-VD-OPh treatment did decrease the amount of apoptosis seen following treatment with either lenalidomide or lenalidomide+anti-CD138-IFNα14 (Figure 3B). Thus, lenalidomide but not anti-CD138-IFNα14 appears to work through a pathway involving caspase activation. However, it should be noted that inhibition of caspase activation does not completely negate the apoptosis seen following treatment with either lenalidomide or lenalidomide+anti-CD138-IFNα14, suggesting that non-caspase dependent pathways also play a role.
Glucose utilization by both glycolysis and OXPHOS plays a role in the induction of apoptosis by lenalidomide+anti- CD138-IFNα14.
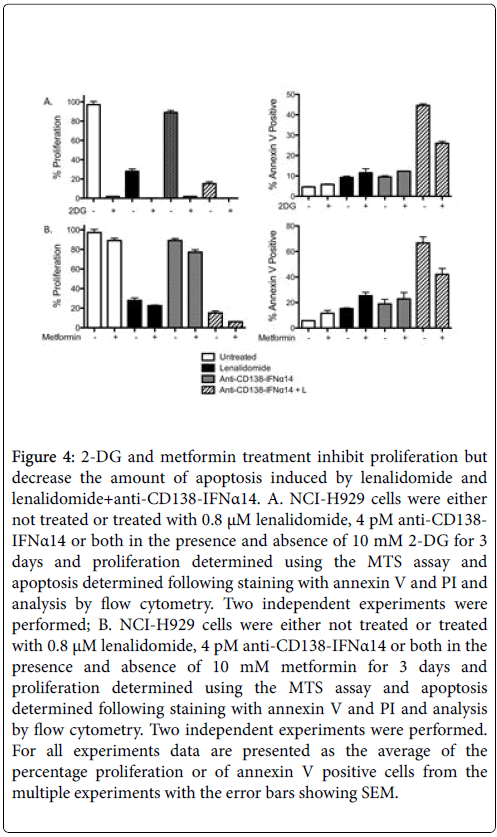
High cellular glucose metabolism is a hallmark of cancer cells. They are characterized by accelerated glycolysis (Warburg Effect) while maintaining the level of OXPHOS seen in normal cells [26]. The glucose analogue 2-DG, which competitively inhibits the production of glucose-6-PO4 from glucose, inhibits proliferation but this inhibition of proliferation did not result in increased apoptosis. Instead, interfering with glycolysis using 2-DG interfered with the synergistic induction of apoptosis seen by anti-CD138-IFNα14+lenalidomide (p=. 004, Figure 4A).
Figure 4: 2-DG and metformin treatment inhibit proliferation but decrease the amount of apoptosis induced by lenalidomide and lenalidomide+anti-CD138-IFNα14. A. NCI-H929 cells were either not treated or treated with 0.8 μM lenalidomide, 4 pM anti-CD138- IFNα14 or both in the presence and absence of 10 mM 2-DG for 3 days and proliferation determined using the MTS assay and apoptosis determined following staining with annexin V and PI and analysis by flow cytometry. Two independent experiments were performed; B. NCI-H929 cells were either not treated or treated with 0.8 μM lenalidomide, 4 pM anti-CD138-IFNα14 or both in the presence and absence of 10 mM metformin for 3 days and proliferation determined using the MTS assay and apoptosis determined following staining with annexin V and PI and analysis by flow cytometry. Two independent experiments were performed. For all experiments data are presented as the average of the percentage proliferation or of annexin V positive cells from the multiple experiments with the error bars showing SEM.
Metformin disables OXPHOS by inhibiting mitochondrial complex I-dependent mitochondrial respiration and oxidative phosphorylation [27,28] resulting in reduced glucose metabolism through the citric acid cycle [29]. Inhibition of OXPHOS resulted in a decrease in cellular proliferation (Figure 4B). Metformin forces a glycolytic phenotype which may augment sensitivity to MCT1 inhibitors and it causes some increase in apoptosis in cells treated with lenalidomide alone although the increase was not statistically significant (p=0.06). In contrast, it caused a statistically significant (p=.021) decrease in apoptosis in cells treated with anti-CD138-IFNα14+lenalidomide suggesting that generation of energy through OXPHOS contributes to the induction of apoptosis seen following treatment with anti-CD138- IFNα14+lenalidomide (Figure 4B). Thus interfering with glucose utilization by either inhibiting glycolysis or OXPHOS results in both inhibition of proliferation and decreased apoptosis following treatment with anti-CD138-IFNα14+lenalidomide. Inhibition of both glycolysis and OXPHOS using treatment with both 2-DG and metformin results in cell death (Data not shown).
Treatment with anti-CD138-IFNα14+lenalidomide results in mitochondrial membrane hyperpolarization, accumulation of ROS, and degradation of Chk1
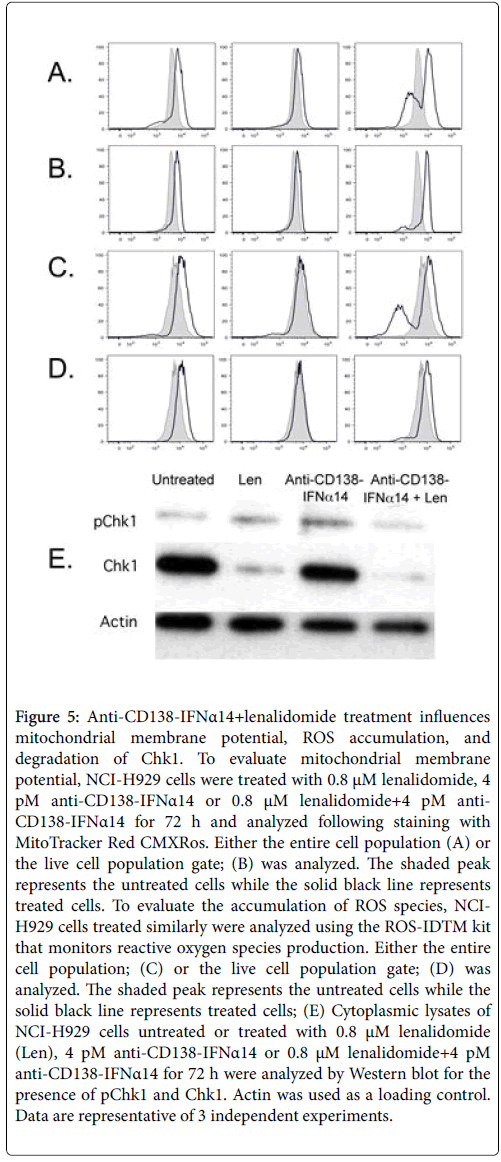
Mitochondrial membrane potential (Δψm) is an important parameter of mitochondrial function. Staining with MitoTracker Red CMXRos , shows one peak with enhanced membrane potential and a second with greatly decreased membrane potential (Figure 5A); this second peak indicates dead cells as it can be decreased or eliminated when dead cells are excluded from the flow cytometry gate (Figure 5B). Increased accumulation of ROS is also seen in cells treated with lenalidomide and anti-CD138-IFNα14+lenalidomide, but not in cells treated with anti-CD138-IFNα14 alone (Figure 5C); a population with decreased ROS indicates dead cells as it can be eliminated by excluding dead cells from the gate (Figure 5D). Increased levels of ROS can promote the incorporation of oxidized nucleotides that can cause the replication fork to stall resulting in replicative stress. Lenalidomide and lenodlidamide+anti-CD138-IFNα14 treatment of H929 results not only in the production of increased ROS but also in the degradation of Chk1 (Figure 5E) which is required for appropriate DNA replication [30].
Figure 5: Anti-CD138-IFNα14+lenalidomide treatment influences mitochondrial membrane potential, ROS accumulation, and degradation of Chk1. To evaluate mitochondrial membrane potential, NCI-H929 cells were treated with 0.8 μM lenalidomide, 4 pM anti-CD138-IFNα14 or 0.8 μM lenalidomide+4 pM anti- CD138-IFNα14 for 72 h and analyzed following staining with MitoTracker Red CMXRos. Either the entire cell population (A) or the live cell population gate; (B) was analyzed. The shaded peak represents the untreated cells while the solid black line represents treated cells. To evaluate the accumulation of ROS species, NCIH929 cells treated similarly were analyzed using the ROS-IDTM kit that monitors reactive oxygen species production. Either the entire cell population; (C) or the live cell population gate; (D) was analyzed. The shaded peak represents the untreated cells while the solid black line represents treated cells; (E) Cytoplasmic lysates of NCI-H929 cells untreated or treated with 0.8 μM lenalidomide (Len), 4 pM anti-CD138-IFNα14 or 0.8 μM lenalidomide+4 pM anti-CD138-IFNα14 for 72 h were analyzed by Western blot for the presence of pChk1 and Chk1. Actin was used as a loading control. Data are representative of 3 independent experiments.
Lenalidomide+anti-CD138-IFNα14 provides synergistic protection against tumor growth in vivo
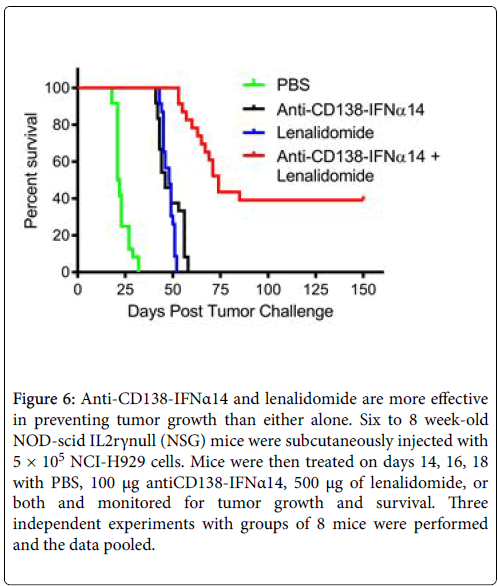
NCI-H929 cells were subcutaneously injected into NOD-scid IL2rγnull (NSG) mice that were then treated on days 14, 16, and 18 with PBS, 100 μg of anti-CD138-IFNα14, 500 μg of lenalidomide, or both and monitored for survival. Groups of 8 mice were treated and the experiment was repeated 3 times and the data pooled (Figure 6). Median survival was 21.5 days for the mice treated with PBS, 46 days for those treated with anti-CD138-IFNα14, 48 days for those treated with lenalidomide and 74 days for those treated with lenalidomide +anti-CD138-IFNα14. There was no statistically significant difference between the efficacy of anti-CD138-IFNα14 and lenalidomide alone (p=0.81), however combined treatment with lenalidomide and anti- CD138-IFNα14 was more effective than either treatment alone (p<0.0001). Thus, similar to what was observed in vitro , the combined treatment with lenalidomide and anti-CD138-IFNα14 was much more protective against tumor growth. Synergistic inhibition of H929 tumor growth has also been seen following treatment with the combination of anti-CD38-IFNα(attenuated) and lenalidomide [8].
Figure 6: Anti-CD138-IFNα14 and lenalidomide are more effective in preventing tumor growth than either alone. Six to 8 week-old NOD-scid IL2rγnull (NSG) mice were subcutaneously injected with 5 × 105 NCI-H929 cells. Mice were then treated on days 14, 16, 18 with PBS, 100 μg antiCD138-IFNα14, 500 μg of lenalidomide, or both and monitored for tumor growth and survival. Three independent experiments with groups of 8 mice were performed and the data pooled.
Discussion
We have shown previously that targeting IFNα and IFNβ fusion proteins is an effective strategy against lymphoma and MM [3,4,6,7]. Based on these studies, we initiated phase 1 clinical testing of anti- CD20-IFNα to treat B-cell lymphomas (ClinicalTrials.gov Identifier NCT02519270), underscoring the therapeutic potential of this approach. We have shown that combined treatment of MM with the clinically approved drug bortezomib and anti-CD138-IFNα14 resulted in synergistic efficacy both in vitro and in vivo in xenograft models, with only the combination having the ability to cure most tumors [5]. We have now sought to extend these studies to determine if combined treatment with anti-CD138-IFNα14 fusion protein and a different FDA-approved drug, lenalidomide, would result in synergistic antitumor efficacy. While some synergistic activity is seen in several model cell lines, the most potent synergy is seen with the NCI-H929 cell line (Suppl. Figure 1).
Signal transducer and activator of transcription (STAT) activation may play an important role in providing anti-tumor protection. On engagement, the interferon-α receptor (IFNAR) activates Janus kinase 1 (JAK1) and tyrosine kinase 2 (TYK2). Phosphorylation of the receptor by these kinases results in the recruitment of STAT proteins, their phosphorylation, and phosphorylated STATs dimerize and migrate to the nucleus where they bind to cis-acting elements in the promoters of responsive genes. STAT1 directly contributes to controlling tumor cell expansion by switching on many pro-apoptotic and anti-proliferative genes in the cancer cells. Consistent with the enhanced anti-tumor efficacy of treatment with lenalidomide+anti- CD138-IFNα14, we see enhanced and prolonged STAT1 activation following treatment with them and interfering with STAT1 activation decreases the inhibition of proliferation and apoptosis induced by lenalidomide+anti-CD138-IFNα14. In contrast, STAT3 activation counteracts inflammation and promotes cell survival/proliferation and immune tolerance [20]. For STAT3, similar activation is seen following treatment with anti-CD138-IFNα14 and the combination of lenalidomide+anti-CD138-IFNα14.
MYC, IRF-4 and PARP, all of which play an important role in MM growth and survival, are all down-regulated following treatment with lenalidomide+anti-CD138-IFNα14, with more effective down regulation seen following treatment with the combination than with either agent alone. Thus, their down regulation may make a significant contribution to the synergistic anti-tumor activity of this treatment. Myc activation is the most common mutation in MM [31] ans inhibition of c-Myc activity efficiently induces myeloma cell death [32]. Expression of IRF4, a member of the IRF family of transcription factors restricted to the immune system, has been found to be essential for the viability of MM cells [24,33] with even small changes in IRF4 levels resulting in MM cell death. IRF4 is a direct target of Myc transactivation with IRF4 and Myc forming a positive autoregulatory circuit [34]. However, IRF4 inhibition is toxic to myeloma cell lines regardless of transforming oncogenic mechanism. Although earlier studies had shown that lenalidomide leads to a rapid downregulation of IRF4 in MM cells including NCI-H929 cells [35], we now find the greatest decrease in IRF4 expression following treatment with lenalidomide+anti-CD138-IFNα14. High levels of IRF4 expression had been found to increase the sensitivity of MM cell lines to lenalidomide and that among MM patients with high levels of IRF4 expression, treatment with lenalidomide led to a significantly longer overall survival [35]. Treatment with lenalidomide+anti-CD138-IFNα14 may be especially effective in this patient population.
Several factors appear to contribute to the enhanced apoptosis seen following treatment with lenalidomide+anti-CD138-IFNα14. Although mitochondrial depolarization is usually taken as a hallmark of apoptosis, mitochondrial hyperpolarization sometimes is found to be a sensitizing feature in the apoptotic process [36]. Indeed, we have now observed mitochondrial hyperpolarization prior to depolarization and cell death following treatment with lenalidomide and anti-CD138- IFNα14 (Figures 5A and 5B). While ROS can act as signaling molecules, excessive ROS levels can lead to oxidative stress. Oxidative stress has been associated with many forms of programmed cell death and there is strong evidence that ROS and oxidative stress can induce the apoptotic process [36]. We now see accumulation of ROS in cells treated with lenalidomide and lenalidomide+anti-CD138-IFNα14 (Figures 5C and 5D). NCI-H929 is PTEN deficient [37] and loss of PTEN leads to an increase in sensitivity to oxidative stress [38] possibly explaining why H929 is so sensitive to the combination treatment.
Chk1, a Ser/Thr kinase, is essential for cell viability after DNA damage [39]. Replicative stress induces the phosphorylation of Chk1 [40]. However, this is a two-edged sword as phosphorylation of Chk1 delivers a signal that both activates Chk1 and marks it for proteolytic degradation [40]. We now find that that the replicative stress induced by treatment with lenalidomide and lenalidomide+anti-CD138- IFNα14 results not only in the production of increased ROS but also in the degradation of Chk1 (Figure 5E). In the absence of Chk1, replication is inappropriately initiated from multiple origins which exhausts replication factors and leads to fork stalling and subsequent collapse [30]. Consistent with this, a decrease in the percentage of cells in S-phase is seen in cells treated with lenalidomide (Data not shown). Chk1 down regulation has not been previously noted to be mechanism of action for lenalidomide-based therapies in myeloma.
Conclusions
Our studies demonstrate that treatment with anti-CD138-IFNα14 plus lenalidomide results in synergistic anti-tumor efficacy against the myeloma NCI-H929 both in vitro and in vivo . This synergism is the consequence of the combined effects of multiple, complementary antitumor activities. Several different pathways are implicated as playing a role including STAT activation, down regulation of c-Myc, PARP and IRF4 as well as contributions made by the glucose metabolism pathway. In addition to influencing ROS accumulation and mitochondrial membrane potential, we made the unexpected observation that lenalidomide and lenalidomide+anti-CD138-IFNα14 treatment results in cellular stress and the degradation of Chk1. Based on our findings, clinical testing of combination therapy with lenalidomide and anti-CD138-IFN fusion proteins for the treatment of multiple myeloma may be warranted.
Conflicts of Interest
SLM has a financial interest in Qwixel Therapeutics which has entered into a LOI with the Regents of the University of California to license intellectual property invented by SLM.
Acknowledgements
This work was supported by a Senior Research Award and the Dean Assink MMRF Senior Research Award from the Multiple Myeloma Research Foundation, and by the National Institutes of Health grant CA200910. The funding sources had no direct involvement in the conduct of the research and preparation of the report.
References
- Borden EC, Lindner D, Dreicer R, Hussein M, Peereboom D (2000) Second-generation interferons for cancer: clinical targets. Semin Cancer Biol 10: 125-144.
- Borden EC, Sen GC, Uze G, Silverman RH, Ransohoff RM, et al. (2007) Interferons at age 50: past, current and future impact on biomedicine. Nat Rev Drug Discov 6: 975-990.
- Huang TH, Chintalacharuvu KR, Morrison SL (2007) Targeting IFN-alpha to B cell lymphoma by a tumor-specific antibody elicits potent antitumor activities. J Immunol 179: 6881-6888.
- Trinh KR, Vasuthasawat A, Steward KK, Yamada RE, Timmerman JM, et al. (2013) Anti-CD20-interferon-beta fusion protein therapy of murine B-cell lymphomas. J Immunother 36: 305-318.
- Vasuthasawat A, Yoo EM, Trinh KR, Lichtenstein A, Timmerman JM, et al. (2016) Targeted immunotherapy using anti-CD138-interferon alpha fusion proteins and bortezomib results in synergistic protection against multiple myeloma. MAbs 8: 1386-1397.
- Xuan C, Steward KK, Timmerman JM, Morrison SL (2010) Targeted delivery of interferon-alpha via fusion to anti-CD20 results in potent antitumor activity against B-cell lymphoma. Blood 115: 2864-2871.
- Yoo EM, Trinh KR, Tran D, Vasuthasawat A, Zhang J, et al. (2014) Anti-CD138-Targeted Interferon Is a Potent Therapeutic Against Multiple Myeloma. J Interferon Cytokine Res 35: 281-291.
- Pogue SL, Taura T, Bi M, Yun Y, Sho A, et al. (2016) Targeting Attenuated Interferon-alpha to Myeloma Cells with a CD38 Antibody Induces Potent Tumor Regression with Reduced Off-Target Activity. PloS one 11: e0162472.
- Wijdenes J, Vooijs WC, Clement C, Post J, Morard F, et al. (1996) A plasmocyte selective monoclonal antibody (B-B4) recognizes syndecan-1. Br J Haematol 94: 318-323.
- Pestka S, Krause CD, Walter MR (2004) Interferons, interferon-like cytokines, and their receptors. Immunol Rev 202: 8-32.
- Lavoie TB, Kalie E, Crisafulli-Cabatu S, Abramovich R, DiGioia G, et al. (2011) Binding and activity of all human alpha interferon subtypes. Cytokine 56: 282-289.
- Petzold G, Fischer ES, Thoma NH (2016) Structural basis of lenalidomide-induced CK1alpha degradation by the CRL4(CRBN) ubiquitin ligase. Nature 532: 127-130.
- Kronke J, Udeshi ND, Narla A, Grauman P, Hurst SN, et al. (2014) Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 343: 301-305.
- Lu G, Middleton RE, Sun H, Naniong M, Ott CJ, et al. (2014) The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science 343: 305-309.
- Bergsagel PL, Chesi M (2016) Promiscuous mechanisms underlie the antitumor effects of thalidomide analogs. Nat Med 22: 706-707.
- Fang J, Liu X, Bolanos L, Barker B, Rigolino C, et al. (2016) A calcium- and calpain-dependent pathway determines the response to lenalidomide in myelodysplastic syndromes. Nat Med 22: 727-734.
- Eichner R, Heider M, Fernandez-Saiz V, Van Bebber F, Garz AK, et al. (2016) Immunomodulatory drugs disrupt the cereblon-CD147-MCT1 axis to exert antitumor activity and teratogenicity. Nat Med 22: 735-743.
- Chou TC (2006) Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev 58: 621-681.
- Arulampalam V, Kolosenko I, Hjortsberg L, Bjorklund AC, Grander D, et al. (2011) Activation of STAT1 is required for interferon-alpha-mediated cell death. Exp Cell Res 317: 9-19.
- Regis G, Pensa S, Boselli D, Novelli F, Poli V (2008) Ups and downs: the STAT1:STAT3 seesaw of Interferon and gp130 receptor signalling. Semin Cell Dev Biol 19: 351-359.
- Ghoreschi K, Jesson MI, Li X, Lee JL, Ghosh S, et al. (2011) Modulation of innate and adaptive immune responses by tofacitinib (CP-690,550). J Immunol 186: 4234-4243.
- Cheung WC, Kim JS, Linden M, Peng L, Van Ness B, et al. (2004) Novel targeted deregulation of c-Myc cooperates with Bcl-X(L) to cause plasma cell neoplasms in mice. J Clin Invest 113: 1763-1773.
- Chesi M, Robbiani DF, Sebag M, Chng WJ, Affer M, et al. (2008) AID-dependent activation of a MYC transgene induces multiple myeloma in a conditional mouse model of post-germinal center malignancies. Cancer cell 13: 167-180.
- Shaffer AL, Emre NC, Lamy L, Ngo VN, Wright G, et al. (2008) IRF4 addiction in multiple myeloma. Nature 454: 226-231.
- Chipuk JE, Green DR (2005) Do inducers of apoptosis trigger caspase-independent cell death? Nat Rev Mol Cell Biol 6: 268-275.
- Hay N (2016) Reprogramming glucose metabolism in cancer: can it be exploited for cancer therapy? Nat Rev Cancer 16: 635-649.
- Bridges HR, Jones AJ, Pollak MN, Hirst J (2014) Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. Biochem J 462: 475-487.
- Cheong JH, Park ES, Liang J, Dennison JB, Tsavachidou D, et al. (2011) Dual inhibition of tumor energy pathway by 2-deoxyglucose and metformin is effective against a broad spectrum of preclinical cancer models. Mol Cancer Ther 10: 2350-2362.
- Andrzejewski S, Gravel SP, Pollak M, St-Pierre J (2014) Metformin directly acts on mitochondria to alter cellular bioenergetics. Cancer Metab 2: 12.
- Dobbelstein M, Sorensen CS (2015) Exploiting replicative stress to treat cancer. Nat Rev Drug Discov 14: 405-423.
- Chng WJ, Huang GF, Chung TH, Ng SB, Gonzalez-Paz N, et al. (2011) Clinical and biological implications of MYC activation: a common difference between MGUS and newly diagnosed multiple myeloma. Leukemia 25: 1026-1035.
- Holien T, Vatsveen TK, Hella H, Waage A, Sundan A (2012) Addiction to c-MYC in multiple myeloma. Blood 120: 2450-2453.
- Shaffer AL, Emre NC, Romesser PB, Staudt LM (2009) IRF4: Immunity. Malignancy! Therapy? Clin cancer res : an official journal of the American Association for Cancer Research 15: 2954-2961.
- Verdelli D, Nobili L, Todoerti K, Intini D, Cosenza M, et al. (2009) Molecular targeting of the PKC-beta inhibitor enzastaurin (LY317615) in multiple myeloma involves a coordinated downregulation of MYC and IRF4 expression. Hematol Oncol 27: 23-30.
- Lopez-Girona A, Heintel D, Zhang LH, Mendy D, Gaidarova S, et al. (2011) Lenalidomide downregulates the cell survival factor, interferon regulatory factor-4, providing a potential mechanistic link for predicting response. Br J Haematol 154: 325-336.
- Giovannini C, Matarrese P, Scazzocchio B, Sanchez M, Masella R, et al. (2002) Mitochondria hyperpolarization is an early event in oxidized low-density lipoprotein-induced apoptosis in Caco-2 intestinal cells. FEBS let 523: 200-206.
- Stengel C, Jenner E, Meja K, Mayekar S, Khwaja A (2013) Proliferation of PTEN-deficient haematopoietic tumour cells is not affected by isoform-selective inhibition of p110 PI3-kinase and requires blockade of all class 1 PI3K activity. Br J Haematol 162: 285-289.
- Gorrini C, Harris IS, Mak TW (2013) Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov 12: 931-947.
- Park C, Suh Y, Cuervo AM (2015) Regulated degradation of Chk1 by chaperone-mediated autophagy in response to DNA damage. Nat Commun 6: 6823.
- Zhang YW, Otterness DM, Chiang GG, Xie W, Liu YC, et al. (2005) Genotoxic stress targets human Chk1 for degradation by the ubiquitin-proteasome pathway. Mol Cell 19: 607-618.
Citation: Trinh KR, Vasuthasawat A, King RY, Timmerman JM, Morrison SL (2018) Synergistic Inhibition of Multiple Myeloma Growth by Anti-CD138-Interferon-alpha14 Fusion Protein and Lenalidomide. J Mol Immunol 3: 120.
Copyright: © 2018 Trinh KR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 7012
- [From(publication date): 0-2018 - Dec 18, 2025]
- Breakdown by view type
- HTML page views: 5958
- PDF downloads: 1054