Synthesis, Characterization and Effectiveness of Chelated Mineral as Aflatoxin Absorbents
Received: 18-Aug-2015 / Accepted Date: 12-Nov-2015 / Published Date: 20-Nov-2015 DOI: 10.4172/2572-0406.1000103
Abstract
Manganese, Chromium, Cobalt, Copper and Zinc complexes of methionine were prepared and characterized by IR, electronic absorption spectra, elemental analysis, magnetic susceptibilities, the differential thermal analysis (DTA) and thermal gravimetric analysis (TGA) of the complexes pointed to their stability. The mechanism of the thermal decomposition is detected .The thermodynamic parameters of the dissociation steps are evaluated. The absorption ability of complexes to aflatoxin has been studied. All complexes reduced the toxic effect of aflatoxin.
Keywords: Chelated minerals; Infrared spectra; Electronic spectra; Thermal analysis; Microorganism; Aflatoxin
9567Introduction
The bioinorganic projects focus the synthesis of transition complexes using ligand – peptide conjugates that will impart coordination environments similar to those found in biological systems [1]. The design of new complexes plays an important role in bioinorganic chemistry [2-4]. A large number of metal-binding substances such as amino acids and proteins are present in biological systems. Low– molecular weight substances are involved in the absorption and transport of metal ions and metalloproteins play various roles, such as enzymatic catalysis, oxygen transport, and metal ion storage and transport [5,6]. Proteins such as metallothioneins protect organisms from the toxic effects of exogenous metal ions [7]. Most transition metal amino acid complexes have considerable biological activity, such as antitumor properties [8]. Amino acids usually increase the diffusibility of complexes and enhance their biological action inside the cell [9]. Such systems are widely used in the field of chemotherapy. Structural studies of many transition metal complexes have shown that amino acids coordinate in various ways, dependent upon the metal ion, its oxidation state and the primary structure of the amino acid [10]. Methionine is one of the nine essential amino acids needed by human beings and its copper (II) complex showed some antiulcer activity as revealed by animal model studies [11] and has also found some interest in veterinary medicine, for copper supplementation [12-14].
Materials and Methods
Preparation of the complexes
Solutions of 0.08 mole of Mn, Cr, Co, Cu and Zn sulfate were mixed with 0.08 mole of methionine. The reaction mixture was refluxed for two hours and then left overnight where the complexes were precipitated. Then filtered, washed with distilled water and dried in vacuum desiccators over P4O10. The melting points of the complexes are over 300ºC.
Analysis of the metal content
The complexes were digested and decomposed with aqua regia. The metal ion contents were determined by atomic absorption (Table 1) spectra.
| Complexes | Formula | Found Calculated(%) | l (nm) | µ (298)K | Metal(%) | ||
|---|---|---|---|---|---|---|---|
| C | H | N | |||||
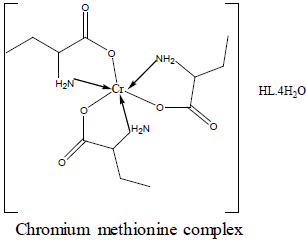
| Cr-methionine | Cr(L)3(HL).4H2O | 33.29 33.19 | 6.65 6.79 | 7.76 7.65 | 249-286-428 | 3.88 | 7.20 |
| Cr-methionine | Mn(L)3.2H2O | 33.42 33.38 | 6.31 6.49 | 7.73 7.56 | 245,248,251,261 263,267,444 | 5.88 | 10.19 |
| Co-methionine | Co(L)3(HL).4H2O | 32.81 32.91 | 6.75 6.57 | 7.65 7.44 | 249,433 | 5.8 | 7.80 |
| Cu-methionine | Cu(L).3H2O | 23.55 23.65 | 5.60 5.63 | 5.22 5.12 | 252,255,265, 270,274 | 1.5 | 23.5 |
| Zn-methionine | Zn(L)2 ( HL).H2O | 33.77 33.61 | 6.19 6.22 | 7.87 7.67 | - | 12.25 | |
Table 1: Analytical data, electronic spectra and μeff for methionine complexes
Carbon, hydrogen and nitrogen analysis
These were done at the Micro Analytical Laboratory (Table 1), Faculty of Science, Tanta University, Egypt.
Instruments and working procedures
IR spectra: The KBr IR spectra were recorded using Perkin –Elmer spectrophotometer model 1430 covering the frequency range 200-4000 cm-1.
UV-Vis spectra: The spectral studies were measured using PYEUnicam spectrophotometer model 1750 covering the wavelength range 190-900 nm. The complexes were measured in nujol mull following the method described by Lee et al. [15].
Magnetic susceptibility measurements: Molar magnetic susceptibility corrected for diamagnetic using Pascal?s constant were determined at room temperature applying the Faraday’s method. The apparatus was calibrated with Hg [Co(SCN)4] [16].
Thermal analysis: Differential thermal analyses (DTA) and thermo gravimetric analyses (TGA) were carried out using Shimadzu DTA-50. The rate of heating was 20ºC/min.
Effect complexes on aflatoxin concentration: One liter of yeast extract broth was divided into 10 flasks. Each flask’s content was mixed with one of the tested compounds to reach a final concentration of 2000 μg/ml. All flasks were artificially contaminated with 104 (c.f.u.) of toxigenic Aspergillus flavus strain and incubated at 25-27ºC for 21 days after which autoclaving of the media was done at 121ºC for 30 min. then mycotoxin concentration was determined using Fluorometer Vicam V 1.3 Series 4, according to [17].
Objective
To study physical and chemical properties of chelated mineral and its effect on aflatoxin.
Results And Discussion
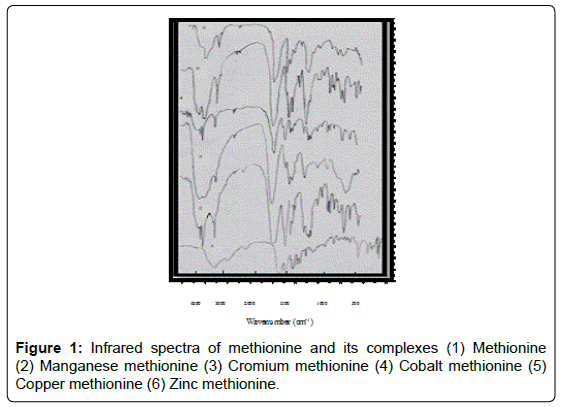
Infrared spectra of amino acid complexes
Methionine complexes: The most important ir spectral bands for methionine and its complexes are given in (Figure 1). The bands a 3342-3269, 3409-3349-3277, 3413.9-3237, 3317 cm-1 for Mn, Cr, Co, Cu and Zn methionine complexes respectively, arise the coordination of H2O molecules and asymmetric stretching vibration of NH2 [18]. The presence of new band at 3269, 3277, 3237 and 3317 cm-1 in Mn, Co, Cu and Zn methionine complexes respectively, are due to γs NH2. So, the nitrogen of the amino group is involved in coordination [19]. It is worthwhile to mention that the free methionine exist as zwitterions (NH3. AA. COO-) due to the presence of characteristic γ (NH3) and σ NH3. The amino acid in complexes does not exist in zwitterions, NH3 get deprotonated and binds to metals through the neutral NH2 group. The transformation of NH3to NH2 must result in upward shift in γs NH2 and δNH2 [20,21]. On complexation, the two δNH2 stretching vibration at 1695 and 1590 cm-1 together with some deformational motions of this group could be observed. The position of these bands clearly supports the involvement of this group in bonding. Moreover, the γ (C-N) stretching mode at 1352 cm-1 is affected to different extent in complexation [22]. The IR spectra showed strong evidence in support of the involvement of carboxylate group in coordination. The γas COO- and γs COO- at 1610 and 1410 cm-1 record shifts in complexes. New ir bands appeared at 588-440 cm-1 are due to γ M-O and γ M-N. So, methionine act as bidentate ligand.The band at 2370cm-1 attributted to γ (SH) unaffected on complexation indicated that (S) atom is not involved in chelation.
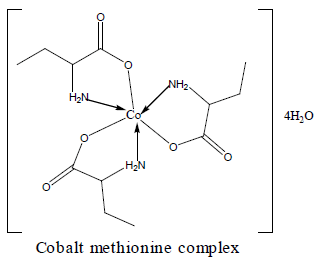
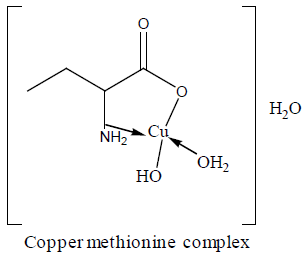
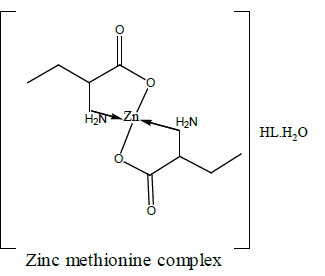
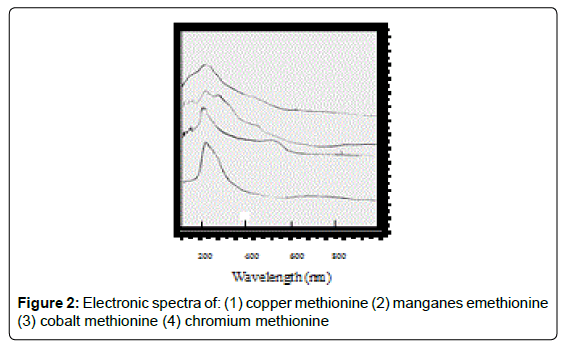
Electronic spectra and magnetic moments of methionine complexes
For the chromium complex (Figure 2), three spin allowed transition have been observed at 249,286 and 428 nm assigned to charge transfer, but for 428nm is due to 4A2g (F) ? 4T1g(F) respectively [23]. The observed magnetic moment is 3.88 B.M due to the existence of octahedral geometry [23]. Mn methionine complex exhibit absorption bands at 245, 248, 251, 261, 263, 267and 444 nm are due to d-d transitions. Its magnetic moments is 5.88 typified the existence of octahedral structure [24]. The nujol mull spectra of cobalt methionine showed two bands at 249 and 434 nm, the band at 249 nm is due to charge transfer but the band at 434 nm is due to4T1g(F) ? 4T1g(P) transition. The Ueff= 5.8 B.M is consistent with octahedral geometry [25].Cu methionine complex exhibit absorption bands at 252,255,265,270 and 274 due to charge transfer type. The Ueff =1.5 B.M is consistent with tetrahedral structure [26].
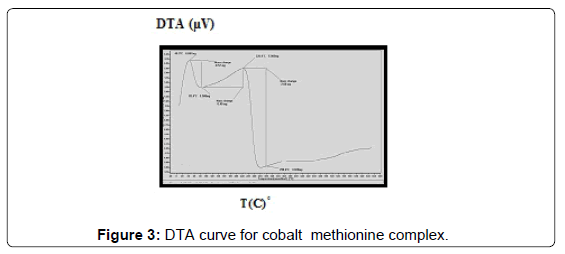
Thermal analysis of complexes
The DTA of Co methionine Co (L)3 (HL).4H2O, (Figure 3) gave one endothermic peak at 205.3°C and two exothermic peaks at 305.7°C, 509.2°C, the order of reactions is 2.2, 0.95 and 1.56 respectively, i.e., the first and second types [27]. The first broad DTA peak at 205.3°C (56.3-236.3)°C is due to dehydration of lattice and coordination water molecules [27]. The broad features of DTA peak may be due to strong thermal agitation accompanying the water elimination and loss of water molecules occurs in more than one step. This is confirmed by appearance of TGA peak at (53-233.2)°C [28] .The two strong exothermic peaks at 305.7°C and 509.2°C are due thermal agitation and decomposition steps of metal complex as evident from TGA. The latter peak at 509.2 and the associated TGA (415.2-560°C) and (560-700)°C is assigned to the decomposition of the complex with weight loss 18.8 and 10.2% ended with the formation of CoO.
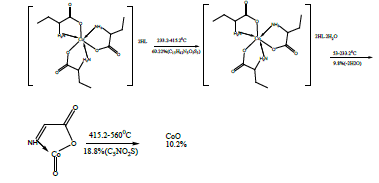
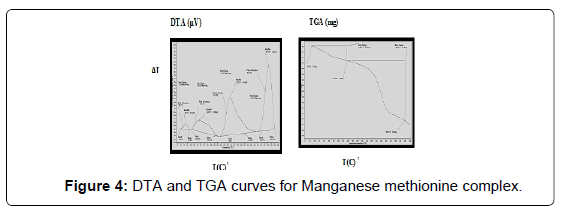
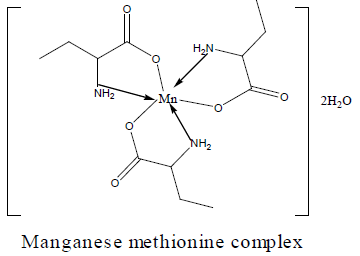
The DTA and TGA of manganese methionine Mn(L)3. 2H2O, (Figure 4) gave one endothermic peak at 99.3 and exothermic peaks at 247.8°C and 513°C.The first peak is assigned to dehydration process of the lattice and coordinated water molecules with activation energy 353.3 KJ/mole, the larger the Ea values is may be due to the thermal agitation accompanying the water elimination [27].The two exothermic peaks at 247 and 513°C with activation energies 79.2 and 220.15 KJ/ mole are corresponding to the thermal agitation and decomposition of complexes ended with the formation of MnO 13.4% [28].
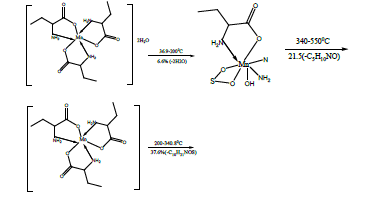
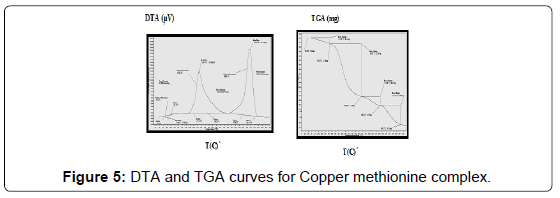
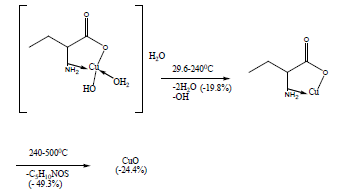
The TG and DTA curves of copper methionine complex Cu (L).3H2O, (Figure 5), pointed to that the dehydration process of water molecules takes places in one step in the temperature range (29.6-240)°C with activation energy 160.4 KJ/mole [28]. The strong exothermic peak at 250°C is due thermal agitation and decomposition steps of metal complexes as evident from TG. The latter broad band peak with maximum at 350°C and the associated TGA peaks with the temperature range (240-500)°C showed weight loss of 49.33% is assigned to the decomposition of complex ended with the formation of Cu O as final product [29].
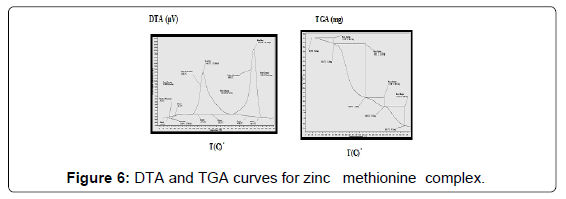
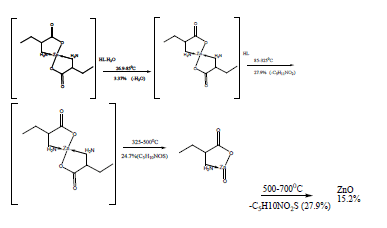
The TG and DTA curves of zinc methionine complex Zn (L)2 (HL). H2O, (Figure 6) gave four exothermic peaks at 69.5, 175, 334 and 563.9°C with activation energies of 93, 42.6,101.6 and 240.5 KJ/mole, their order of decomposition is 1.34 ,1.12 , 0.19 and 0.93. The first band at 69.5°C (40.8-94)°C is due to dehydration of H2O molecule this is confirmed by TGA peaks in the range (26.9-85)°C. The three strong exothermic peaks are due to thermal agitation and decomposition which occurs in three steps with weight loss 27.9, 24.7 and 27.9 ended with the formation of ZnO 15.2% as evident from TG [30,31].
Biological activity against aflatoxin
Table 2 show that the addition of methionine complexes results in reduction of aflatoxin percentage to different degree. The complexes have the ability to absorb mycotoxin with high affinity. In vitro results suggested that the complexes are a strong binder for aflatoxin. An inert, stable and insoluble complex between chelated mineral and aflatoxin was assumed to be responsible for toxin absorption [32]. Absorptions by all metal complexes increase with increasing their levels. The activity order of complexes are copper methionine>zinc methionine>cobalt methionine. The geometric structure of the complexes plays an important role for adsorption of aflatoxin. So, the tetrahedral structure has the great ability to bind aflatoxin than octahedral structure [33-37].
| Aflatoxin concentration % | Copper methionine | Zinc methionine | Cobalt methionine |
|---|---|---|---|
| 0.05% | 47 | 36.74 | 45.02 |
| 0.1% | 72.43 | 64.18 | 65.76 |
| 0.2% | 82.94 | 81.36 | 79.37 |
| 0.4% | 87.77 | 86.36 | 89.25 |
| 0.6% | 88.47 | 87.45 | 89.73 |
| 0.8% | 98.02 | 99.03 | 94.8 |
Table 2: The effect of complexes on the concentration (%) of aflatoxin.
Conclusion
Transition metalamino acid complex (copper, zinc and cobalt methionine) have considerable biological activity against aflatoxin and the geometry structure of complex play an important role for adsorption of aflatoxin.
References
- Joshi JD, Vora JJ, Sharma S, Chaudhary RH, Patel RA, et al. (2006) Equilibrium Studies on Binary Chelate Formation ofVitamin-U with Some Alkaline Earth Metal Ions. Asian Journal of Chemistry 18: 1517.
- Szabó-Plánka T, Rockenbauer A, Korecz L (1999) An ESR study of co-ordination modes in copper (II) complexes of l-serine in aqueous solution at ligand excess above pH 7. Polyhedron 18: 1969-1974.
- Magnuson A, Frapart Y, Abrahamsson M, Horner O, Ã…kermark B, et al. (1999) A biomimetic model system for the water oxidizing triad in photosystem II. J Am Chem Soc 121: 89-96.
- Place C, Zimmermann JL, Mulliez E, Guillot G, Bois C, et al. (1998) Crystallographic, Electrochemical, and Pulsed EPR Study of Copper (II) Polyimidazole Complexes Relevant to the Metal Sites of Copper Proteins. J Inorg Chem 37: 4030.
- Chen Y, Pasquinelli R, Ataai M, Koepsel R, KortesA,et al. (2000)Coordination of Two High-Affinity Hexamer Peptides to Copper(II) and Palladium(II) Models of the Peptide-Metal Chelation Site on IMAC Resins. Inorg Chem 39: 1180-1186.
- Facchin G, Torre MH, Kremer E, Piro OE, CastellanoEE et al. (2001) J Inorg Biochem 89: 174.
- Sorenson JRJ (1976) Copper chelates as possible active forms of the antiarthritic agents.J Med Chem 19: 135-148.
- Sorenson JRJ (1982)Metal Ions in Biological Systems. (Sigel H Ed) M Dekker, New York 14: 77.
- Sorenson JRJ (1995)Handbook of Metal-Ligand Interactions in Biological Fluids (GBerthon Ed) M Dekker, New York2: 128.
- Kishore VTM, Ramakrishna KR and Sorenson JRJ(1982) Inflammatory Diseases and Copper (JRJ Sorenson Ed) Humana Press, Clifton 363: 73.
- Boila RJ, Devlin TJ, DrysdaleRA, Lillie LE (1984) Can J Anim Sci 64: 365.
- McDowell LR (2002) Minerals in Animal and Human Nutrition. Academic Press, San Diego.
- Lee RH, Griswold E, Kleinberg J (1964) Studies on the stepwise controlled decomposition of 2, 2'-bipyridine complexes of cobalt (II) and nickel (II) chlorides. Inorg Chem 3: 1278-1283.
- Figgis BN, Lewis J (1967) Modern Coordination Chemistry. Interscience, New York.
- Cuevas A, Vlera I, Torre MH, Kremer E, Etchevery SB, et al. (1999) Vibrational spectrum of the copper (II) complex of anthranilic acid. Acta Farm Bonaerense 56: 263.
- MasoudMS, Abd El-Hamid OH (1989)Structural chemistry of amino acid complexes. Trans Met Chem 14: 233-234.
- Uemura T, Shimura T, Nakamishi H,Tomahiro T, Nagawa Y, et al. (1991) 13C and 195Pt NMR of anticancer platinum pyrimidine greens. Inorg Chim Acta 181: 11-14.
- Iakovidis A, Hadjiliadis N, Schollhorn H, ThewaltU, Trotscher G (1989)Interaction of cis-Pt(NH3)2Cl2 with amino acids. The crystal structures of cis-[Pt(NH3)2(gly)] (NO3), cis-[Pt(NH3)2(ala)](NO3) and cis-[Pt(NH3)2(val)](NO3). Inorganica Chimica Acta 164: 221-229.
- Baran EJ, Wagner CC, Torre MH, Kremer E,Kogesler P (2000)Acta Farm Bonaerense 19: 231.
- Konig F (1971) Ligand field theory and the energy of the so-called "third band" in chromium(III) and vanadium(II) complexes. InorgChem 10: 2632-2633.
- FiggisBN, Lewis J (1964)Progress in Inorganic Chemistry(ed.) F A Cotton (New York Interscience ).
- Lever ABLP (1968) Inorganic Electronic Spectroscopy. Elsevier, New York : 243.
- MasoudMS, and Hagag SS (1992) Thermal properties of some transition metal nitroso and thiobarbiturate complexes: conductivity, magnetism and thermogravimetry.Thermochimica Acta 196: 221-223.
- Traore K (1972)Analysethermiquedifferentielleetcinetique de reaction. J Thermal Anal 4: 135-145.
- MasoudMS, Haagag SS, Ramadan AM, Mahmoud SA (1998)Nitrosophenol complexes of transition metal salts. Trans Met Chem 23: 343-348.
- Mallikarjum KG, Naidu RS (1992)Thermal decomposition kinetics of Cu(II) chelates of substituted chalcones. Thermochimica Acta 206: 273-278.
- Allan JR, MacloyB, Paton AD (1993)Thermal, structural and electrical studies of the chloro complexes of cobalt, nickel and copper with 4,7-phenanthroline. Thermochimica Acta 214: 211-217.
- Shehata SA, El melegy KM, Ebrahim MS, AbouYousef RA (2009) Journal of Arab Aquaculture Society4: 55-72.
- Yalcin I, Oren I, TemizO,Sener EA (2000)QSARs of some novel isostericheterocyclics with antifungal activity. Acta Biochemica Polonica 47: 481-486.
- Concon JM (1986) Food toxicology: Contaminants and additives. Part B, Marcel Dekker, New York pp: 677.
- Miazzo RD, Peralta MF, Magnoli C, Salvano M, Ferrero S, et al. (2005) Efficacy of sodium bentonite as a detoxifier of broiler feed contaminated with aflatoxin and fumonisin. Poultry Science 84: 1- 8.
- Khadem AA, Sharifi SD, Barati M, Borji M (2012) Evaluation of the Effectiveness of Yeast, Zeolite and Active Charcoal as Aflatoxin Absorbents in Broiler Diets. Global Veterinaria 8: 426-432.
Citation: Ashry GME, Melegy KME (2015) Synthesis, Characterization and Effectiveness of Chelated Mineral as Aflatoxin Absorbents. J Chem Biol Ther 1: 103. DOI: 10.4172/2572-0406.1000103
Copyright: © 2015 Ashry GME, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 15489
- [From(publication date): 2-2016 - Aug 30, 2025]
- Breakdown by view type
- HTML page views: 14150
- PDF downloads: 1339