The Association between Minerals and Gastric Cancer
Received: 24-May-2017 / Accepted Date: 09-Jun-2017 / Published Date: 20-Jun-2017
Abstract
Purpose: Study the role of minerals in gastric carcinogenesis.
Materials and methods: Three hundred blood samples were used to analyze the status of concentration of Selenium (Se), Copper (Cu), Zinc (Zn) and Iron (Fe) in gastric cancer (GC) patients and healthy individuals by using atomic absorption spectrophotometer.
Results: There was a significant (p<0.05) decline in the concentration of Se in serum samples of GC patients as on comparison with the healthy individuals and comparison within the genders among GC patients the level of Se concentration remained insignificant. Concentration of Cu of GC patients was increased significantly (p<0.05) on comparison with the healthy individuals and on comparison within the genders among GC patients there was a significant (p<0.05) decline in the concentration of Cu in male on comparison with the female group, There was a significant (p<0.05) decline in the concentration of Zn in GC patients on comparison with the healthy individuals. Within genders among GC patients the level of Zn concentration remained insignificant p>0.05. Level of difference of Fe remain insignificant (p>0.05) in serum of GC patients and within gender among GC patients. There was a significant decline in the concentration of Se and Zn while high level of serum copper in gastric cancer patients, as compared with normal healthy controls.
Conclusion: There is an association between serum selenium, zinc and copper with cancer gastric cancer, so increase or decrease in above minerals may be one of the factors which can lead to the gastric cancer.
Keywords: Gastric cancer (GC); Serum minerals; Selenium (Se); Copper (Cu); Zinc (Zn); Iron (Fe)
Introduction
Gastric cancer (GC) continues to be the second most common malignant neoplasm around the world with varied regional incidences due to different factors [1] and in Kashmir among all the cancers GC has been reported to be a highly prevalent malignancy, constitutes about 30-40% of all malignancies [2]. Various studies have been done on incidence rate and distribution of gastrointestinal cancers in Kashmir [3], but there was no information regarding the mineral status of the GC patients till date. GC has remained a main clinical challenge due to its poor prognosis, limited treatment options, relatively resistance to chemotherapy/radiotherapy and late diagnosis of the disease. Several possible mechanisms have been proposed for the probable role of Selenium (Se), Copper (Cu), Zinc (Zn), and Iron (Fe) in GC etiology [4-6]. Since the beginning of the 1970s the minerals has received a lot of attention as per the variations of mineral concentration in serum has been related to increased risk for various types of cancer in humans [7-10]. She plays a vital role in cancer prevention and appears to have important structural and enzymatic roles such as antioxidant activity. It is well established that oxidative stress plays an important role in the carcinogenic process, as reactive oxygen species (ROS) which induce oxidative damage, DNA damage and protein damage. However, in the literature there are many controversial studies regarding the protective/ therapeutic role of Se in human cancer [11-13]. Fe and Cu can produce the reactive oxygen species which can attack DNA and cause DNA mutation, and can act as an element in the pathological process of cancer, as Fe may be a limiting nutrient to the growth and replication of cancer cells in the humans [14]. Cu can be concerned in the activation of several organic peroxide and making them more carcinogenic [15]. Zn plays an anti-carcinogenic role by stabilizing the structure of DNA, RNA and ribosome [16] also Zn is necessary to the functions of several transcriptional factors, proteins that recognize certain DNA sequences and control gene transcription [17]. Zn protects against free radical damage and may influence immune response [18,19]. The aim of the present study was to determine the real status of Se, Zn, Cu and Fein serum of GC patients against the serum of healthy individuals in Kashmir Valley India.
Materials and Methods
A collaborative study of stomach cancer was conducted, December 2013 to march 2015 jointly by the Division of Veterinary Biochemistry of FVSC and AH, SKUAST-K, Department of Biochemistry of GMC, Srinagar, and Department of Oncology, GMC, Srinagar. Two hundred patients (male 150 and female 50) diagnosed with gastric carcinoma admitted to Government Medical College, Srinagar were considered for the study. Venous blood samples were collected into plain vials from patients (200) and control group (100) and were transported to the laboratory on ice and centrifuged at 3000 rpm for 15 minutes and serum was stored at -4°C until the day of mineral estimation, by using Atomic Absorption Spectroscopy AAS-ECIL. For statistical analysis, SPSS software was used and p-values less than 0.05 were regarded as statistically significant.
Preparation of Serum for Mineral Estimation
1. 4 ml of Diacid (3 ml nitric acid: 1 ml picric acid) were added to 1 ml of serum in volumetric flask of 100 ml volume).
2. The flask was then kept at room temperature for overnight.
3. Then on next day flask was put on a hot plat at simmering heat till the volume in the flask was reduced up to 0.5 ml.
4. Then final volume of flask was made up to 10 ml by diluting by distilled water.
5. These 10 ml were used for the examination of mineral estimation by using the Atomic absorption spectrophotometer.
Results
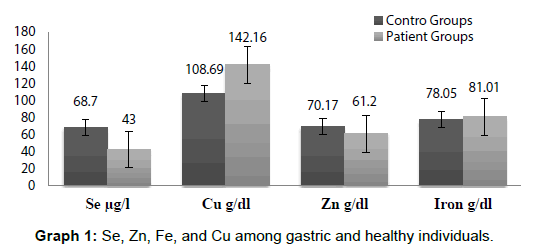
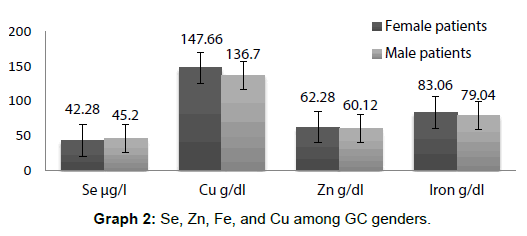
All values of Se, Zn, Cu and Fe are expressed as means ± Se at each time interval. Th e level of significance was set at p<0.05 and data was analyzed by SPSS. Graph 1 represents the serum concentration of Se, Zn, Fe, and Cu among GC patients and controls and Graph 2 showing among GC ganders.
The mean serum Se levels were significantly p<0.05 different in GC patients (44.00 ± 6.3 μg/l)and in the control group of healthy individuals (69.70 ± 4.5 μg/l) and remained insignificant for the two genders (43 ± 6.3) in female and 42 ± 5.9 in male p>0.05. The mean serum Cu levels (p<0.05 different in GC patients (142.16 ± 18.72) and in the control group of healthy individuals (108.69 ± 16.47) and on correlation between serum concentration and gender the mean serum Cu levels were significantly p<0.05 different for the two genders, in female 147.66 ± 18.72 and in male136.79 ± 17.79. The mean serum Zn levels p<0.05 different in GC patients (61.20 ± 5.57) and in the control group of healthy individuals (70.17 ± 6.43) and on correlation between serum concentration and gender the mean serum Zn levels were insignificantly different for the two genders p>0.05, in female 62.28 ± 5.57 and in male 60.12 ± 6.01. The mean serum Fe levels were insignificantly (p>0.05) different in GC patients (78.05 ± 3.25) and in the control group of healthy individuals (81.01 ± 2.15) and on correlation between serum concentration and gender the mean serum Fe levels were also insignificantly p>0.05 different for the two genders, in female 83.06 ± 3.21 and in male79.04 ± 4.00.
Discussion
In the past studies on minerals it was showed that there was an inhibitory effect of some minerals on the cell growth, DNA, RNA and protein synthesis in transformed cells, [12] in view of the above data we analyze the status of Se, Zn, Fe, and Cu in serum of gastric and healthy individuals. Results of our study showed that there was a significant decline in the concentration of Se in serum samples of GC patients on comparison with the healthy individuals and on comparison within the genders among GC patients the level of Se concentration remained insignificant which was in agreement with the findings of many coworkers [7,20,21]. The decline of Se may be due to over uptake of it by malignant cells and thus may be there was an increase in the concentration of Se in the tumor cells. In our study the concentration of Cu in serum samples of GC was increased significantly on comparison with the healthy individuals and on comparison within the genders among GC patients there was a significant decline in the concentration of Cu in male on comparison with the female group, which was in agreement with the findings of many coworkers in different cancers, bladder cancer [22], breast cancer [23], colorectal cancer [24], GC [25]. As Cu can be concerned in the activation of several organic peroxides [26,27] and can produce the hydroxyl radicals which cause mutation in DNA which may be one of the causes of cancer development, but we do not understand why there was a significant decline in Cu concentration in male as compared to female group patients. There was a significant decline in the concentration of Zn in serum samples of GC patients in our study on comparison with the healthy individuals and on comparison within the genders among GC patients the level of Zn concentration remained insignificant which was inconsistent with the most finding of the earlier studies which have reported higher serum Zn levels in the cases of cancer [28]. Zn plays an important role in stabilizing the structure of DNA, RNA and ribosome, it is also necessary for functioning of several transcription factors, proteins that recognize certain DNA sequences and control gene transcription and protects against free radical damage. So may be due to decline in the concentration of Zn in GC patients any of the above process get disturbed and may be acting as a causative agent for cancer. In our study there was found no significant difference in the levels of Fe in serum of GC patients as compared with the healthy individuals and on correlation within gender among GC the mean serum Fe levels were insignificantly different for the two genders, and are in contradict with the findings of Weinberg.
Conclusion
Minerals appear to have important structural and enzymatic roles in body, as there are some minerals having role in antioxidant activities like Se or some having role in stabilization of DNA, RNA, ribosome and protein structures like Fe and Cu or some producing oxygen reactive species like Zn. We detected in our study, there was a significant decline in the concentration of Se and Zn while high level of serum Cu in gastric cancer patients, as compared with normal healthy controls. This shows an association of serum selenium, zinc and copper with cancer gastric cancer. The increase or decrease of mineral concentration in serum of gastric cancer patients may be one of the factors which can lead to other biological processes to cause gastric cancer. If there is really happen so then there is need of such studies in future whey and which biological process is going under or overtakes of minerals during cancer development.
Acknowledgments
Authors would like to thank the Indian Council of Medical Research (ICMR), RamalingaswamiBhawan Ansari Nagar, Post Box-4911, New Delhi-110029) for their support vide Research grant as senior research fellowship to the first author.
Conflict of Interest
Authors declare that they have no conflict of interests.
Author’s Contribution
All authors read and approved the final manuscript.
References
- Prakash S, Sarran L, Socci N, DeMatteo RP, Eisenstat J, et al. (2005) Gastrointestinal stromal tumors in children and young adults: a clinicopathologic, molecular, and genomic study of 15 cases and review of the literature. J PediatrHematoloncol 27: 179-187.
- Nawroz H, Koch W, Anker P, Stroun M, Sidransky D (1996) Microsatellite alterations in serum DNA of head and neck cancer patients. Nat Med 2: 1035-1037.
- Qurieshi MA, Masoodi MA, Kadla SA (2011) Gastric Cancer in Kashmir. Asia Pac J Cancer Prev 12: 303-307.
- Magalora V, Bella A, Brtkova (1999) Copper, zinc, super oxide dismutase in precancerous, benign disease and gastric, colorectal and breast cancer. Neoplasm 46: 100-104.
- Jayadeep A, Raveendran PK, Kannan S (1997) Serum levels of copper, zinc, iron and ceruplasmin in oral leukoplakia and squamous cell carcinoma. J Exp Clin Cancer Res 16: 295-300.
- Rayman MP, Dipple A (1973) Studies of the mechanism of tumour initiation. Br J Cancer 1: 85.
- Challis BC, Rayman MP (1973) Potential alkylating agents from the oxidation of carcinogenetic cyclic N-nitrosamines. Br J Cancer 1: 84.
- Kallistratos G, Evangelou A, Seferiadis K, Vezyraki P, Barboutis K (1985) Selenium and haemodialysis: serum selenium levels in healthy persons, non-cancer and cancer patients with chronic renal failure. Nephron 41: 217-222.
- Rayman M (2000) The importance of selenium to humans and health. Cancer 356: 233-241.
- Rayman M (2005) Selenium in cancer prevention: a review of the evidence and mechanism of action. Proc NutrSoc 64: 527-542.
- Rayman MP, Rayman MP (2002) The argument for increasing selenium intake. Proc Nutr Soc 61: 203-215.
- Toyokuni S (1996) Iron-induced carcinogenesis: the role of Redox regulation. Free Radic Biol Med 20: 553-566.
- Stevens RG, Kalkwarf DR (1990) Iron, radiation, and cancer. Environ Health Persp 87: 291-300.
- Massa EM, Giulivi C (1993) Alkoxyl and methyl radical formation during cleavage of tertbutylhydroperoxide by a mitochondrial membrane-band redox active copper pool: An EPP study. Free Radic Biol Med 14: 559-565.
- Clogg MS, Keen CL, Hurley IS (1989) Biochemical pathologies of zinc deficiencies. In: Mills CF (ed.) Zinc in human biology. International Life Science Institute, London.
- Kaim W, Schwederski BV (1994) Bioinorganic chemistry: Inorganic Elements in the chemistry of life. John Wiley and Sons, New York.
- Burke JP, Fenton MR (1985) Effect of a zinc deficient diet on lipid peroxidation in liver and tumour subcellular membranes. P Soc Exp Biol Med 179: 187-197.
- Prasad AS (2009) Impact of the discovery of human zinc deficiency on health. J Am Coll Nutr 28: 257-265.
- Charalabopoulos K, Kotsalos A, Batistatou A, Charalabopoulos A, Vezyraki P, et al. (2006) Selenium in serum and neoplastic tissue in breast cancer: correlation with CEA. Br J Cancer 95: 674-676.
- Charalabopoulos K, Kotsalos A, Karkabounas S, Vezyraki P, Kalfakakou V, et al. (2006) Low selenium levels in serum and increased concentration in neoplastic tissues in patients with colorectal cancer: correlation with serum carcinoembryonic antigen. Scand J Gastroenterol 41: 359-360.
- Mazdak H, Yazdekhasti F, Movahedian A, Mirkheshti N, Shafieian M (2010) The comparative study of serum iron, copper, and zinc levels between bladder cancer patients and a control group. Int Urol Nephrol 42: 89-93.
- Yiicel I, Arpaci F, Ozet A (1994) Serum copper and zinc levels and copper/zinc ratio in patients with breast cancer. Biol Trace Elem 40: 31-38.
- Gupta SK, Shukla VK, Vaidya MP, Roy SK, Gupta S (1993) Serum and tissue trace elements in colorectal cancer. J Surg Oncol 52: 172-175.
- Liu XG (1991) Serum and tissue copper, zinc and selenium levels in patients with gastric carcinoma. Chung Hua Chung Liu Tsa Chih 13: 93-96.
- Linder MC, Hazegh Azam M (1996) Copper biochemistry and molecular biology. Am J Clin Nutr 63: 797-811.
- Huang YL, Sheu JY, Lin TH (1999) Association between oxidative stress and changes of trace elements in patients with breast cancer. Clin Biochem 32: 131-136.
- Prasad AS (1983) Clinical biochemical and nutritional spectrum of zinc deficiency in human subjects: An update. Nutr Rev 41: 187-208.
- Weinberg ED (1996) The role of iron in cancer. Eur J Cancer prevention 5: 19-36.
Citation: Bhat SA (2017) The Association between Minerals and Gastric Cancer. J Oncol Res Treat 2: 113.
Copyright: © 2017 Bhat SA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 5791
- [From(publication date): 0-2017 - Nov 17, 2025]
- Breakdown by view type
- HTML page views: 4791
- PDF downloads: 1000