The Impact of Gut Microbiota on Host Obesity
Received: 11-Nov-2018 / Accepted Date: 23-Jan-2018 / Published Date: 30-Jan-2018 DOI: 10.4172/2161-069X.1000591
Abstract
Obesity is a critical medical condition present in the twenty-first century. Multiple factors contribute to its aetiology. Among those factors, the gut microbiota contribution is larger. Changes in the composition of two main phyla (increased Firmicutes and decreased Bacteroidetes) impact on mechanisms such as lipid metabolism and inflammation. Obese individuals indicate higher Firmicutes: Bacteroidetes ratio than their lean counterparts. Daily intake of high-fat diet also alters the gut microbiota composition. Nevertheless, direct bacterial influence in these mechanisms is still indistinct. Therefore, further investigations are required, which will also help to improve therapeutic strategies than those being used nowadays to treat obesity.
Keywords: Gut microbiota; Obesity; Gastrointestinal tracts; Gut lumen; Hypertension
Introduction
Obesity is a serious medical condition which increases the likelihood of chronic disorders such as type-2 diabetes mellitus, cardiovascular diseases, hypertension, atherosclerosis and certain types of cancers [1]. Obesity has been categorized as a disease by the American Medical Association. As shown in Figure 1, several factors contribute to the onset of obesity [2,3].
Gut microbiota is a group of microorganisms that reside in the gastrointestinal tracts of humans and animals. Each individual has a unique gut microbiota composition, but gut microbiota mainly belongs to four phyla, Firmicutes, Bacteroidetes, Actinobacteria and Proteobacteria [4]. The number of bacterial cells in an adult human body is ten times more than the cells constituting the human body. Factors such as diet, medications disease state and host genetics affect the gut microbiota composition [5,6].
Recently, it has been observed that changes in gut microbiota composition play a major role in the pathophysiology of metabolic disorders, especially in obesity. In earlier studies, mouse models of obesity have been shown shifts in the inclusive proportions of two main phyla, Firmicutes and Bacteroidetes [7,8].
These two phyla have been found to play a prevailing role while other phyla attribute to a lesser degree. Obese individuals and mouse models indicate a tendency towards a dysbiosis which also comprises a higher ratio of Firmicutes: Bacteroidetes [9].
The alteration of bacterial compositions in the gut lumen affects the capability of the microbiota to produce energy from indigestible carbohydrates, suppress several gene expressions thereby increase the fatty acid deposition in adipocytes [10].
This gut microbiota tends to get into the host’s immune system because of a leaky intestinal barrier, which in turn induces inflammation [7,11]. As mentioned above, several mechanisms have been proposed that gut microbiota may impact on host obesity. This review has been made an attempt to discuss two major mechanisms which involve in causing obesity.
Metabolic Mechanisms of the Gut Microbiota in the Genesis of Obesity
The gut microbiota impact on host functions through several mechanisms, suggesting that they might involve in the onset of obesity [5]. The correlation between host, microbiota, and diet is very complicated, but the procedures involved are well connected.
Figure 2, illustrates some mechanisms such as short-fatty acid production, lipoprotein metabolism, suppression of particular gene expressions and microbiota-associated inflammation where gut microbiota involved in the onset of obesity [12].
Microbiota Associated-Inflammation
It has been found that obesity is associated with systemic inflammation, which triggers an innate immune response to bacterial Lipopolysaccharide (LPS). LPS is a cell wall component of gramnegative bacteria which is considered as an endotoxin [13,14].
LPS is an amphiphilic molecule which contains both hydrophobic and hydrophilic components in it. It has shown that during cell shedding or bacteria lysis, LPS get released from bacterial cell walls. Thus, it is considered as a Pathogen-Associated Molecular Pattern (PAMP) [14,15].
LPS concentrations found to be low in gut lumens of normal, healthy individuals and cannot penetrate the intestinal epithelium. Nevertheless, high concentrations of LPS have been found in obese individuals. These findings have also shown diet as a determinant factor [16,17].
Both human and animal models which were on a High-Fat Diet (HFD) have been shown compositional changes in the gut microbiota (increase Firmicutes and decrease Bacteriodetes) and higher LPS levels in systemic circulation, which resulted in metabolic endotoxemia [17,18].
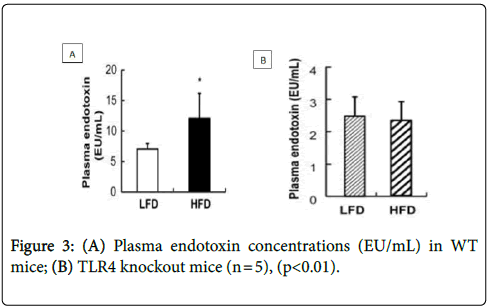
A study was carried out by Kim et al. [19]. They observed the correlation of HFD with systemic endotoxemia. As shown in Figure 3A, plasma endotoxin levels were higher in Wild-Type (WT) mice, which were on an HFD than WT mice which were on a Low-Fat Diet (LFD).
As shown in Figure 3B, there was no significant difference observed in the plasma endotoxin levels between TLR4 Knock Out (KO) mice which were on an HFD or an LFD. Thus, suggested HFD induces inflammation by increasing plasma LPS levels.
There is a tight junction barrier which tightly regulates the intestinal mucosal permeability. This tight junction barrier is made up of proteins like claudin, zonula occludens-1 (ZO-1), and occludin [20]. It has been found that HFD intake cause reduction in the synthesis of these proteins. Thus, increases gut permeability. Study of the de La Serre et al. has shown that gut permeability increases following an HFD [21].
Kim et al. also observed the influence of HFD on occludin and claudin-1 protein levels [19]. As shown in Figure 4A, WT mice showed a drastic reduction in the expression of occludin and claudin-1 which is responsible for intestinal permeability after switching to an HFD. As shown in Figure 4B, no decrease was observed in TLR4 KO mice, which were on an HFD.
There is a molecule called Glucagon-Like Peptide-2 (GLP-2) synthesized by intestinal L-cells. It has found that GLP-2 regulates the synthesis of those tight junction proteins that are important in promoting intestinal growth and regulating gut permeability [22]. It has been suggested that continuous consumption of HFD decreases the expression of GLP-2. Thus, declines the mRNA expression for the synthesis of proteins of the enterocyte-tight junctions, which results in increased gut permeability [23].
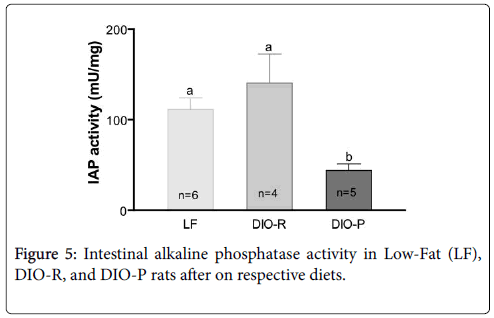
There is an LPS detoxifying enzyme called small Intestinal Alkaline Phosphate (IAP). It has found that IAP expression decrease with higher intake of HFD. In the study of de La Serre et al. they used two types of Sprague-Dawley rats [21]. Those were Obesity-Prone (DIO-P) and Obesity-Resistant (DIO-R) phenotype. These rats were fed with LFD or an HFD for a few weeks. As shown in the Figure 5, when compared to LF and DOI-R, DIO-P rats had a significant decrease in IAP activity.
As confirmed by the studies LPS has an ability to cross the gut mucosa through intestinal leaky tight junction barrier [24]. It has been shown that LPS has a high affinity towards chylomicrons thus easily integrates into it. As chylomicron formation increases after an HFD, systemic LPS levels also increase. A dietary survey was done by Amar et al. involving 1015 randomly chosen healthy French men [16].
These data suggested that bacterial LPS was efficiently transported in fat from the gut lumen into the systemic circulation and consumption of HFD induces plasma LPS concentration. Previously described studies have also shown that mice fed with HFD have higher LPS levels in them. Hence it is evident, that the higher amount of LPS enters the systemic circulation via the lymph following an HFD.
Once LPS reach the host bloodstream, it penetrates into tissues such as liver or adipose tissue. These tissues contain Toll-Like Receptors (TLRs) which act as Pattern Recognition Receptors (PRRs). From several types of TLRs, TLR4 is found to be functional in adipose tissues [25]. TLR4 is known to play a major role in this mechanism by acting as an LPS receptor which needs to trigger an innate immune response.
This was confirmed by the study of Kim et al. where it showed that TLR4 KO mice had low levels of LPS than their WT mice. As a result of that, TLR4 KO mice also didn’t gain weight, which suggested that TLR4 is an important component in this process. The study of Lin et al. also showed that LPS effectively signalled through TLR-4 [26].
There are receptor proteins called a cluster of differentiation-14 (CD14) on the plasma membranes of macrophages in adipose tissue [24]. LPS activates these CD14 after binding to the plasma LPSBinding Protein (LBP) and form a complex. A study done by Hailman et al. showed that CD14 has the ability to bind with LPS without LBP [27].
It further confirmed that the main function of LBP is to accelerate the binding of LPS to CD14. Cani et al. did a comparative study using CD14 KO and WT mice [28]. Mice were administered LPS for 2 weeks. The WT mice gained weight, which was similar to mice fed an HFD for 2 weeks, but CD14 KO mice didn’t gain weight. Hence it confirmed that LPS results in the development of obesity.
The LPS-CD14 complex binds with TLR4 via Myeloid Differentiation factor-2 (MD-2) in the intracellular space on the plasma membrane of the macrophages (Park et al. [29]). Activation of this TLR4 was also measured by de La Serre et al. using immunolocalization [10]. They quantified the immunoreactivity of the tissues obtained from the rats. Results showed higher immunoreactivity in DOI-P when compared to LF and DOI-R, suggesting that HFD increases intestinal TLR4 activation.
TLR4 has a downstream signalling molecule called myeloid differentiation primary response-88 (MyD88). It has been found that TLR4 recruit this protein after getting activated by LPS. Activation of MyD88 elicits an induction of Mitogen-Activated Protein Kinase (MAPK) pathways and IKKβ [29,30]. MAPK pathway signals to activate three molecules; extracellular signal-regulated kinase-1 and 2 (ERK1/2), p38 and c-Jun N-terminal Kinases (JNK).
These three molecules together activate activator protein-1 (AP-1). As Tuncman et al. observed that, mice lack JNK1 (an isoform of JNK) had diminished obesity-induced JNK activity, following a high-caloric diet [31]. IKKβ activates nuclear factor-κB (NF-κB). Cai et al. observed elevated levels of NF-κB and IKK-β activities in the livers of fat rats (fa/fa) compared to their lean counterparts, suggested that the NF-κB signalling pathway is activated by HFD [32].
When the up-regulation of AP-1 and NF-κB activity occurs, they bind to the gene promoters of interleukin-6 (IL-6) and Tumour Necrosis Factor alpha (TNF-α) in the adipocytes, increase their proinflammatory cytokine expressions [33]. In order to support this fact, Eggesbø et al. did a comparative study and examined LPS induced cytokine expression in whole blood [34].
They were using seven people with high levels and seven people with low levels of High-Density Lipoprotein (HDL) in their serum. People who had higher levels of HDL exhibited increased cytokine (IL-1β, IL-6, IL-8, and TNF-α) expression than those who had lower HDL. The study of Cai et al. also indicated elevated levels of mRNAs encoding for IL-6, IL-1β, and TNF-α after an HFD [32].
It has been found that IL-6 increases lipolysis and fat oxidation where TNF-α promote lipolysis. TNF-α also found to downregulate the adipogenic genes, thus inhibits pre-adipocyte conversion to mature adipocytes [33]. This lets further recruitment of undifferentiated cells that leads to further development of adipose tissue mass. Weisberg et al. did a study using both human and mouse models [35].
Results of both showed that macrophages in adipose tissue are responsible for TNF-α and IL-6 expression [36]. Thus, suggested the amount of macrophages increase and induce inflammation in adipose tissues of obese individuals.
Increased Fatty Acid Metabolism
Lipoprotein Lipase (LPL) is an enzyme which is found to be important in lipid metabolism by regulating the lipid homeostasis and energy balance in the human body. LPL synthesized by parenchymal cells in adipocytes, skeletal and cardiac muscles [36,37]. LPL transported to the capillary endothelial cell surface and attached to it via heparan sulphate proteoglycans [38].
It is known to be involved in plasma Triglycerides (TG) clearance by catalysing the hydrolysis of TG rich lipoproteins, chylomicrons, and Very Low-Density Lipoproteins (VLDL) into Free Fatty Acids (FFA) [39]. LPL deliver these hydrolysed metabolites to skeletal muscles, cardiac muscles and adipose tissue. It has been found that once getting into the adipocytes, these fatty acids re-esterified into TG and stored as fat [40].
Fasting-induced adipocyte factor or angiopoietin-like protein-4 (Fiaf/Angptl4) is a glycoprotein. It is known to be essential for peroxisome proliferator-activated receptor proteins (PPARα and PPARγ) [41].
It is produced in the liver, large intestinal epithelial cells, and white/ brown adipose tissues. It has found that Fiaf/Angptl4 has the ability to regulate fatty acid oxidation in adipose tissue by inhibiting LPL activity. Thus restrict the TG accumulation in adipocytes [40,42].
Experimental data has been suggested that the gut microbiota alteration due to HFD intake suppresses the expression of Fiaf/ Angptl4. Thus induces the activity of circulatory LPL, where the hydrolysis of plasma TG into FFAs increases. Hence, the storage of FFAs in adipose tissue increases and induces host adiposity [43,44].
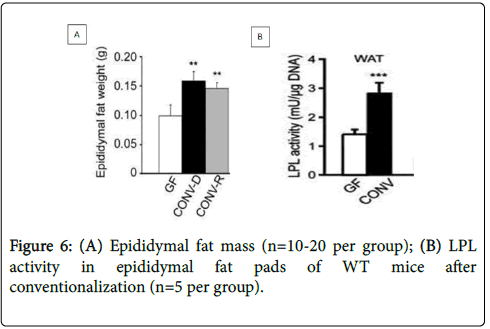
Bäckhed et al. have been confirmed above hypothesis by transplanting gut microbiota of Conventionally Raised (CONV-R) mice into Germ-Free (GF) mice [43]. As shown in Figures 6 A and B, GF mice had an increase in epididymal fat mass by 61% and LPL activity by 122% after conventionalization.
In a subsequent study, where the GF WT and Fiaf KO mice were switched to an HFD and body weights were monitored. As shown in Figure 7A, Fiaf expression was decreased in the small intestines of both genotypes after switching to an HFD (Western) but the GF WT mice had higher Fiaf expression than the conventionalized WT mice, regardless of diet.
As shown in Figure 7B, they observed GF Fiaf KO mice become obese on an HFD than the GF WT mice. Therefore, suggested that the Fiaf expressions in GF WT mice are suppressed by the gut microbiota in them, which in turn increases LPL activity and fat accumulation in adipocytes [43].
Gut Microbiota Composition and Obesity
It has been found that gut microbial composition alters following HFD thus results in obesity. Several human and animal studies have been done to prove this hypothesis. Recently, Ley et al. did a study using genetically obese ob/+ mothers and their ob/ob mice, lean on/+ and wild-type offspring [45].
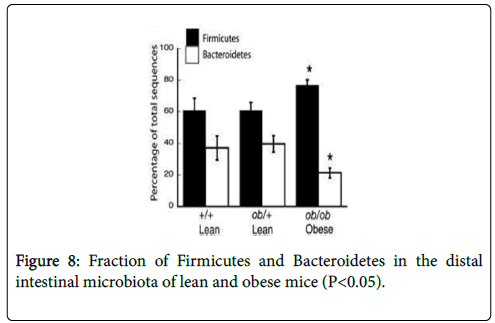
The results were examined by feeding them with same caloric diet. Ob/Ob animals had a reduction in the abundance of Bacteroidetes by 50% and a greater fraction (P<0.05) of Firmicutes comparatively to their lean counterparts (Figure 8). Thus, suggested that gut microbiota diversity can be affected by obesity, regardless of gender.
A comparative study done by Turnbaugh et al. identified an increase in the abundance of Firmicutes (specifically Mollicutes) in the mice that got diet-induced obesity than that of their lean counterparts [46]. Those obese mice have also lost their weight when they were undergoing fat-restricted low caloric diet.
Thus, it suggested diet as a determinant factor for obesity-associated changes in the gut microbiota. This was further confirmed by Murphy et al. [47]. They observed an increased Firmicutes: Bacteroidetes ratio in both Ob/Ob mice and HFD fed mice compared with lean mice. This ratio was much higher in HFD fed mice than in Ob/Ob mice.
Hildebrandt et al. observed the influence of HFD on gut microbiota composition [10]. They used five Resistin-Like Molecule-β (RELMβ) knockout mice, a model which is resistant to HF- induced obesity and five WT mice. Both types were lean on a standard chow diet, but after switching to an HFD, WT mice became obese while RELMβ KO mice remained relatively lean.
As shown in Figure 9, 16S rDNA sequencing results of WT mice, which were on an HFD showed extreme changes. Both genotypes showed an increase in phylum Firmicutes and decrease in phylum Bacteroidetes after moving to an HFD.
Even though so many studies have proven the gut microbial impact on host obesity, the direct involvement of these gut microbiota is still unclear. Influence of bacterial compositional changes on obesity also indistinct. Thus, studying further about above mechanisms is important. Another advantage of understanding these mechanisms and functions of the gut microbiota on these mechanisms is to use their interactions as possible targets to develop new personalized treatments and clinical management strategies to treat obesity.
Therapeutic Studies to Treat Obesity
There are several human and animal experimental studies have been carried out to gain therapeutic approaches that helped to alter the gut microbiota composition in a positive way to treat obesity. Dietary interventions are preferable than other available intervention approaches (antibiotics and surgery) due to its low cost and safety. Probiotics and prebiotics have become promising since they have a direct impact on the gut microbiota [48,49].
Makris and Foster recommended few dietary interventions to lose weight based on experimental data published between 2005 and 2011 [50]. Sackner-Bernstein et al. suggested low carbohydrate diet approaches are more effective and safer in weight management of obese individuals [51].
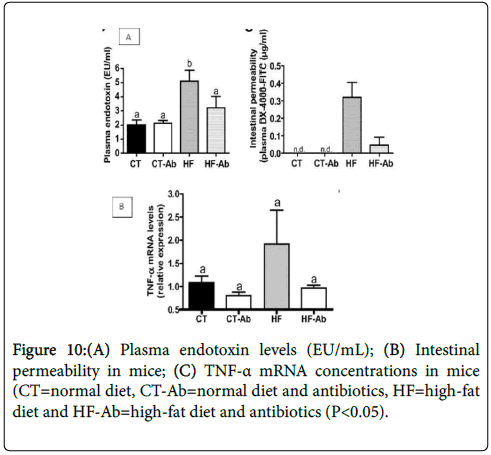
Study of Cani et al. found that plasma endotoxin levels and TNFalpha mRNA levels in HF-fed mice were higher than the chow-fed mice but those levels were reduced after treated with antibiotics [18]. It also demonstrated that HF feeding increase gut permeability and it can be reversed by giving antibiotics (Figures 10 A-C). Thus, suggested that gut microbiota composition and metabolic activities could be affected by antibiotic treatment regardless of the diet.
Everard et al. did a study by administrating Akkermansia muciniphila daily to HFD- induced obese mice for a few weeks. Mice have shown a weight reduction without any food intake changes [52]. HF-fed mice have effectively lost their body weight and fat mass, when they were supplemented with probiotic that contains Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 [53]. A recent study of Everard et al. examined anti-obesity properties of probiotic yeast called, Saccharomyces boulardii Biocodex [54]. Obese mice experienced a reduction in weight gain and body fat mass where the Bacteroidetes: Firmicutes ratio of mice had also increased.
However, the most recent evidence from animal and human studies suggests gut or faecal microbial transplantation could be used as a therapeutic intervention against obesity [55-57]. Figure 11 shows the procedure of faecal transplantation and Table 1 shows the studies done based on gut or faecal microbiota transplantation.
| Mouse Model | Treatment | Outcome | References |
|---|---|---|---|
| Adult germ- free C57BL/6 mice | Colonized with normal microbiota harvested from the caecum of adult conventionally raised mice and fed on a low fat-polysaccharide-rich diet. |
Increase in body fat content and insulin resistance despite reduced food intake. |
Bäckhed et al. [44] |
| Adult germ- free C57BL/6J mice | -Transplantation of microbes taken from the caecum of:-Obese (ob/ob) mice with a greater relative abundance of Firmicutes. -Lean (+/+) donors with a smaller relative abundance of Firmicutes. |
Increase in relative abundance of Firmicutes and body fat. Decrease in the relative abundance of Firmicutes and body fat. |
Turnbaugh et al. [47] |
| Adult germ- free C57BL/6J mice | Transplanted germ-free mice with faecal microbiota from adult human female twin pairs; discordant for obesity and those mice were fed on low-fat, high polysaccharide diet. |
Mice transplanted with microbiota from an obese twin developed higher adiposity than mice with the microbiota from a lean twin. |
Ridaura et al. [58] |
Table 1: Animal studies on gut or faecal microbiota transplantation.
Conclusion
The prevalence of obesity has increased in epidemic fractions in adults and children in worldwide. Numerous factors have recognized to explain the aetiology and pathophysiology of obesity. However, gut microbiota is one known reason which affects the host weight through several mechanisms. This evidence has obtained from tools such as 16S rRNA sequencing gene clone libraries, DNA microarrays, metagenomics, gnotobiotic knockout mouse models and human studies. These studies have shown that the host and its gut microbiota have equally useful and supportive interactions. These studies have also shown an increase in the Firmicutes: Bacteroidetes ratio, which is known to be associated with increased inflammation and lipid metabolism. Studies have further explained the role of LPS and Fiaf expression in the pathophysiology of obesity. These studies have also shown diet as a determinant factor of gut microbiota composition.
Even though these findings are promising, the direct involvement of these gut bacteria in the mechanisms is still indistinct thus required further study. The mechanisms responsible for the comparative fractions of Firmicutes and Bacteroidetes should be explored. In particular, the hereditary and ecological factors that define the unique features of each individual’s gut microbiota must be recognized. Specific gut bacterial influences on host obesity should be observed. Recent studies have carried out to examine the effect of dietary interventions, prebiotic, probiotic and gut microbiota/faecal transplantation to treat obesity. Some studies havedd shown positive outcomes where some methods (gut microbiota/faecal transplantation) need further investigation. Thus, more clinical trials should be done to evaluate whether modulating the gut microbiota could help to reduce weight.
Conflicts of Interest
Conflict of interest disclosed was none.
References
- Flegal KM, Carroll MD, Kit BK , Ogden CL (2012) Prevalence of obesity and trends in the distribution of body mass index among us adults, 1999-2010. JAMA 307: 491-497.
- Hruby A, Hu FB (2014) The epidemiology of obesity: A big picture. Pharmacoeconomics 33: 673-689.
- Robinson CJ, Bohannan BJM, Young VB (2010) From structure to function: The ecology of host-associated microbial communities. Microbiol Mol Biol Rev 74: 453-476.
- Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, et al. (2011) Enterotypes of the human gut microbiome. Nature 473: 174-180.
- Ding T, Schloss PD (2014) Dynamics and associations of microbial community types across the human body. Nature 509: 357-360.
- Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, et al. (2013) Metabolites produced by commensal bacteria promotes peripheral regulatory T-cell generation. Nature 504: 451-455.
- Cox A, West NP, Cripps AW (2015) Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol 3:207-215.
- Dethlefsen L, Huse S, Sogin ML, Relman DA (2008) The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol 6: 280.
- Hildebrandt MA, Hoffmann C, Sherrill–Mix SA, Keilbaugh SA, Hamady M, et al. (2009) High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology 137: 1716-1724.
- Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, et al.(2013) Richness of human gut microbiome correlates with metabolic markers. Nature 500: 541-546.
- Graham C, Mullen A, Whelan K (2015) Obesity and the gastrointestinal microbiota: A review of associations and mechanisms. Nutr Rev 73: 376-385.
- Silhavy TJ, Kahne D, Walker S (2010) The bacterial cell envelope. Cold Spring Harb Perspect Biol 2: a000414.
- Papo N, Shai Y (2005) A molecular mechanism for lipopolysaccharide protection of gram-negative bacteria from antimicrobial peptides. J Biol Chemistry 280: 10378-10387.
- Amar J, Burcelin R, Ruidavets JB, Cani PD, Fauvel J, et al. (2008) Energy intake is associated with endotoxemia in apparently healthy men. Am J Clin Nutr 87: 1219-1223.
- Boutagy NE, McMillan RP, Frisard MI, Hulver MW (2016) Metabolic endotoxemia with obesity: Is it real and is it relevant? Biochimie 124: 11-20.
- Erridge C, Attina T, Spickett CM, Webb DJ (2007) A high-fat meal induces low-grade endotoxemia: Evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr 86: 1286-1292.
- Kim K, Gu W, Lee I, Joh E, Kim D (2012) High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the tlr4 signaling pathway. PLoS One 7: 47713.
- Anderson JM, Van Itallie CM (2009) Physiology and function of the tight junction. Cold Spring Harb Perspect Biol 1: a002584.
- de La Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge JC, et al. (2010) Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol 299: G440-G448.
- Yu C, Jia G, Deng Q, Zhao H, Chen X, et al. (2016) The effects of glucagon-like peptide-2 on the tight junction and barrier function in ipec-j2 cells through phosphatidylinositol 3-kinase–protein kinase b–mammalian target of rapamycin signaling pathway. Asian-Australas J Anim Sci 29: 731–738.
- Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, et al. (2009) Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2- driven improvement of gut permeability. Gut 58: 1091-1103.
- Guo S, Al-Sadi R, Said HM, Ma TY (2013) Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am J Pathol 182: 375-387.
- Brightbill HD, Modlin RL (2000) Toll-like receptors: Molecular mechanisms of the mammalian immune response. Immunology 101: 1-10.
- Lin Y, Lee H, Berg AH, Lisanti MP, Shapiro L, et al. (2000) The lipopolysaccharide-activated toll-like receptor (tlr)-4 induces synthesis of the closely related receptor TLR-2 in adipocytes. J Biol Chem 275: 24255-63.
- Hailman E, Lichenstein HS, Wurfel MM, Miller DS, Johnson DA, et al.(1994) Lipopolysaccharide (LPS)-binding protein accelerates the binding of LPS to CD14. J Exp Med 179: 269-277.
- Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, et al. (2007) Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56: 1761-1772.
- Park BS, Lee JO (2013) Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp Mol Med 45: e66.
- Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, et al. (2004) The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA 101: 15718-15723.
- Krishnan J, Selvarajoo K, Tsuchiya M, Lee G, Choi S (2007) Toll-like receptor signal transduction. Exp Mol Med 39: 421-438.
- Tuncman G, Hirosumi J, Solinas G, Chang L, Karin M, et al. (2006) Functional in vivo interactions between JNK1 and JNK2 isoforms in obesity and insulin resistance. Proc Natl Acad Sci USA 103: 10741-10746.
- Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, et al. (2005) Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nat Med 11: 183-190.
- Cawthorn WP, Sethi JK (2008) TNF-alpha and adipocyte biology. FEBS Lett 582: 117–131.
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, et al. (2003) Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112: 1796-1808.
- Eggesbø JB, Hjermann I, Høstmark AT, Kierulf P (1996) LPS induced release of IL-1 beta, IL-6, IL-8 and TNF-alpha in EDTA or heparin anticoagulated whole blood from persons with high or low levels of serum HDL. Cytokine 8: 152-160.
- Wang H, Eckel R (2009) Lipoprotein lipase: from gene to obesity. Am J Physiol Endocrinol Metab 297: E271-E288.
- Kusunoki M, Tsutsumi K, Sato D, Nakamura T (2012) Lipoprotein lipase and obesity. Health 4: 1405-1412.
- Peterson J, Fujimoto WY, Brunzell JD (1992) Human lipoprotein lipase: relationship of activity, heparin affinity, and confirmation as studied with monoclonal antibodies. J Lipid Res 33: 1165-1170.
- Mead J, Irvine S, Ramji D (2002) Lipoprotein lipase: Structure, function, regulation, and role in disease. J Mol Med 80: 753-769.
- Walton RG, Zhu B, Unal R, Spencer M, Sunkara M, et al. (2015) Increasing adipocyte lipoprotein lipase improves glucose metabolism in high fat diet-induced obesity. J Biol Chem 290: 11547-11556.
- Kersten S, Mandard S, Tan NS, Escher P, Metzger D, et al. (2000) Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. J Biol Chem 275: 28488-28493.
- Mandard S, Zandbergen F, van Straten E, Wahli W, Kuipers F, et al. (2005) The fasting-induced adipose factor/angiopoietin-like protein 4 is physically associated with lipoproteins and governs plasma lipid levels and adiposity. J Biol Chem 281: 934-944.
- Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI (2007) Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci 104: 979-984.
- Festi D, Schiumerini R, Eusebi LH, Marasco G, Taddia M, et al. (2014) Gut microbiota and metabolic syndrome. World J Gastroenterol 20: 16079.
- Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, et al. (2005) Obesity alters gut microbial ecology. Proc Natl Acad Sci USA 102: 11070–11075.
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, et al. (2006) An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444: 1027-1031.
- Murphy EF, Cotter PD, Healy S, Marques TM, O'Sullivan O, et al. (2010) Composition and energy harvesting capacity of the gut microbiota: Relationship to diet, obesity and time in mouse models. Gut 59: 1635-1642.
- Holmes E, Li J, Marchesi JR, Nicholson JK (2012) Gut microbiota composition and activity in relation to host metabolic phenotype and disease risk Cell Metab 16: 559-564.
- Dahiya DK, Renuka PM, Shandilya UK, Dhewa T, Kumar N, et al. (2017) Gut microbiota modulation and its relationship with obesity using prebiotic fibers and probiotics: A review. Frontx Microbiol 8: 563.
- Makris A, Foster GD (2011) Dietary approaches to the treatment of obesity. Psychiatr Clin North Am 34: 813-827.
- Sackner-Bernstein J, Kanter D, Kaul S, Siegel A (2015) Dietary intervention for overweight and obese adults: Comparison of low-carbohydrate and low-fat diets. A meta- analysis PLoS One 10: e0139817.
- Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, et al. (2013) Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA 110: 9066-9071.
- Park DY, Ahn YT, Park SH, Huh CS, Yoo SR, et al. (2013) Supplementation of Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 in diet-induced obese mice is associated with gut microbial changes and reduction in obesity. PLoS One 8: e59470.
- Everard A, Matamoros S, Geurts L, Delzenne NM, Cani PD (2014) Saccharomyces boulardii administration changes gut microbiota and reduces hepatic steatosis, low-grade inflammation, and fat mass in obese and type 2 diabetic db/db mice. MBio 8: e01011-e01014.
- Jayasinghe TN, Chiavaroli V, Holland DJ, Cutfield WS, O'Sullivan JM (2016) The new era of treatment for obesity and metabolic disorders: Evidence and expectations for gut microbiome transplantation. Front Cell Infect Microbiol 6: 15.
- Marotza CA, Zarrinpar A (2016) Treating obesity and metabolic syndrome with fecal microbiota transplantation. Yale J Biol Med 89: 383–388.
- Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, et al. (2013) Cultured gut microbiota from twins discordant for obesity modulate adiposity and metabolic phenotypes in mice. Science 341: 1241214.
Citation: Manchanayake LN (2019) The Impact of Gut Microbiota on Host Obesity. J Gastrointest Dig Syst 9: 591. DOI: 10.4172/2161-069X.1000591
Copyright: © 2019 Manchanayake LN, This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6161
- [From(publication date): 0-2019 - Dec 22, 2025]
- Breakdown by view type
- HTML page views: 5185
- PDF downloads: 976