The Molecular Mechanism of Intestinal Flora Imbalance through the LPS/TLR4 Pathway to Regulate IgA1 Secretion to Induce IgA Nephropathy
Received: 09-Aug-2021 / Accepted Date: 22-Sep-2021 / Published Date: 29-Sep-2021 DOI: 10.4172/1165-158X.1000206
Abstract
Objective: IgA nephropathy has been the most common primary glomerular disease in China. This study aimed to investigate the detail molecular mechanism of intestinal flora imbalance through the LPS/TLR4 pathway to regulate IgA1 secretion to induce IgA nephropathy.
Methods: A mouse model of IgA nephropathy was established. Collect all mice blood and urine, and ELISA was also used to analyze IL-6, TNF-α, MCP-1, COX2, Gd-IgA1 level. Analyze the distribution and content of IgA+B220+B lymphocytes in all mouse intestinal tissues with tissue immunofluorescence tracking technology. HE staining was used to analyze the pathological damage characteristics of kidney tissue. Tissue immunofluorescence technique was used to analyze glomerular IgA deposition. Protein TIL4, BAFF, APRIL expression in mesenteric lymphoid tissues was detected by western blot analysis.
Results: For 5 groups of mice (normal control group + 4 experimental groups), the amount or degree of the physiological indicators and inflammatory factors detected by ELISA, B lymphocytes and IgA sedimentation measured by immunofluorescence tracing, kidney pathological detected by HE staining and the expression of immune-related proteins measured with western blotting, all showed a common trend: IgA-N group> IgA-N+GEC group> IgA-N+anti- TLR4 group> IgA-N+anti-TLR4+GEC group> NC group.
Conclusion: The TLR4 antibody and GEC for the treatment of intestinal tract regulated and repaired intestinal function, so that IgA nephritis was also relieved at the same time. The results proved the relation between the LPS/TLR4 pathway regulating mesenteric B cells to secrete low-glycosylated poly-IgA1 and IgA nephritis, which provided a new potential therapeutic plan for IgA nephritis.
Keywords: IgA nephropathy; Intestinal flora imbalance; LPS/TLR4 pathway; Mesenteric B cell
Introduction
IgA nephropathy (IgA-N) is pathologically characterized by the deposition of IgA (or containing IgG) immune complexes in the glomerular mesangial area and capillary loops. Recent studies have shown that low-glycosylated poly IgA1 (Galactose-deficient IgA1, Gd- IgA1) is called ‘nephrogenic IgA’ [1,2]. IgA nephropathy is the most common primary chronic glomerular disease in the world, so the research on this disease is very important [3]. For a long time, people have been keeping exploring the mechanism of IgA nephropathy. In clinical studies, the correlation between IgA nephropathy and the microbiota of excreta was found [4,5]. Other researchers speculated that the intestinal-derived IgA complex is deposited in the glomerular mesangial cells, causing IgA nephropathy [6]. Then the mechanism was begun to be explored.
The normal intestinal flora plays an important role in maintaining the intestinal mucosal barrier function and the immune homeostasis. When the intestinal flora is imbalanced, the potential pathogenic bacteria increase and produce a variety of toxins. The toxins cause inflammatory damage to the intestinal mucosal barrier. Increased mucosal permeability increases the absorption of antigens such as Lipopolysaccharide (LPS), and mediates systemic inflammation [7]. LPS is a phospholipid that forms the outer membrane of gramnegative bacilli. Toll-like Receptors (TLRs), the receptors of LPS, are highly expressed in intestinal mucosal epithelial cells and mesenteric lymphocytes. When LPS directly contacts intestinal mucosal epithelial cells, which can cause intestinal mucosal epithelium permeability increasing and intestinal mucosal function damage [8,9]. In addition, when B cells are activated, TLRs on the surface of B cells recognize Pathogen-Associated Molecular Patterns (PAMPs), which can induce B cell activation to produce low-affinity IgM and IgA single Cloned antibodies [10].
Although there have been some scattered advances in the research of the mechanism, the research on the whole specific regulation mechanism is still relatively rare, and the pathogenesis of IgA-N is not completely clear [11]. This has also led to the limitation of the therapeutic effect of IgA-N. Exploring the relationship between intestinal flora imbalance and IgA-N can provide new methods and perspectives for treatment [12,13]. Therefore, this work explored the relevant molecular mechanism of intestinal flora dysregulation through the LPS/TLR4 pathway to regulate mesenteric B cells secreting low-glycosylated poly IgA1 to induce IgA nephropathy. In this work, a mouse model of IgA nephropathy was established, and then glutamine enteric-coated capsules were gavaged and TLR4 antibody was injected into the tail vein. Collect all mice blood, urine, intestinal tissue and kidney tissue for experimental testing. Enzyme- Linked Immunosorbent Assay (ELISA), tissue immunofluorescence tracking technology, HE staining, Western blot and other methods were used to confirm the successful establishment of the model and analyze the mechanism of the entire pathway.
Materials and Methods
Experiment design
Sixty SPF C57BL/6 (B6, H-2b) male mice were purchased from Shanghai Slack Laboratory Animal Co., Ltd. All the mice were 6-8 weeks old, weighing 20-25 grams. After one week of adaptive feeding, they are randomly divided into 5 groups. Among them, one group was set as the normal control group, and the other 4 groups were used to establish IgA nephropathy mouse models by the BSA+SEB combined method. The specific implementation plan is as follows: gavage with 0.1% BSA acidified water (0.4 ml/mouse), once every other day, for 5 consecutive weeks; from the 6th week, 1% BSA buffer (0.4 ml/mouse) was injected into the tail vein, once a day, for 3 days, for the 7th and 8th week; from the 9th week, SEB (0.4 mg/kg) was injected into the tail vein, once a week, continuously 3 weeks, the end of the experiment was the 12th week. In addition, the normal healthy mice in control group were given 6 mmol/L equal amount of acidified water by gavage and 0.01mmol/L PBS buffer by tail vein injection at the same time. For the mice in the mouse IgA nephropathy model group, group 1 was no more subsequent processing; group 2 was given the tail vein injection of TLR4 antibody; group 3 was given glutamine enteric-coated capsules by gavage. Both the doses in group 2 and 3 were 216 mg/(kg⋅d), once a day. Group 4 was given the tail vein TLR4 antibody + glutamine enteric-coated capsules were injected intravenously. All the treatments for group 1-4 ended at the 12th week. The mice were the anesthetized with isopentane. All the mice blood, urine, intestinal tissue and kidney tissue were collected for experimental testing.
ELISA analysis
The mice blood samples were centrifuged at 3000g for 15 minutes in order to collect the serum for ELISA analysis. The Inflammatory factors such as IL-6, TNF-α, MCP-1, COX2, Gd-IgA1(rat IL-6, TNF-α, MCP-1, COX2, Gd-IgA1 kits are from R & D Systems, Co.) were detected by the ELISA technology. Ten standard wells were set on the ELISA-coated plate [14]. The standard samples were added to the wells according to the concentration requirements and serially diluted. The sample volume in each well was 50 μl. Then a blank control well and the sample well to be tested were set. The blank control well would not add sample and enzyme-labeled reagent; the other steps were the same with the sample well. Add 40 μl of sample diluent to the sample well, and then add 10 μl of the sample. The final dilution of the sample is 5 times. Seal the plate with a sealing film and incubate at 37ºC for 30 minutes. After incubation, wash the plate with washing solution and dry it. After drying, add 50 μl of enzyme-labeled reagent to each well except the blank well. Repeat the incubation and washing steps. Then add 50 μl of developer A and 50 μl of developer B in each well sequentially, and develop color at 37ºC for 15 minutes in the dark, and lastly add 50 μl of stop solution to stop the reaction (the blue turns to yellow immediately). Set the blank control well as zero, and measure the absorbance (OD value) of each well in sequence at 450 nm wavelength.
Tissue immunofluorescence tracer
The intestinal tissues and kidney tissues of mice of each group were collected for acid hydrolysis. After acid hydrolysis, they were encapsulated with slicing fluid, and slices of each tissue were made using a microtome [15].
Place the tissue sections in a 65ºC incubator for 1 hour to dewax, and then use 0.5% Triton X-100 to permeate at room temperature for 10 min. Use citrate buffer for antigen retrieval, then incubate the slices with 3% H2O2 at room temperature for 30 min to inactivate endogenous peroxidase. 1% BSA was applied for blocking at room temperature for 30 min to block non-specific epitopes. Incubate the specific primary antibody according to the recommended instructions for the antibody and let it stand overnight in a humidified box at 4ºC. Take out the slices the next day and rewarm at room temperature for 30 min. Select the corresponding immunofluorescence secondary antibody and then incubate at 37ºC for 30 min in the dark. Under dark conditions, stain the nucleus with DAPI. Add anti-fluorescence quencher for mounting. Finally, use a fluorescence microscope to observe and take pictures.
HE staining
We observed the morphological changes of kidney tissue with hematoxylin-eosin staining (HE staining) under light microscope. The detailed steps of HE staining are as followed.
After fixing with 4% Paraformaldehyde (PFA) at 4ºC overnight, the kidney tissues were dehydrated by gradient ethanol, permeabilized by xylene and embeds with paraffin. Following this, tissues were cut into 4 μm slices and stained with hematoxylin-eosin solution (Nanjing Jiancheng, Nanjing, Jiangsu, China) according to the manufacturers’ protocols. Finally, slices were mounted with neutral resins and analyzed by a light microscope (Nikon Eclipse 1000, Tokyo, Japan).
Western blot analysis
Mesenteric lymphoid tissue cells of each group were collected and 200 μl of cell lysate was added to each six-well plate. After sonication, the cells were lysed on ice for 1 hour. The lysed cell sample was centrifuged at 12,500 g at 4ºC for 15 minutes. Then, the supernatant in the centrifuge tube was transferred to a clean centrifuge tube. The BCA protein quantification kit was used to quantify protein concentration determined concentration of protein samples were stored at -80ºC. In Western blotting electrophoresis, the protein loading concentration was 50 micrograms per well [16]. After SDS-PAGE electrophoresis, the membrane was transferred and blocked. TLR4, BAFF, APRIL, Actin (1: 500, anti-human, Abcam, USA) primary antibody Dilute to use concentration. The samples were incubated on a 4°C shaker overnight. After washing the samples in PBS, the samples were incubated with secondary antibodies (1:1000, Abcam, USA) in the dark at room temperature for 30 minutes. Finally, the developer was used for development and photographing.
Statistical analysis
The experimental results are expressed as mean ± standard deviation. The paired t test was used in the comparison within the same group. The two independent sample t tests were applied to compare the relapse scores between the two groups. Statistical analysis was performed using SPSS 22.0 software and P<0.05 was considered to be statistically different. The figures were produced with Origin 2020 software.
Results
Expression of specific physiological indicators
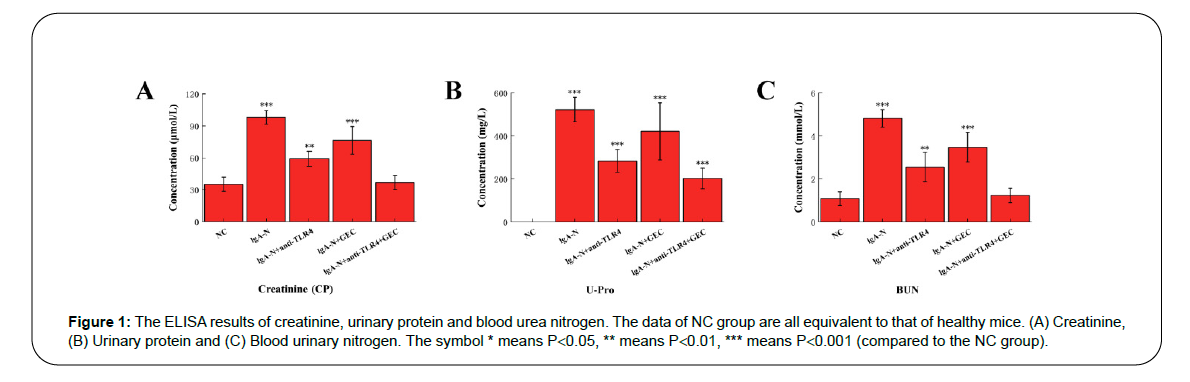
The data obtained by ELISA method showed that the kidney function of the mice in the NC group was normal (urinary protein could not be detected in the urine of normal animals), while the blood creatinine, urinary protein and urea nitrogen of the mice with IgA nephropathy (IgA-N group) increased significantly. In the three groups of IgA-N+anti-TLR4, IgA-N+GEC and IgA-N+anti-TLR4+GEC, the characteristic physiological indicators all decreased to different degrees. Among them, the IgA-N+GEC group had the least decrease, and the combination of anti-TLR4 and GEC had the most decrease (Figure 1).
Figure 1: The ELISA results of creatinine, urinary protein and blood urea nitrogen. The data of NC group are all equivalent to that of healthy mice. (A) Creatinine, (B) Urinary protein and (C) Blood urinary nitrogen. The symbol * means P<0.05, ** means P<0.01, *** means P<0.001 (compared to the NC group).
Expression of inflammatory factors
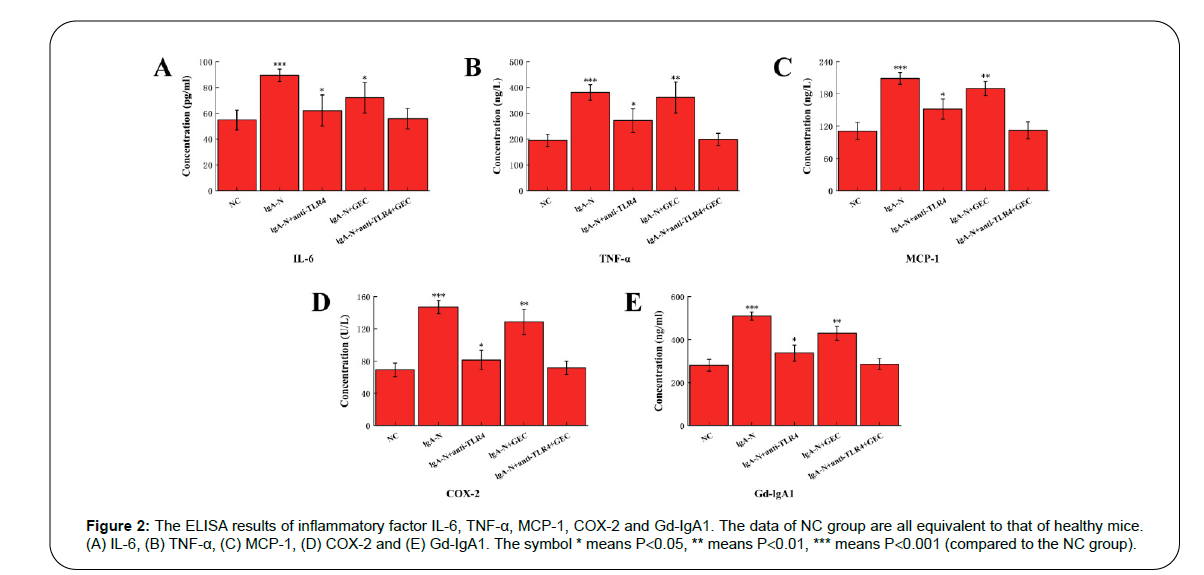
Inflammatory factors Interleukin-6 (IL-6), Tumor Necrosis Factor (TNF-α), Monocyte Chemotactic Protein 1 (MCP-1), Cyclooxygenase-2 (COX-2) and Galactose-Deficient IgA1 (Gd-IgA1) all have a significant increase during the period of IgA-N in mice, which means that there is a strong inflammatory response in the body. Inflammation mainly occurs around the mesentery and glomerulus. By applying anti- TLR4, GEC and the combination of the two into the diseased mice, the inflammatory response was alleviated to a certain extent. Among the three groups, the level of inflammatory factors in IgA-N+anti- TLR4+GEC group was basically reduced to the normal level, which indicated the best therapeutic effect (Figure 2).
Figure 2: The ELISA results of inflammatory factor IL-6, TNF-α, MCP-1, COX-2 and Gd-IgA1. The data of NC group are all equivalent to that of healthy mice. (A) IL-6, (B) TNF-α, (C) MCP-1, (D) COX-2 and (E) Gd-IgA1. The symbol * means P<0.05, ** means P<0.01, *** means P<0.001 (compared to the NC group).
Distribution and content of IgA+B220+B lymphocytes in intestinal tissue
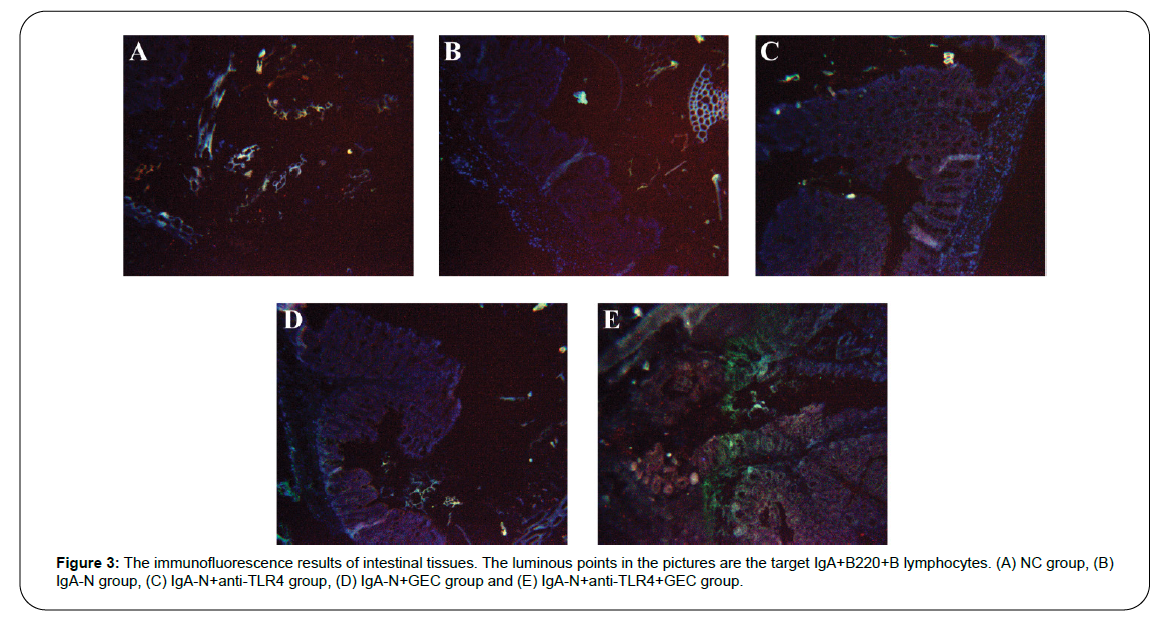
Tissue immunofluorescence tracking technology was used to determine the distribution and content of IgA+B220+B lymphocytes in the intestinal tissue, and the results are shown in Figure 3. It can be seen from the figure that the trend of IgA+B220+B lymphocytes content is as following: IgA-N group> IgA-N+GEC group> IgA-N+anti-TLR4 group> IgA-N+anti-TLR4+GEC group> NC group.
Pathological damage of kidney tissue
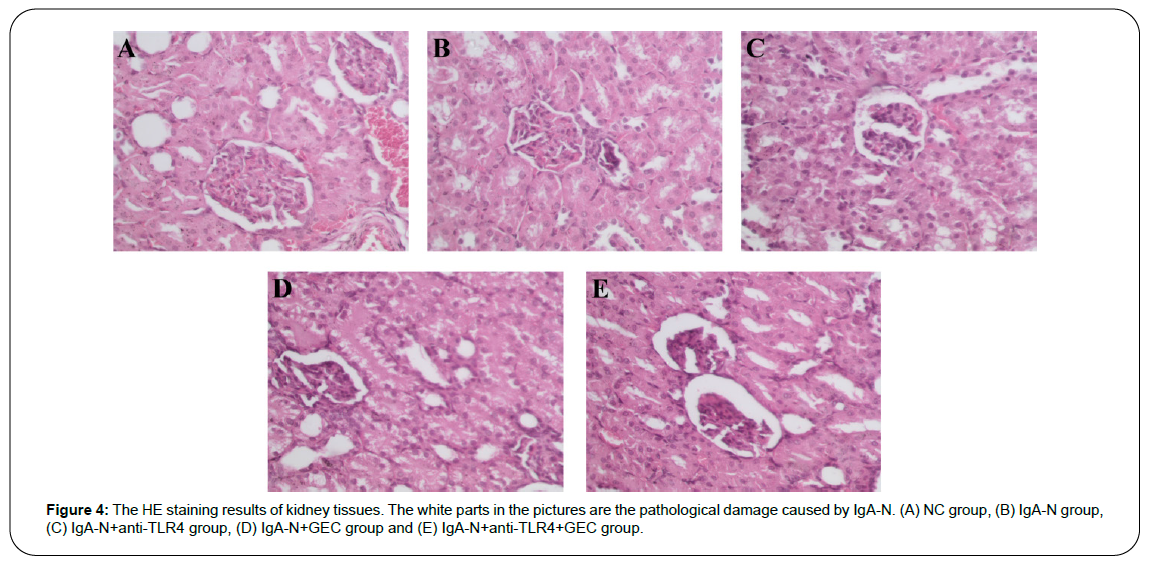
HE staining technology was used to analyze the characteristics of pathological damage of kidney tissue, and the results are shown in Figure 4. Changes in the permeability of the mesentery could cause pathological damage to the kidney. Therefore, through the repair of the mesentery by anti-TLR4 or/and GEC, the kidney damage would also be reduced. It can be seen from the figure that the degree of kidney damage from high to low was as following: IgA-N group> IgA-N+GEC group> IgA-N+anti-TLR4 group> IgA-N+anti-TLR4+GEC group> NC group. The simultaneous use of anti-TLR4 and GEC made the kidney damage almost the same as NC group.
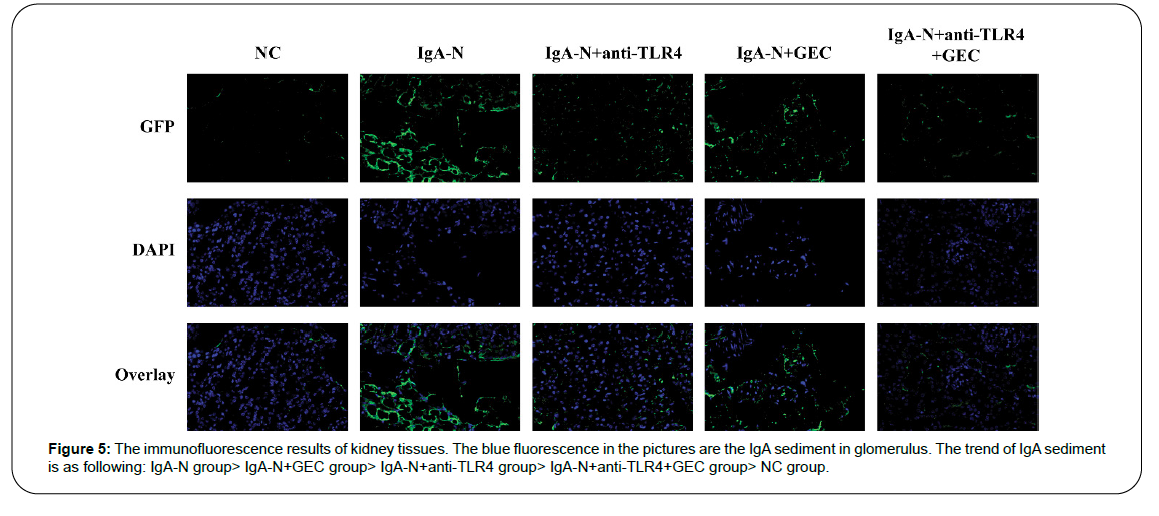
IgA sedimentation in the glomerulus
The sedimentation of IgA in the glomerulus is the direct cause of IgA-N. By measuring the sedimentation of IgA with immunofluorescence technology, the therapeutic effect of the drug used on kidney disease can be determined. The results proved that the effect of using GEC alone is the worst; the effect of using anti-TLR4 alone is the second, and the effect of using both at the same time is the best (Figure 5).
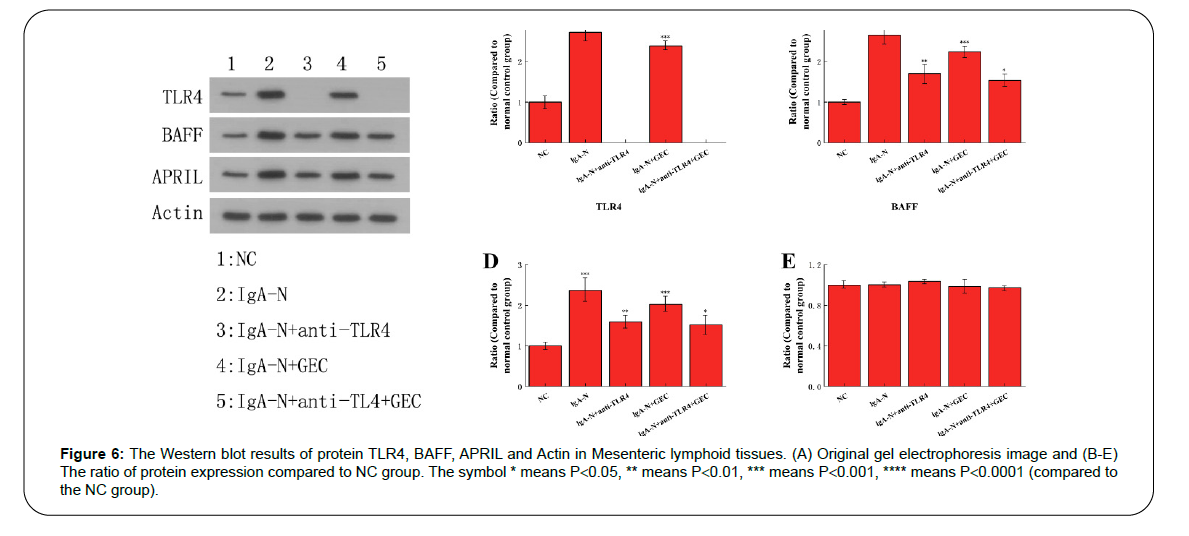
The expression level of TLR4, BAFF, APRIL
The expression of TLR4, BAFF, and APRIL can directly affect the amount and activity of B cells, which indirectly leads to the overexpression and sedimentation of IgA. The results obtained from Western blot analysis fully confirmed the inhibitory effect of anti-TLR4 and GEC on these three immune-related proteins (Figure 6). Actin was the internal reference protein in this Western blot analysis experiment.
Figure 6: The Western blot results of protein TLR4, BAFF, APRIL and Actin in Mesenteric lymphoid tissues. (A) Original gel electrophoresis image and (B-E) The ratio of protein expression compared to NC group. The symbol * means P<0.05, ** means P<0.01, *** means P<0.001, **** means P<0.0001 (compared to the NC group).
Discussion
Immune tolerance may lead to changes in the intestinal flora and cause abnormal reactions to the microbiota, including increased absorption of digestive tract antigens and bacterial toxins, thereby triggering Mucosa-Associated Lymphoid Tissue (MALT) activation and subclinical intestinal inflammation. Synthesizing abnormally glycosylated polymeric IgA1 can cause abnormal reactions to digestive tract antigens or symbiotic microorganisms, and ultimately cause kidney deposits to enter the circulatory system. [17,18] Some studies have genetically verified the correlation between IgA-N and intestinal diseases [19]. However, the detail mechanism is still unclear, and this present study is carried out to investigate the specific correlation between IgA-N and intestinal diseases.
LPS/TLR4 signal way play a key role in cell physiological function, and it can regulate the disease progression through cell proliferation, differentiation and apoptosis. LPS/TLR4 signal way has been implicated in IgA nephropathy in previous relative papers [20]. In the process of establishing a model to study the relationship between the LPS/TLR4 pathway regulating mesenteric B cells to secrete low-glycosylated poly-IgA1 and IgA nephritis, the content of inflammatory factors and immune-related specific proteins in the mice body played the key role.
In our study, the western blot results show that the level of TLR4, BAFF, APRIL and Actin protein for all mice is IgA-N group> IgA-N+GEC group> IgA-N+anti-TLR4 group> IgA-N+anti-TLR4+GEC group> NC group, and the level of these protein show significant difference. These findings demonstrate that the protective effect of GEC and anti- TLR4 on mice with IgA-N, which indicated that LPS/TLR4 signal way is closely connected with on mice with IgA-N. This result consists with the previous relative study [21].
Some studies have reported that inflammatory cytokines such as IL-6, TNF-α, MCP-1, COX2, Gd-IgA1 can promote IgA-N disease progression. IL-6 can make B cell precursors become B cells that produce antibodies. TNF-α can promote T cells to produce various inflammatory factors, thereby promoting the occurrence of inflammation. MCP- 1 has chemotactic activity on monocytes and activates monocytes and macrophages [22]. The activity of COX-2 in normal tissue cells is extremely low. When the cells are stimulated by inflammation, its expression level in inflammatory cells can increase to 10-80 times the normal level, leading to inflammation and tissue damage [23]. Gd- IgA1 is called “nephrogenic IgA”, whose overexpression can lead to IgA1 sedimentation in the glomerular mesangial and finally nephritis [24]. These five inflammatory factors will increase rapidly during the occurrence of IgA-N, which are the main specific indicators for diagnosing IgA-N. Through the use of anti-TLR4 and GEC drugs for the intestine, the content of inflammatory factors was significantly reduced, which directly proved the correlation between the two. The ELISA results show that show that the level of IL-6, TNF-α, MCP-1, COX2, Gd-IgA1 inflammatory cytokines for all mice is IgA-N group> IgAN+ GEC group> IgA-N+anti-TLR4 group> IgA-N+anti-TLR4+GEC group> NC group, and the level of these inflammatory cytokines show significant difference. These observations indicate that GEC and anti- TLR4 can inhibit expression of these inflammatory cytokines on mice with IgA-N, and the result consists with that the previous studies [25].
When TLR4-mediated LPS contacts intestinal mucosal epithelial cells, it will directly damage intestinal mucosal function and stimulate B cell activation to produce low-affinity IgA, and then induce IgA-N to occur [26]. The TNF family (BAFF) is an important B cell survival factor. Transgenic mice overexpressing BAFF have mesangial deposited IgA and abnormally glycosylated high circulating levels of polymerized IgA [27,28]. Therefore, B cell immunotherapy also provides new ideas for the treatment of IgA-N [29]. The intestinal flora is very important in the development of IgA-N in mice that produce high levels of IgA and transgenic mice with BAFF overexpression. The gut-renal axis regulated by the microbiota and diet is a potential way for treating IgA-N [30]. APRIL acts in the later stages of immune-related diseases, cooperating with BAFF together to regulate the function and survival of effector B cells [31].
Overall, the TLR4 antibody and GEC for the treatment of intestinal tract regulated and repaired intestinal function, so that IgA nephritis was also relieved at the same time. The results proved the relation between the LPS/TLR4 pathway regulating mesenteric B cells to secrete low-glycosylated poly-IgA1 and IgA nephritis, which provided a new potential therapeutic plan for IgA nephritis.
Conclusion
Increasing the expression of TLR4 antibody and GEC for the treatment of intestinal tract regulated and repaired intestinal function, so that IgA nephritis was also relieved at the same time. The results proved the relation between the LPS/TLR4 pathway regulating mesenteric B cells to secrete low-glycosylated poly-IgA1 and IgA nephritis, which provided a new potential therapeutic plan for IgA nephritis.
Acknowledgments
We would like to acknowledge everyone for their helpful contributions on this paper.
Authors’ Contributions
HDH, YYT, MLS conceived and designed experiments; HDH, YYT, MLS performed experiments and data analysis; WQS, XDX provided technical support, data collection and analysis; and HDH, YYT, MLS wrote the manuscript. All authors provided their final approval of the submitted and published version.
Ethics Approval and Consent to Participate
The research protocol was reviewed and approved by the Ethical Committee and Institutional Review Board of the Minhang District Central Hospital.
Competing Interests
The authors declare that they have no competing interests.
Funding
The Science and Technology Commission of Shanghai Municipality of the project of Shanghai Key Laboratory of kidney and Blood Purification (No. 14DZ2260200). National Natural Science Foundation of China (No. 81774060). Scientific research project of Shanghai Health and Family Planning Commission (No. 20184Y0040).
References
- Schwarzbich, M. A., & Witzens-Harig, M. Cellular Immunotherapy in B-Cell Malignancy. Oncol Res Treat., 2017;40(11): 674-681.
- Suzuki, H., Yasutake, J., Makita, Y., Tanbo, Y., Yamasaki, K., Sofue, T., et al. IgA nephropathy and IgA vasculitis with nephritis have a shared feature involving galactose-deficient IgA1-oriented pathogenesis. Kidney Int., 2018;93(3): 700-705.
- Wyatt, R. J., & Julian, B. A. IgA Nephropathy. N Engl J Med., 2013;368: 2402-2414.
- Suzuki, H., Kiryluk, K., Novak, J., Moldoveanu, Z., Herr, A. B., Matthew, B., et al. The Pathophysiology of IgA Nephropathy. J Am Soc Nephrol., 2011;22: 1795-1803.
- De Angelis, M., Montemurno, E., Piccolo, M., Vannini, L., Lauriero, G., Maranzano, V., et al. Microbiota and metabolome associated with immunoglobulin A nephropathy (IgAN). PLoS One., 2014;9: e99006.
- Terasaka, T., Uchida, H. A., Umebayashi, R., Tsukamoto, K., Tanaka, K., Kitagawa, M., et al. The possible involvement of intestine- derived IgA1: a case of IgA nephropathy associated with Crohn’s disease. BMC Nephrology., 2016;17: 122.
- Li, L., Wan, G. W., Han, B., & Zhang, Z. W. Echinacoside alleviated LPS-induced cell apoptosis and inflammation in rat intestine epithelial cells by inhibiting the mTOR/STAT3 pathway. Biomed Pharmacother., 2018;104: 622-628.
- Erridge, C., Kennedy, S., Spickett, C. M., & Webb, D. J. Oxidized phospholipid inhibition of Toll-like receptor (TLR) signaling is restricted to TLR2 and TLR4 - Roles for CD14, LPS-binding protein, and MD2 as targets for specificity of inhibition. J Biol Chem., 2008;283(36): 24748-24759.
- Kumari, A., Singh, D. K., Dash, D., & Singh, R. Intranasal curcumin protects against LPS-induced airway remodeling by modulating toll-like receptor-4 (TLR-4) and matrixmetalloproteinase-9 (MMP- 9) expression via affecting MAP kinases in mouse model. Inflammopharmacol., 2019;27(4): 731-748.
- Kauffman, R. C., Adekunle, O., Yu, H., Cho, A., Nyhoff, L. E., Kelly, M., et al. Impact of Immunoglobulin Isotype and Epitope on the Functional Properties of Vibrio cholerae O-Specific Polysaccharide-Specific Monoclonal Antibodies. mBio., 2021;12(2): e03679.
- Eduardo, G., Fernando, C. F., Leonella, L., Enrique, M., Marina, A., & Manuel, P. A Personalized Update on IgA Nephropathy: A New Vision and New Future Challenges. Nephron., 2020;144(11): 555-571.
- Rauen, T. Intensive Supportive Care plus Immunosuppression in IgA Nephropathy. N Engl J Medicine., 2015;373(23): 2225-2236.
- Pei, G. Q., Tan, J. X., Tang, Y., Tan, L., Zhong, Z. X., Zhou, L., et al. Corticosteroids or immunosuppressants were not superior to supportive care in IgA nephropathy patients with mild proteinuria. Med., 2020;99: 24.
- Wang, M., Lv, J., Zhang, X., Chen, P., Zhao, M., & Zhang, H. Secondary IgA Nephropathy Shares the Same Immune Features With Primary IgA Nephropathy. Kidney Int Rep., 2019;5(2): 165-172.
- Rizk, D. V., Maillard, N., Julian, B. A., Knoppova, B., Green, T. J., Novak, J., et al. The Emerging Role of Complement Proteins as a Target for Therapy of IgA Nephropathy. Front Immunol., 2019;10: 504.
- Kurano, M., Tsuneyama, K., Morimoto, Y., Nishikawa, M., & Yatomi, Y. Apolipoprotein M suppresses the phenotypes of IgA nephropathy in hyper-IgA mice. FASEB J., 2019;33(4): 5181-5195.
- Floege, J., & Feehally, J. The mucosa-kidney axis in IgA nephropathy. Nat Rev Nephrol., 2016;12(3): 147-156.
- Rosanna, C. The intestine–renal connection in IgA nephropathy. Nephrol Dial Transplant., 2015;30: 360-366.
- Kiryluk, K. Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat Genet., 2014;46(11): 1187-1196.
- Zhang, J., Mi, Y., Zhou, R., Liu, Z., Huang, B., Guo, R., et al. The TLR4-MyD88-NF-κB pathway is involved in sIgA-mediated IgA nephropathy. J Nephrol., 2020;33(6): 1251-1261.
- Sheng, X., Zuo, X., Liu, X., Zhou, Y., & Sun, X. Crosstalk between TLR4 and Notch1 signaling in the IgA nephropathy during inflammatory response. Int Urol Nephrol., 2018;50(4): 779-785.
- Kaczynski, T., Miskiewicz, A., Gorski, B., Radkowski, M., Strzemecki, D., Kryczka, T., et al. The influence of glycyrrhetinic acid (enoxolone) toothpaste on periodontal treatment outcomes and salivary levels of IL-8, TNF-alpha, IL-17, MCP-1 and VEGF in patients with chronic periodontitis. Postepy Higieny I Medycyny Doswiadczalnej. 2018;72: 1097-1103.
- Lim, S. C., Hwang, H., & Han, S. I. Ellagic Acid Inhibits Extracellular Acidity-Induced Invasiveness and Expression of COX1, COX2, Snail, Twist 1, and c-myc in Gastric Carcinoma Cells. Nutri., 2019;11: 12.
- Zheng, N., Xie, K., Ye, H., Yu, D., Bing, W., Luo, N., et al. TLR7 in B cells promotes renal inflammation and Gd-IgA1 synthesis in IgA nephropathy. JCI Insight., 2020;5(14): e136965.
- Gao, R., Wu, W., Wen, Y., & Li, X. Hydroxychloroquine alleviates persistent proteinuria in IgA nephropathy. Int Urol Nephrol., 2017;49(7): 1233-1241.
- Guo, S. H., Al-Sadi, R., Said, H. M., & Ma, T. Y. Lipopolysaccharide Causes an Increase in Intestinal Tight Junction Permeability in Vitro and in Vivo by Inducing Enterocyte Membrane Expression and Localization of TLR-4 and CD14. American J Pathol., 2013;182(2): 375- 387.
- Sang, M., Li, J., Wei, Z., Wu, X., Wang, Z., Ma, L., et al. Molecular structure, expression, and bioactivity of B-cell-activating factor of the TNF family (BAFF) and its receptor BAFF-R in cats (Felis catus). Mol Immunol., 2019;112: 59-71.
- Schwarzbich, M. A., & Witzens-Harig, M. ellular Immunotherapy in B-Cell Malignancy. Oncol Res Treat., 2017;40(11): 674-681.
- Rose, W. A., Okragly, A. J., Hu, N. N., Daniels, M. R., Martin, A. P., Koh, Y. T., et al. Interleukin-33 Contributes Toward Loss of Tolerance by Promoting B-Cell- Activating Factor of the Tumor-Necrosis-Factor Family (BAFF)-Dependent Autoantibody Production. Frontiers Immunol., 2018;9: 2871.
- Coppo, R. The gut-kidney axis in IgA nephropathy: role of microbiota and diet on genetic predisposition. Pediatr Nephrol., 2018;33(1): 53-61.
- Alturaiki, W. The roles of B cell activation factor (BAFF) and a proliferation-inducing ligand (APRIL) in allergic asthma. Immunol Lett., 2020;225: 25-30.
Citation: He H, Huan H, Shen M, Tang Y, Sun W, et al. (2021) The Molecular Mechanism of Intestinal Flora Imbalance through the LPS/TLR4 Pathway to Regulate IgA1 Secretion to Induce IgA Nephropathy. Cell Mol Biol 67: 206. DOI: 10.4172/1165-158X.1000206
Copyright: © 2021 He H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3291
- [From(publication date): 0-2021 - Nov 29, 2025]
- Breakdown by view type
- HTML page views: 2504
- PDF downloads: 787