The New FDA Approved Drug for Alzheimerâs disease, Aducanumab, and what Patients should know?
Received: 04-Aug-2021 / Accepted Date: 18-Aug-2021 / Published Date: 25-Aug-2021 DOI: 10.4172/2161-0460.s5.1000019
How does Aducanumab Work in the Brain?
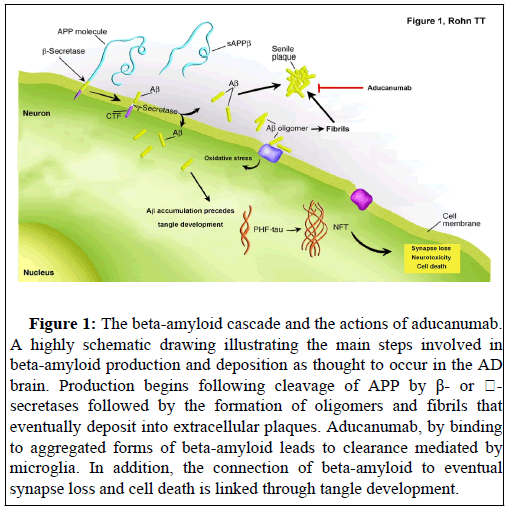
AD is a neurodegenerative disorder characterized by extensive neuronal loss leading to cognitive impairment and dementia. AD is diagnosed based upon the extent of senile plaques composed of betaamyloid and neurofibrillary tangles (NFTs) containing abnormally phosphorylated tau [1]. For more than 25 years, the beta-amyloid hypothesis (or amyloid cascade hypothesis) has been a central theory in the field of AD, positing that beta-amyloid is the primary cause of AD, which promotes tau aggregation into NFTs, ultimately triggering neuronal death [2]. The assumption is that when beta-amyloid aggregates (into fibrils or more specifically into oligomers), it triggers neurodegenerative processes that lead to the loss of memory and cognitive ability in AD. Strong support of this hypothesis comes from the knowledge that all known mutations that lead to early-onset AD have the overall effect of increasing the levels of beta-amyloid [3]. Therefore, if the progression of AD is believed to align with the betaamyloid hypothesis, it follows that pharmaceutical intervention aimed at any discrete step along the hypothesis with regards to oligomer or plaque formation or persistence should function to retard both betaamyloid load and cognitive decline. This framework provides a logical backbone for the development of pharmaceuticals aiming at targeting the pathogenic mechanisms of AD. These pharmaceutical approaches include those targeting clearance mechanisms of beta-amyloid from the brain including aducanumab (Figure 1).
Figure 1: The beta-amyloid cascade and the actions of aducanumab. A highly schematic drawing illustrating the main steps involved in beta-amyloid production and deposition as thought to occur in the AD brain. Production begins following cleavage of APP by β- or ��- secretases followed by the formation of oligomers and fibrils that eventually deposit into extracellular plaques. Aducanumab, by binding to aggregated forms of beta-amyloid leads to clearance mediated by microglia. In addition, the connection of beta-amyloid to eventual synapse loss and cell death is linked through tangle development.
Previous studies have shown that the build-up of beta-amyloid in the brain can occur years, even decades before symptoms manifest [4,5] and this has led to the development of therapeutics that directly target the clearance of beta-amyloid out of the brain. Aducanumab (brand name Aduhelm) is a monoclonal antibody designed to bind to aggregated forms of beta-amyloid, which in turn facilitate its removal from the brain through the actions of microglia [6]. Because betaamyloid is thought to represent the earliest step in the molecular cascade leading to AD, the expectation is that once beta-amyloid is removed this will prevent neuronal cell death or synapse loss and in turn improve cognitive outcomes.
What were the Major Outcomes Following Human Clinical Trials with Aducanumab?
In a dose-escalation trial of aducanumab, patients with mild AD who received one year of monthly infusions had reduced beta-amyloid levels in a dose-dependent manner. Moreover, aducanumab reduces clinical decline when measured using a Clinical Dementia Rating scale and Mini-Mental State Examination (MMSE) scores [6]. A 12- month follow-up indicated that almost half of these patients with mild AD no longer had cerebral amyloid on PET imaging [6]. Two subsequent phase III clinical trials led to the accelerated approval of aducanumab by the US FDA to treat mild cognitive impairment [7]. Accelerated approval was given based on the results of only one of these two trials. The findings from these trials have since undergone extensive review and have contributed to the confusion over whether aducanumab is effective or not [8,9]. To summarize, the sponsor of the two clinical trials halted both phase III trials after an interim futility analysis showed that it was unlikely to improve cognition for people with mild AD. However, highly critical post hoc analyses led the sponsor to assert that there was sufficient efficacy to justify a new drug application for approval by the FDA. A recent analysis of this trial concluded that aducanumab’s efficacy as a treatment for cognitive decline in AD cannot be proven by clinical trials with divergent outcomes [10]. Furthermore, the authors of this study did not agree with the sponsor’s claim for efficacy of aducanumab based on a review of data presented publicly in December 2019 [10].
Even taken into account the potential efficacy that aducanumab may have, this benefit appears to be small. Based on the two objective measures used in the clinical trials, the high dose led to a 0.6-point change on the 30-point MMSE and on the 85-point AD Assessment Scale-Cognitive Subscale=13, it made a 1.4-point change [11]. Taken together, it appears the potential impact this may have on cognitive decline may be minimal at best.
Cost and Safety
Patients who will be prescribed aducanumab will require intravenous infusions every four weeks indefinitely. In the phase III clinical trials, 30% of those who took aducanumab had a reversible swelling of the brain termed ARIA (amyloid-related imaging abnormalities). It is important to note that this edema was primarily transient and manageable using a titration schedule and safety monitoring with MRI [6]. In addition, roughly 10% of trial participants exhibited tiny brain bleeds. In terms of cost, the sponsor estimates that an annual cost of $56,000 per year [12] can be expected and as of yet, it is not clear whether Medicare, Medicaid, or even private insurance companies will help pay for this medication.
Controversial Approval
A key aspect to how drugs are ultimately approved through the FDA consists of advisory committees, who provide FDA with independent opinions and recommendations from outside experts on applications to market new drugs [13]. In general, advisory committees weigh the available evidence and provide scientific and medical advice on the safety, effectiveness, and appropriate use of drugs that the FDA regulates. However, it is important to note that FDA advisory committees are just that, advisory, in nature. The FDA can choose to not follow the recommendations of the committee, which is what appears, have occurred with aducanumab. In a nonbinding vote, no member voted for approval of aducanumab although all members nearly unanimously agreed that the results provide a signal that the drug might have a clinical effect but may not affect disease progression. Members of the committee concluded that the totality of evidence did not amount to substantial evidence of efficacy from adequate, well-controlled trials that the law requires, and that patients and physicians should expect for traditional approval [14].
Conclusion
Given that the approval of aducanumab has proven to be highly controversial and that the results from two phases III randomized clinical trials were far from conclusive, patients that are prescribed this new drug should be done so under total transparency in terms of potential benefits or lack thereof. Another important ethical consideration is the exorbitant price for this medication set at about $56,000 per year per person. Thus, given the controversial history of aducanumab a careful analysis of cost/benefit should be performed between the patient and physician before prescribing this medication. To date, the clinical trial data on aducanumab are incomplete and contradictory. In the end, prescribing any medication is a risk-benefit calculus inherent in the decision to start or stay on any medication. But with a drug like aducanumab with its contentious history of efficacy, high price tag, and risk of serious side effects, the math is even more complicated and the stakes higher.
Funding
This work was funded by National Institutes of Health Grant 2R15AG042781-02A1. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Golde TE, Dickson D, Hutton M (2006) Filling the gaps in the Aβ cascade hypothesis of Alzheimer's disease. Curr Alzheimer Res 3:421-430.
- Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer's disease: Progress and problems on the road to therapeutics. Science 297:353-356.
- Kametani F, Hasegawa M (2018) Reconsideration of amyloid hypothesis and tau hypothesis in Alzheimer's disease. Front. Neurosci 12: 25.
- Beason-Held LL, Goh JO, An Y, Kraut MA, O'Brien RJ, et al. (2013) Changes in brain function occur years before the onset of cognitive impairment. J Neurosci 33: 18008-10014.
- Jack Jr CR, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, et al. (2013) Tracking pathophysiological processes in Alzheimer's disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurol 12: 207-216.
- Sevigny J, Chiao P, Bussière T, Weinreb PH, Williams L, et al. (2016) The antibody aducanumab reduces Abeta plaques in Alzheimer's disease. Nature 537: 50-56.
- Food and Drug Administration (FDA) News release (2021) FDA grants accelerated approval for Alzheimer’s drug.
- Kuller LH, Lopez OL (2021) ENGAGE and EMERGE: Truth and consequences? Alzheimers Dement 17: 692-695.
- Alexander GC, Emerson S, Kesselheim AS (2021) Evaluation of aducanumab for Alzheimer disease: Scientific evidence and regulatory review involving efficacy, safety, and futility. JAMA 325: 1717-1718.
- Knopman DS, Jones DT, Greicius MD (2021) Failure to demonstrate efficacy of aducanumab: An analysis of the EMERGE and ENGAGE trials as reported by Biogen, December 2019. Alzheimers Dement 17:696-701.
- Schneider L (2020) A resurrection of aducanumab for Alzheimer's disease. Lancet Neurol 19: 111-112.
- Mullard A (2021) Landmark Alzheimer's drug approval confounds research community. Nature 594: 309-310.
- Food and Drug Administration (FDA) News Release (2017) The FDA's drug review process: Ensuring drugs are safe and effective in U.S.F.a.D. Administration.
- Alexander GC, Knopman DS, Emerson SS, Ovbiagele B, Kryscio RJ, etal. (2021) Revisiting FDA Approval of Aducanumab. N Engl J Med.
Citation: Rohn TT (2021) The New FDA Approved Drug for Alzheimer’s disease, Aducanumab, and what Patients should know? J Alzheimers Dis Parkinsonism S5: 019. DOI: 10.4172/2161-0460.s5.1000019
Copyright: © 2021 Rohn TT. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2641
- [From(publication date): 0-2021 - Nov 10, 2025]
- Breakdown by view type
- HTML page views: 1927
- PDF downloads: 714