The Oral and Conjunctival Microbiotas in Cats with Feline Immunodeficiency Virus Infection
Received: 09-Sep-2022 / Manuscript No. omoa-22-74562 / Editor assigned: 12-Sep-2022 / PreQC No. omoa-22-74562 (PQ) / Reviewed: 24-Sep-2022 / QC No. omoa-22-74562 / Revised: 27-Sep-2022 / Manuscript No. omoa-22-74562 (R) / Published Date: 30-Sep-2022
Abstract
The human oral microbiome is known to play a significant role in human health and disease. While less well studied, the feline oral microbiome is thought to play a similarly important role. To determine roles oral bacteria play in health and disease, one first has to be able to accurately identify bacterial species present. 16S rRNA gene sequence information is widely used for molecular identification of bacteria and is also useful for establishing the taxonomy of novel species. The objective of this research was to obtain full 16S rRNA gene reference sequences for feline oral bacteria, place the sequences in species-level phylotypes, and create a curated 16S rRNA based taxonomy for common feline oral bacteria. Clone libraries were produced using "universal" and phylum-selective PCR primers and DNA from pooled sub gingival plaque from healthy and periodontally diseased cats. Bacteria in subgingival samples were also cultivated to obtain isolates. Full-length 16S rDNA sequences were determined for clones and isolates that represent 171 feline oral taxa. A provisional curated taxonomy was developed based on the position of each taxon in 16S rRNA phylogenetic trees. The feline oral microbiome curated taxonomy and 16S rRNA gene reference set will allow investigators to refer to precisely defined bacterial taxa. A provisional name such as "Propionibacterium sp. feline oral taxon FOT-327" is an anchor to which clone, strain or GenBank names or accession numbers can point. Future next-generation-sequencing studies of feline oral bacteria will be able to map reads to taxonomically curated full-length 16S rRNA gene sequences.
Keywords
Microbiotas; Conjunctivitis; microflora; 16S rRNA
Introduction
Conjunctivitis, keratoconjunctivitis, and corneal sequestration are common clinical problems in cats. Based on research over the last few decades, characteristics of the bacterial flora in feline conjunctival sacs show a similar composition, and the occurrence of particular species of bacteria varies by frequency of their isolation [1]. However, Gelatt described the feline conjunctival and corneal surface as being generally colonized to a lower degree than in other domestic species. Among bacteria isolated from the conjunctiva, staphylococci are the most representative group [2].
A second group of frequently isolated microorganisms are hemolytic and no hemolytic streptococci. Previous studies based on the microbiological identification of bacteria or the sequencing of amplicons generated from microbial DNA have also been considered conjunctival commensals, which in some circumstances may be involved in conjunctival pathology. Chlamydophila felis has been identified as an indisputable pathogen of feline conjunctiva. This Gram-negative bacterium has already been isolated from a number of feline conjunctivitis cases. There is also evidence that other Chlamydia-related microorganisms like Chlamydophila pneumoniae and Neochlamydia hartmannellae may be associated with conjunctiva. Investigating conjunctival infections in cats with lepromatous lesions [3]. Identified Mycobacterium spp. to have occurred. Based on a phylogenetic analysis, a novel species in the Mycobacterium simiaerelated group was identified.
Most of the previous research has investigated feline ocular microflora using a classical microbiology approach involving the culture and further characterization of isolates. The aim of the present study was to examine the suitability of the methodology which may disclose microbial diversity within feline conjunctivas of healthy cats and animals with conjunctivitis symptoms, using partial sequencing of the 16S rRNA gene [4]. To the best of our knowledge, it is a frontier research in the field of veterinary ophthalmology and a preliminary study linked to our next project concerning next generation sequencing (NGS).
Materials and Methods
Conjunctival swabs obtained from three clinically healthy cats with no ocular disorders and from three cats with conjunctivitis symptoms were included in the study [5]. Based upon our own clinical experience with chronic conjunctivitis in cats and for the purpose of the study, sick animals comply with criteria such as manifestation of conjunctivitis lasting about six months and insufficient response to the standard ophthalmological treatment. Sick cats were also tested by PCR and RTPCR to determine the presence of Chlamydia felis, feline herpesvirus-1 and Mycoplasma felis infections, according to published protocols by Chalker et al., Marsilio et al., and Helps et al., whereby specific DNA was not detected [6]. Additionally, an ophthalmic examination was performed on each cat; eyelash and cartilage abnormalities and incorrect positioning of the eyelids were ruled out. Irregularities of the drainage system were eliminated with a 1% fluorescein test and by irrigation via a 26 G catheter.
Results
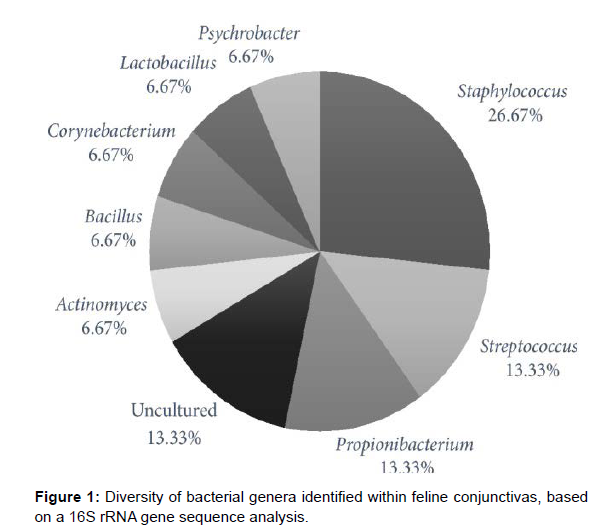
A total of 48 sequence reads were obtained in the study; only the 30 high-quality sequence reads were used in further analysis of the diversity of bacterial flora in the feline conjunctiva. Eight genera were identified among the sequences from clinically healthy and diseased animals (Figure 1). Taking into consideration the maximal 16S rRNA distance scores <1%, the following species were recognized: Bacillus subtilis, Psychrobacter faecalis, Psychrobacter pulmonis [7], Propionibacterium acnes, Staphylococcus caprae, Staphylococcus capitis, Staphylococcus succinus, Streptococcus infantarius, and Streptococcus lutetiensis. The low similarity in microflora composition at the genus level was observed between diseased and healthy conjunctivas.
Discussion
The limited capacity of culture-based methods for the identification of bacteria from the feline conjunctiva makes standard procedures incomplete [8]. This is mainly due to the limited viability of some microbial species, coinfections, or the presence of uncultivable or as yet unknown species. The monitoring of feline conjunctiva using alternative methods is not commonly applied as a standard for analyzing the diversity of conjunctival microflora in cats. DNA-based approaches were already used to assess the diversity of microbial communities or to monitor population dynamics. The analysis of bacterial taxa in conjunctival swabs by DNA sequencing provided evidence that feline conjunctiva may be settled by microorganisms not yet isolated. Our results, compared with those of culture-based studies [9], suggest that the diversity of bacterial flora within feline conjunctiva can vary more than previously believed. We found that our results based on sequence analysis methods were concordant with the culture-based analysis previously applied to the same material in terms of genera such as Bacillussp, Staphylococcus sp., and Streptococcus sp. Bacteria belonging to these genera had already been identified in cat conjunctivas [10]. A comparison of eye microflora of clinically healthy animals and those with signs of conjunctivitis indicated no qualitative differences. The results of our study revealed some species that had not been reported earlier in feline conjunctiva, including Bacillus subtilis, Staphylococcus caprae, Staphylococcus succinus, Streptococcus infantarius, Streptococcus lutetiensis, Psychrobacter faecalis, and Propionibacterium acnes.
Psychrobacter sp. belongs to the gamma Proteobacteria family and includes bacteria isolated from the skin of fish and chickens, meat products, clinical sources, and sea water [11]. In our study, bacteria from Psychrobacter taxon constituted a considerable subpopulation. Psychrobacter faecalis is a new species, isolated from pigeon feces and from human samples. In the present study, one sequence also showed a similarity to Psychrobacter pulmonis, a novel subline within the genus Psychrobacter, isolated previously from lambs and humans. Staphylococcus succinus and Staphylococcus caprae belong to the coagulase-negative staphylococci (CNS) [12]. They may colonize the skin surface and mucous membranes of mammals. To the best of our knowledge, there were no reports on the isolation of these species from cat conjunctivas. S. capraewas originally associated with goats and identified as an etiologic agent of intramammary infections. These bacteria were also detected in humans with bloodstream, urinary tract, bone, and joint infections as well as a commensal on human skin. S. succinus ubiquitously occurs in the environment, but it was also isolated from clinical samples from humans with various clinical disorders.
Lactobacillus salivarius was isolated from the gastrointestinal tract and oral cavity of hamsters and from the intestinal tract of swine and chickens. It serves as a common component of probiotic substances. Thus, its presence in cat conjunctivas can be explained by nursing cats with milk products [13].
It was shown that the 16S rRNA gene sequencing can have low strength for the discrimination of species in the genus Bacillus. In our study, the 16S rRNA gene sequence homology to Bacillus subtilis was 100%. Moreover, isolates assigned to Bacillus were previously identified in cats’ conjunctivas in our laboratory by microbiological means. Three genera belonging to Actinobacteria were found only in cats with signs of conjunctivitis. It was shown that Propionibacterium, Actinomyces, and Corynebacterium may constitute commensal and environmental bacterial flora and could be acquired by pets from human. Propionibacterium acnes are an example of a bacterium of human origin, frequently considered a commensal colonizer of human skin, one which is involved in inflamed acne breakouts. Although Corynebacterium, also referred to as diphtheroids, is considered nonpathogenic, they have been recognized as the cause of serious systemic and ocular infections. Bacteria belonging to Corynebacterium are associated with conjunctivitis, keratitis, and endophthalmitis in humans [14]. Actinomycosis in cats is typically related to the oropharyngeal, thoracic or abdominal cavity infection and associated with the migration of plant foreign bodies. Actinomyces spp. was most frequently isolated from cat pyothorax and subcutaneous wounds. Their role in feline conjunctivitis was not recognized; nevertheless, the bacteria can grow in anaerobic or facultatively anaerobic environment, which can also be found in conjunctival sacs. Concurrent or prior multiplication of facultative aerobic bacteria in tissues may also decrease the oxygen level, creating an environment supporting anaerobic bacteria growth.
Conclusion
The feline conjunctiva may be inhabited by a diverse microbial community consisting of hundreds or thousands of species, with relatively few genera predominating. Our study demonstrates that the feline conjunctival sacs are inhabited by a much more rich and diverse microbial community than could be inferred from culture-based methods. Feline conjunctivas could also be colonized with uncultivable bacteria, which limit their standard diagnostics. In this case, demonstration of such species in the diagnostic context may constitute a new area for research on the etiology of feline conjunctivitis. In our opinion, it is worth focusing on the bacteria, which could be overlooked during a standard bacteriological investigation, for example, as detected in our study, actinomycetes which require customized incubation time. Furthermore, they belong to the leading producers of substances showing biological activity, which could interfere with a selection of antibiotic-resistant strains of other bacteria. As yet, the role of actinomycetes in feline conjunctivitis has not been established, but it is clear that other standards for cultivation or examination targeted at molecular detection should be taken into account. Clinical relevance of this microbiota requires further study.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- Shewen PE, Povey RC, Wilson MR (1980) A survey of the conjunctival flora of clinically normal cats and cats with conjunctivitis. Can Vet J. 21: 231–233.
- W. Lilenbaum (1996) Prevalence of bacteria in the conjunctival sac and on the eyelid margin of clinically normal cats. J Small Anim Pract. 37: 364– 366.
- Lilenbaum W, Nunes ELC, Azeredo MAI (1998) Prevalence and antimicrobial susceptibility of staphylococci isolated from the skin surface of clinically normal cats. Lett Appl Microbiol. 27: 224–228.
- Hartmann AD, Hawley J, Werckenthin C, Lappin MR, Hartmann K (2010) Detection of bacterial and viral organisms from the conjunctiva of cats with conjunctivitis and upper respiratory tract disease. J Feline Med Surg. 12: 775–782.
- Lee-Fowler T (2014) Feline respiratory disease: What is the role of Mycoplasma species? J Feline Med Surg. 16: 563–571.
- Ploneczka-Janeczko K, Kiełbowicz Z, Bania J, Bednarek K (2011) Real-time PCR detection of Mycoplasma felis in domestic cats suffering from chronic conjunctivitis (Poland). Pol J Vet Sci. 14: 679–681.
- Low HC, Powell CC, Veir JK, Hawley JR, Lappin MR (2007) Prevalence of feline herpesvirus 1, Chlamydophila felis, and Mycoplasma spp DNA in conjunctival cells collected from cats with and without conjunctivitis. Am J Vet Res. 68: 643–648.
- Haesebrouck F, Devriese LA, van Rijssen B, Cox E (1991) Incidence and significant of isolation of Mycoplasma felis from conjunctival swabs of cats. Vet Microbiol. 26: 95–101.
- Gruffydd-Jones T, Addie D, Belak S (2009) Chlamydophila ´ felis infection ABCD guidelines on prevention and management. J. Feline Med Surg. 11: 605–609.
- Di Francesco A, Piva S, Baldelli R (2004) Prevalence of Chlamydophila felis by PCR among healthy pet cats in Italy. Microbiologica. 27:199–201.
- Sibitz C, Rudnay EC, Wabnegger L, Spergser J, Apfalter P, et al. (2011) Detection of Chlamydophila pneumoniae in cats with conjunctivitis. Vet Ophthalmol. 14: 67–74.
- Von Bomhard W, Polkinghorne A, Huat Lu Z (2003) Detection of novel chlamydiae in cats with ocular disease. Am J Vet Res. 64:1421–1428.
- Fyfe JA, McCowan C, Brien CR (2008) Molecular characterization of a novel fastidious mycobacterium causing lepromatous lesions of the skin, subcutis, cornea, and conjunctiva of cats living in Victoria, Australia. J Clin Microbiol. 46: 618–626.
- Fox JG, Beaucage CM, Murphy JC, Niemi SM (1984) Experimental Salmonella-associated conjunctivitis in cats. Can j comp med. 48: 87–91.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Adrian P (2022) The Oral and Conjunctival Microbiotas in Cats with Feline Immunodeficiency Virus Infection. Optom Open Access 7: 174.
Copyright: © 2022 Adrian P. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 3053
- [From(publication date): 0-2022 - Nov 30, 2025]
- Breakdown by view type
- HTML page views: 2530
- PDF downloads: 523