The Superiority of Endoscopic Submucosal Dissection to Endoscopic Mucosal Resection for Achieving Negative Margins in Adenocarcinomas of the Gastroesophageal Junction: A Histopathological Evaluation
Received: 04-Aug-2016 / Accepted Date: 17-Aug-2016 / Published Date: 19-Aug-2016 DOI: 10.4172/2161-0681.1000290
Abstract
Background: Due to the significant morbidity associated with esophagectomy, advanced endoscopic therapies have been developed for diagnosis and resection of gastroesophageal junctional (GEJ) adenocarcinomas.
Aim: To compare two commonly employed techniques, namely endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD), for efficacy in removing GEJ adenocarcinomas.
Methods: A retrospective review of all adenocarcinomas at the GEJ removed via EMR or ESD over a ten-year period with coexisting BE (2004-2014) was performed. Thirty-one cases met the study inclusion criteria. All cases were re-reviewed by a gastrointestinal pathologist, and a chi-square analysis of all variables including tumor stage, degree of differentiation, depth of invasion, presence of lymphovascular invasion, and margin status was performed to evaluate for statistically significant differences between EMR and ESD.
Results: ESDs were significantly more likely than EMRs to yield negative margins (64.3% vs. 35.7%; pvalue= 0.026). ESDs also produced fewer positive deep margins than EMRs; however, when the deep margin was analyzed independently, this number did not quite reach statistical significance (30.8% vs. 69.2%; p-value=0.057)
Conclusion: This study demonstrates that ESD is superior to EMR for achieving negative margins in endoscopically resected adenocarcinomas at the GEJ. ESD also allows for better margin assessment histologically by the pathologist. Further, we believe that as the number of ESDs performed increases, we will also see that ESD will be more likely to provide both deep and lateral margin negativity.
Keywords: Barrett’s esophagus; Esophageal adenocarcinoma; Endoscopic submucosal dissection; Endoscopic mucosal resection; Gastroesophageal junction
314134Introduction
Esophageal adenocarcinoma is a disease with significant morbidity and mortality. Five-year survival is reported to be less than 18% and its incidence appears to be rapidly increasing in the Western world [1-5]. Historical therapies, including esophagectomy, carry significant morbidity for patients. Recent advances in endoscopic technology have allowed for novel minimally invasive therapies to emerge. While technical expertise and reimbursement issues hinder their expansion in community practices, they are beginning to gain traction in academic centers with excellent results. Two of these therapies, endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD), have been increasingly employed at our institution and others for management of early esophageal adenocarcinoma and Barrett’s esophagus (BE) with high-grade dysplasia [6,7]. The American College of Gastroenterology (ACG), American Gastroenterological Association (AGA), American Society for Gastrointestinal Endoscopy (ASGE), and the British Society of Gastroenterology (BSGE) all recommend endoscopic therapies for high-grade dysplasia or mucosal irregularities in an attempt to spare patients the morbidity of esophagectomy [8-11]. Outcomes experienced with endoscopic therapy at this point appear to be comparable to surgery in the medium term [12-14].
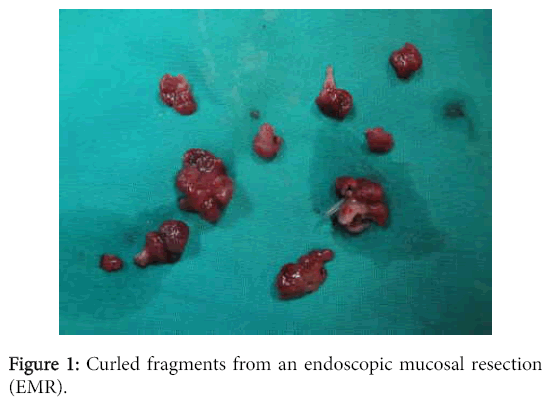
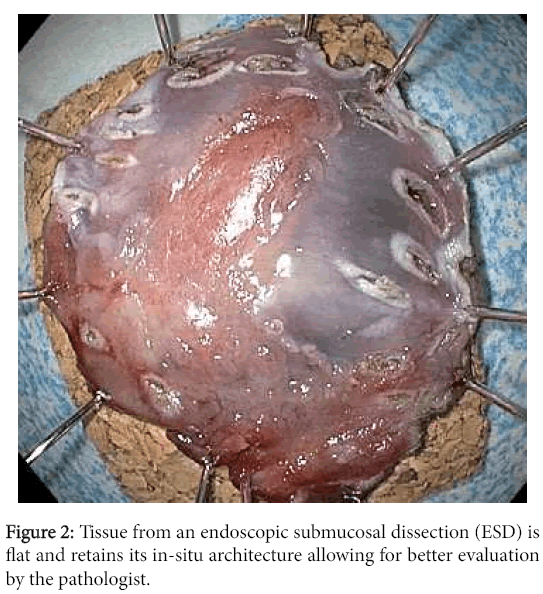
EMR involves injecting fluid into the submucosa of the target lesion, which upon lifting, may be removed via cap and snare or cap and ligation techniques. The size of the resected specimen is limited by the size of the cap and typically lesions greater than 15 millimeters can only be removed by EMR in piecemeal fashion. On the other hand, ESD allows for resection of the target lesion en-block regardless of its size. As such, ESD allows for better microscopic evaluation of the lateral margin and for completeness of the resection which cannot be achieved with piecemeal EMR (Figures 1 and 2).
Nevertheless, most gastroenterological experts believe that EMR and ESD are relatively equivalent therapies, with EMR generally being the preferred technique as it takes less time and is easier to perform [15]. Endoscopic therapies are increasingly being employed for definitive treatment of esophageal dysplasia as evidenced by the AGA recommendations: “The goal of endoscopic eradication therapy is the elimination of all Barrett’s epithelium to prevent neoplastic progression. Complete eradication appears to be more effective than therapy that removes only a localized area of dysplasia in Barrett’s epithelium” [16]. Endoscopists, however, are not only using these techniques to remove Barrett’s-related dysplasia, but also to spare patients an esophagectomy for esophageal adenocarcinoma. The aim of this study was to determine if there were any differences between EMR and ESD with regard to margin status in the treatment of early esophageal adenocarcinoma.
Methods
The study was approved by the University of Florida Institutional Review Board (IRB). We performed a retrospective review by searching our pathology database (PowerPath v 9.5, Sunquest Information Systems, Tucson, AZ) for all adenocarcinomas of the gastroesophageal junction (GEJ) removed via EMR or ESD over a tenyear period with coexisting BE (2004-2014). Thirty-four cases were initially identified. Three cases were excluded from the study as the material was no longer available. This left us with thirty-one cases available for study (18 EMR and 13 ESD). All cases were re-reviewed by a gastrointestinal pathologist, and the parameters recorded were patient age, diagnosis, margin status (including both lateral and deep margins), depth of invasion (tumor stage), and degree of differentiation. Margins were defined as positive if tumor extended to an inked surface. A chi-square analysis of all variables including tumor stage, degree of differentiation, depth of invasion, presence of lymphovascular invasion, and margin status was performed to evaluate for statistically significant differences between EMR and ESD.
Results
The study included 24 men and 7 women with an average patient age of 66 years. When compared head to head, ESDs were significantly more likely than EMRs to produce negative margins and complete tumor resection (64.3% vs. 35.7%; p-value=0.026). ESDs also yielded fewer positive deep margins than EMRs; however, when the deep margin was analyzed independently, this number did not quite reach statistical significance (30.8% vs. 69.2%; p-value=0.057). There were no statistically significant differences between EMR and ESD with respect to tumor differentiation, depth of invasion (stage), or presence of lymphovascular invasion. Tumor stage and depth of invasion also correlated with positive margin status (pT1a (23.1%), pT1b (75%), pT2 (100%); p-value=0.006). Only 25% of the cases with submucosal invasion, and no poorly-differentiated cases, yielded negative margins (Tables 1 and 2).
| Procedure | ||||
|---|---|---|---|---|
| Variable | Level | EMR | ESD | Exact Chi-square test |
| Margins (All) | Negative | 5 (35.7%) | 9 (64.3%) | 0.026 |
| Lateral Positive | 4 (100.0%) | 0 (0.0%) | ||
| Deep Positive | 1 (33.3%) | 2 (66.7%) | ||
| Lateral and Deep Positive | 8 (80.0%) | 2 (20.0%) | ||
| Margins (Deep) | Negative | 5 (35.7%) | 9 (64.3%) | 0.057 |
| Deep Margin Positive | 9 (69.2%) | 4 (30.8%) | ||
| Differentiation | Well | 2 (40.0%) | 3 (60.0%) | 0.857 |
| Moderately | 13 (61.9%) | 8 (38.1%) | ||
| Poorly | 3 (60.0%) | 2 (40.0%) | ||
| Depth of Invasion | LP/MM | 6 (46.2%) | 7 (53.8%) | 0.635 |
| SM | 11 (68.7%) | 5 (31.3%) | ||
| MP | 1 (50.0%) | 1 (50.0%) | ||
| Stage | pT1a | 6 (46.2%) | 7 (53.8%) | 0.635 |
| pT1b | 11 (68.7%) | 5 (31.3%) | ||
| pT2 | 1 (50.0%) | 1 (50.0%) | ||
| LVI | No | 15 (65.2%) | 8 (34.8%) | 0.228 |
| Yes | 3 (37.5%) | 5 (62.5%) | ||
Table 1: Comparison between EMR and ESD with respect to histopathologic variables.
| Variable | Level | Negative Margins | Positive Margins | Exact Chi-square Test |
|---|---|---|---|---|
| Differentiation | Well | 4 (80.0%) | 1 (20.0%) | 0.05 |
| Moderately | 10 (47.6%) | 11 (52.4%) | ||
| Poorly | 0 (0.0%) | 5 (100.0%) | ||
| Depth of Invasion | LP/MM | 10 (76.9%) | 3 (23.1%) | 0.006 |
| SM | 4 (25.0%) | 12 (75.0%) | ||
| MP | 0 (0.0%) | 2 (100.0%) | ||
| Stage | pT1a | 10 (76.9%) | 3 (23.1%) | 0.006 |
| pT1b | 4 (25.0%) | 12 (75.0%) | ||
| pT2 | 0 (0.0%) | 2 (100.0%) | ||
| LVI | No | 12 (52.2%) | 11 (47.8%) | 0.24 |
| Yes | 2 (25.0%) | 6 (75.0%) |
Table 2: Correlation between margin status and histopathologic variables.
Discussion
Esophageal adenocarcinoma is an aggressive malignancy with high morbidity and mortality. Historically, treatment often consisted of esophagectomy, which itself carries significant morbidity. Given the high rate of progression to adenocarcinoma in BE with high-grade dysplasia (6% per 100 person years of follow up), better screening and prevention tools have been sought for quite some time [16].
The advancement of endoscopic technology in recent years has enabled endoscopists to expand their scope of practice and provide patients with increasingly less invasive procedures for both diagnosis and therapy. EMR and ESD are two such procedures that have been developed as minimally invasive treatment approaches. EMR is performed by endoscopically injecting fluid underneath a lesion causing it to lift, and then employing suction or a snare device to excise the tissue of interest, often in piecemeal fashion. ESD is similar, except that incisional cuts are made surrounding a lesion, and then another blade is used to dissect underneath the lesion. ESD is designed to reach the submucosa, and occasionally excises to the inner circular layer of muscularis propria. As a result, it is more technically challenging and takes longer to perform [17]. The ACG, AGA, ASGE, and BSGE recommend endoscopic resection for BE with high-grade dysplasia and/or mucosal irregularities. Often, adenocarcinoma is discovered within these mucosal irregularities. Ideally, these endoscopic techniques should not only serve as diagnostic procedures, but therapeutic as well, yielding negative margins for any adenocarcinoma discovered, and potentially sparing the patient the morbidity and cost associated with radiation therapy and/or esophagectomy.
In a study of 26 esophageal adenocarcinomas resected endoscopically, Dolak-Werner et al. achieved negative margins in 64% of their cases with muscularis mucosae infiltration, but did not achieve negative margins in any case with deep submucosal extension [2]. Their study, however, did not differentiate between procedure types (EMR versus ESD) or lateral and deep margins. In a meta-analysis performed by Park et al. [18] of all esophagogastric junctional (EGJ) adenocarcinomas resected endoscopically via ESD from January 1990- March 2014, they found a complete resection rate of 87% for earlystage EGJ adenocarcinomas resected using ESD in six studies which met their inclusion criteria.
Similar to the meta-analysis by Park et al., our data suggests that ESD is superior to EMR for achieving negative margins in GEJ adenocarcinomas resected endoscopically. Only 30.8% of the ESDs in our study had margins positive for tumor, while 72.2% of the EMRs had positive margins (p=0.03). Endoscopic margins are often discussed in terms of “en bloc resection” and “complete resection.” En bloc resection means that the lateral margins are free of the lesion. Complete resection means that both the deep and lateral margins are free of the lesion. In our study, we simply defined our cases as having either positive or negative margins, designating deep and lateral.
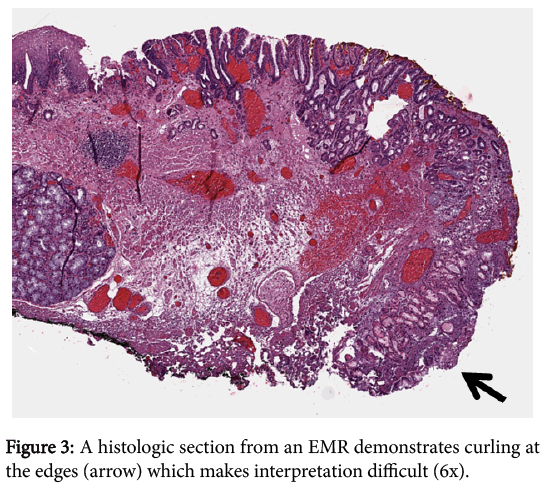
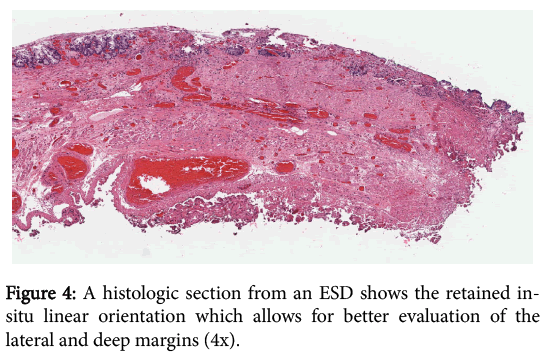
In addition to ESDs being more likely to yield negative margins than EMRs, they also allow the pathologist to reach a better margin assessment histologically. Tissue removed via the EMR technique tends to curl at the edges, which makes it difficult to determine where the lateral margin ends and the deep margin begins. This situation becomes particularly problematic in cases where tumor is present within the tissue curl itself. Tissue removed via ESD largely retains its in-situ architecture and allows for better orientation of the muscularis mucosae in order to facilitate the crucial distinction between intramucosal (T1a) and submucosal (T1b) cancer and for determining whether a positive margin is lateral or deep. The striking difference between the anatomically correct orientation achieved with ESD and the curling with disrupted orientation via EMR is illustrated (Figures 3 and 4).
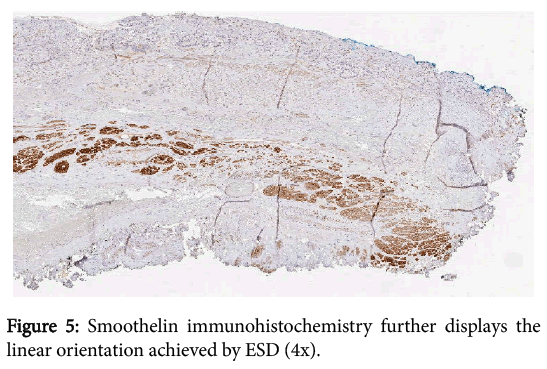
We also believe that as the number of ESDs performed increases, we will see that ESD will be more likely to yield both lateral and deep margin negativity. When we examined the deep margin independently, only 30.8% of ESDs had positive deep margins, compared to 69.2% of EMRs. Unfortunately, these numbers did not quite reach statistical significance (p=0.0569), likely due to a lack of power at the time of the study, as only 13 ESDs had been performed that could be included. Another potential limitation of our study includes possible selection bias in deciding which procedure to employ. The greater the area of mucosal irregularity seen endoscopically, the more likely ESD is to be performed. That being said, there were no statistically significant differences between EMR and ESD in our series with respect to tumor differentiation, depth of invasion (stage), or presence of lymphovascular invasion, suggesting that there was no real bias between the techniques with regard to tumor characteristics (Figure 5).
In all, our study demonstrates that ESD is superior to EMR for achieving negative margins in endoscopically resected adenocarcinomas at the GEJ. Additional studies including prospective randomized trials will be helpful in further evaluating these two procedures for efficacy and clinical utility.
References
- Dolak W, Mesteri I, Asari R, Preusser M, Tribl B et al. (2015) A pilot study of the endomicroscopic assessment of tumor extension in Barrett’s esophagus-associated neoplasia before endoscopic resection. Endoscopy International Open 02: E19-E28.
- Blot WJ, Devesa SS, Kneller RW, Fraumeni JF Jr. (1991) Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA 265: 1287-1289.
- Brown LM, Devesa SS (2002) Epidemiologic trends in esophageal and gastric cancer in the United States. SurgOncolClin N Am 11: 235-256.
- Pohl H, Welch HG (2005) The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst97: 142-146.
- Chevaux JB, Piessevaux H, Jouret-Mourin A, Yeung R, Danse E, et al. (2015) Clinical outcome in patients treated with endoscopic submucosal dissection for superficial Barrett's neoplasia. Endoscopy 47: 103-112.
- Probst A, Aust D, Märkl B, Anthuber N, Messmann H (2014) Early esophageal cancer in Europe: endoscopic treatment by endoscopic submucosal dissection. Endoscopy 47: 113-121.
- Hirota WK, Zuckerman MJ, Adler DG, Davila RE, Egan J, et al. (2006) ASGE guideline: the role of endoscopy in the surveillance of premalignant conditions of the upper GI tract. GastrointestEndosc63: 570-580.
- Fitzgerald RC, di Pietro M, Ragunath K, Ang Y, Kang JY, et al. (2014) British Society of Gastroenterology guidelines on the diagnosis and management of Barrett's oesophagus. Gut 63: 7-42.
- American Gastroenterology Association, Spechler SJ, Sharma P, Souza RF, Inadomi JM, et al. (2011) American Gastroenterological Association medical position statement on the management of Barrett's esophagus. Gastroenterology 140: 1084-1091.
- Wang KK, Sampliner RE (2008) Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett's esophagus. Am J Gastroenterology 103: 788-797.
- Prasad G, Wang KK (2005) Endoscopic mucosal resection: esophageal applications. Curr Treat Options Gastroenterol8: 41-49.
- Prasad GA, Wu TT, Wigle DA, Buttar NS, Wongkeesong LM, et al. (2009) Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett's esophagus. Gastroenterology 137: 815-823.
- Bennett C, Vakil N, Bergman J, Harrison R, Odze R, et al. (2012) Consensus statements for management of Barrett’s dysplasia and early-stage esophageal adenocarcinoma, based on a Delphi Process. Gastroenterology 143: 336-346.
- Rubenstein JH, Shaheen NJ (2015) Epidemiology, Diagnosis, and Management of Esophageal Adenocarcinoma. Gastroenterology 149: 302-317.
- Rastogi A, Puli S, El-Serag HB, Bansal A, Wani S, et al. (2008) Incidence of esophageal adenocarcinoma in patients with Barrett's esophagus and high-grade dysplasia: a meta-analysis. GastrointestEndosc67: 394-398.
- Tsou YK, Chuang WY, Liu CY, Ohata K, Lin CH, et al. (2015) Learning curve for endoscopic submucosal dissection of esophageal neoplasms. Dis Esophagus.
- Park CH, Kim EH, Kim HY, Roh YH, Lee YC (2015) Clinical outcomes of endoscopic submucosal dissection for early stage esophagogastric junction cancer: A systematic review and meta-analysis. Digestive and Liver Disease 47: 37-44.
Citation: Martelli MG, Gonzalo DH, Chang MD, Xiaomin Lu, Draganov PV, et al. (2016) The Superiority of Endoscopic Submucosal Dissection to Endoscopic Mucosal Resection for Achieving Negative Margins in Adenocarcinomas of the Gastroesophageal Junction: A Histopathological Evaluation. J Clin Exp Pathol 6:290. DOI: 10.4172/2161-0681.1000290
Copyright: © 2016 Martelli MG, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 13491
- [From(publication date): 8-2016 - Aug 30, 2025]
- Breakdown by view type
- HTML page views: 12421
- PDF downloads: 1070