To Investigate Role of Glycosylated Hemoglobin (Hba1c) as a Biomarker for Prediction of Dementia and Cognitive Dysfunction in Type 2 Diabetic Patients
Received: 11-May-2017 / Accepted Date: 04-Apr-2018 / Published Date: 11-Apr-2018 DOI: 10.4172/2161-0460.1000437
Abstract
Introduction: Diabetes Mellitus is one the major disease burden globally. One of the significant complications of the uncontrolled Diabetics is Cognitive dysfunction and Dementia. In this study we laid focus on the Evaluation of HbA1C as a Biomarker to predict Dementia and Cognitive Dysfunction in Type 2 Diabetic Mellitus. Aim of the study: A pilot study to investigate HbA1C as a Biomarker for prediction of Dementia and Cognitive Dysfunction in Type 2 Diabetic Mellitus in a Hospital Setting. Methods and results: A prevalence study in which 60 subjects (n=30 Type 2 Diabetics; n=30 non Diabetic) were enrolled. In this study HbA1C values were correlated with that of individual memory and cognition batteries* score. The range of the HbA1C values was 7.1 to 13.3. The mean values of HbA1C in the Diabetic group (n=30) was found to be 9.19.The corresponding values of Pearson’s Correlation “r” in the diabetic group of the w.r.t. various Cognitive batteries were: General Practitioner Assessment of Cognition (GPCOG)=-0.53; Attendant Informant Tool (AI)=-0.43; Memory Impairment Screen (MIS)=-0.37; Negative values of the Pearson’s Correlation “r” indicates that lower the respective battery score, poorer is the cognitive function. In the Diabetic group GPCOG, AI and MIS were found to be significantly correlated with HBA1C. Similarly, in the non- Diabetic group (n=30), no significant Dementia and Cognitive Impairment was recorded when same group of Cognitive Batteries were administered. Conclusion: It is quite evident from the results that HbA1C as biomarker has a great potential to predict Dementia and Cognitive decline in uncontrolled Diabetes. However, the study needs to be conducted on a larger scale along with comparative analysis with tools like Functional MRI and other standard biomarkers.
Keywords: Diabetes; Dementia; Alzheimers
Abbreviations
AI: Attendant Information Tool; CDT: Clock Drawing Test; GPCOG: General Practitioner Assessment of Cognition; HbA1C: Glycosylated Hemoglobin; KFT: Kidney Function Test; MIS: Memory Impairment Screen; MINI COG: Mini Cognition; MCI: Mild Cognitive Impairment; NS: Not Significant; OHA: Oral Hypoglycemic Agent; PPBS: Post Prandial Blood Sugar; RBS: Random Blood Sugar; T2DM: Type 2 Diabetes Mellitus
Introduction
Diabetes mellitus (DM) is globally one of the key disease burdens. It impacts 250 million people worldwide, with approximately 6 million new diagnosed patients every year. The prevalence of Diabetes varies for various age groups. In the people above 80years of age the prevalence is 15% as compared to 12% in population above 65-70 years [1-3].
Research suggests that poorly controlled Diabetes can impact the Cognitive functionality of the patients in a negative way [4-13]. Further, based on the literature the Alzheimer’s type Dementia is found to be linked with the uncontrolled type 2 Diabetes Mellitus [7-13].
In a population based cohort study, impaired insulin metabolism and insulin resistance was found to be linked with increased risk of developing Alzheimer’s disease [8].
Association between type 2 diabetes mellitus (T2DM) and Alzheimer’s disease (AD) common pathology is evident by virtue of various epidemiological researches and other scientific research. Researchers have proposed Type -3 Diabetes to Alzheimer’s disease based on the shared pathophysiology between the two [9].
Structural imaging of brain in Diabetic subjects reveal that cerebral anatomy is distorted which can lead to cognitive impairment. Out of these, subcortical and cortical atrophy being the important structural changes found in Diabetic subjects [4].
Interestingly, the cognitive impairment was found to be significant in those Diabetic subjects who had fairly controlled hypertension, thus indicating the poor Diabetes control as an independent risk factor for predicting Dementia and Cognitive dysfunction in Diabetes Mellitus [10].
In a study conducted by Cosway et al. [11], it was found that duration of Diabetes is significantly linked to impaired functions especially with respect to the verbal memory. In a two year follow up study on patients with early Alzheimer’s disease, Burns et al. found that that insulin resistance was linked with the cognitive dysfunction [12]. Saczynski et al. [13] assessed the cognitive impairment in Diabetic subjects and concluded that patients with Diabetes have greater tendency to develop challenges pertaining to Cognitive functioning.
Cognitive decline is more significant in the obese type 2 Diabetic patients versus the normal weight ones. A recent study clearly indicates that Diabetic subjects with poor control have poor cognitive assessment scores. In a population based Case Control study conducted on 371 patients, association between Diabetes and Early Stage Diabetes was found in male subjects.
In an Animal model study it was found that the Diabetes was associated with multiple complications including cognitive decline in the Rat brain.
In a recent review, it was found that early management of the pre diabetes may help to prevent the multiple complications of Diabetes including Cognitive decline.
In one of recent study conducted in 2017, it was found that patients suffering from Type 2 diabetes with already diagnosed mild cognitive impairment were at a greater risk to develop Dementia.
In a study conducted by Merhan et al. it was found that cognitive decline in the type 2 diabetes may be due to the oxidative stress and lower levels of antioxidant markers.
In the current study we investigated the association of Dementia and Cognitive Dysfunction and uncontrolled type 2 Diabetes using HbA1C as a biomarker.
HbA1C (Glycosylated Hemoglobin) as a biomarker to diagnosis and prognosis of the disease has been reviewed by an International Expert Committee which was set up with members appointed by the American Diabetes Association, the European Association for the Study of Diabetes and the International Diabetes Federation. Following advantages were observed by the International Expert Committee with respect to routine blood sugar testing versus HbA1C.
• HbA1C provides better index of overall glycemic exposure.
• HbA1C predicts better risk for long-term complications
• Comparatively less biologic variability.
• No empty stomach blood samples required.
• Frequency of testing every 3 months.
• Results do not vary with acute illness (e.g. stress).
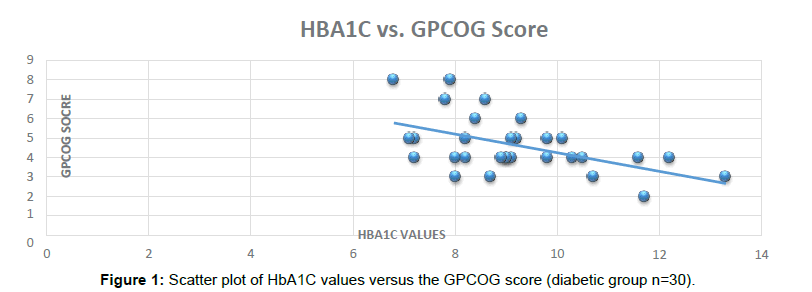
HbA1C has been used widely in India as a biomarker for Diabetic diagnosis and an indicator of diabetes control in Clinical practice. With the benefits of testing requirement every 3 months, accurate results and cost effectiveness HbA1C has become more acceptable as compared to routine investigations like Random Blood Sugar, Post Prandial Blood Sugar, Glucose Tolerance test (Figure 1).
Historically, different approaches have been used to diagnose Dementia and Cognitive decline. Cognitive assessment batteries like General Practitioner Assessment of Cognition (GPCOG), Attendant Informant Tool (AI), Memory Impairment Screen (MIS); MINI COG have been used both for Clinical practice and research. All these tools require training and in routine are not carried out by the general physicians. These tests are more common in Neurology, Psychiatry and Neuropsychology clinics. Other radiological techniques like Magnetic Resonance Imaging, Positron Emission Brain Scans are quite expensive and time consuming for initial Dementia screening.
In this study, we show that that higher values of HbA1C can be a good indicator of poor Cognitive batteries score and therefore Dementia and Cognitive decline in Type 2 Diabetic Patients. In the Indian scenario, Diabetic subjects visiting to the Physicians do get their HbA1C tested every 3 months based on their Physician’s advice. Correlation of individual Cognitive batteries scores with poor Diabetes control can be a promising and cost effective screening for Dementia (Figure 2).
Current study did reveal that there is a correlation between the Cognitive decline and the poor Diabetes control. HbA1C seems to be a great biomarker to predict Dementia and Cognitive decline in Type 2 Diabetes. Higher values of HbA1C can be linked to the Dementia and Cognitive assessment by the experts using more specific screening tools. The study needs to be conducted on a larger scale along with comparative analysis, correlation of HbA1C values with the Functional MRI assessments and other biomarkers.
Research Methodology
Patients and methods
Patients: Thirty (n=30); Type 2 Diabetics were enrolled in the study from the Out-Patient Department and hospitalized patients from Sarvodaya Hospital and Research Center, Faridabad, Delhi, NCR, India. Control group comprised of thirty (n=30) Non-Diabetic subjects as confirmed by Random Blood Sugar testing and hospital/out patient records. Ethical clearance from the University Ethics Committee and written permission from the Hospital was sought before the initiation of the study.
Age group of both Diabetic and control group was between 35- 80 years of age. Patients with Type 2 Diabetes were required to have minimum 5 years of Type 2 Diabetes history as defined by “American Diabetic Association Criteria” [14]. HbA1C (Glycosylated Hemoglobin) greater than 7 in the Diabetic group. Participants should be well versed with written and spoken either Hindi or English language.
Subjects willing to participate and signing off informed consent form in the study were only enrolled in the research project.
Stringent exclusion criteria were adopted to prevent bias. Subjects of age less than 35 years, pregnant women and Children were excluded from the study. Patients with a history of other “chronic systemic diseases, neurological conditions, psychiatric disorders, alcohol and drug abuse were not included in the study.
Methods: In both the groups, demographic details of the participants were recorded as patient information form. The patient information form included age, sex socio economic status, education, marital status, occupation, duration of diabetes and diabetes complications like retinopathy, neuropathy nephropathy, whether on OHA (Oral Hypoglycemic Agents), insulin or both, family history of diabetes and history of other diseases. Pregnancy status of the women subjects was confirmed by history. The participants with diabetes were questioned about preceding episodes of hypoglycemia. All the subjects were clinically examined to rule out any acute ailment which can interfere the study proceedings.
Once the initial screening was over, the participants were explained about the probable benefits about the study participation. Candidates willing to participate in the study were then shared English/Hindi version of the Informed Consent Forms as applicable. The study context was clearly explained to the subjects and before participation informed consent forms were duly signed off by all the participants.
Cognition measurement tools
Cognitive batteries used in the current study were adopted from “The Alzheimer’s Association” [15] (http://www.alz.org) with a written permission from the association.
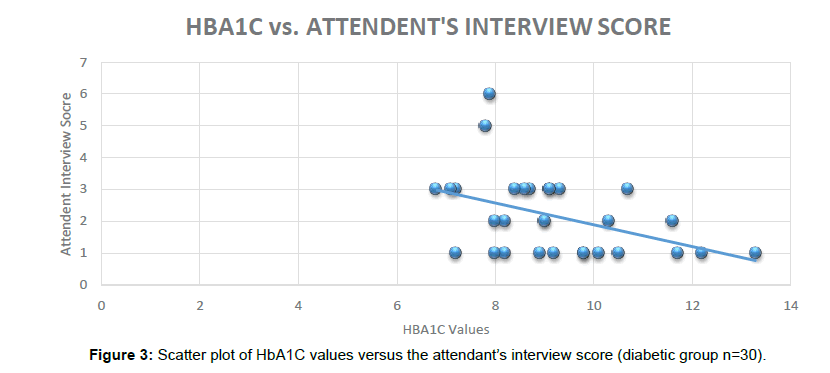
The Cognitive tools are helpful for early detection and diagnosis of dementia and have been developed by a group of clinical dementia experts. The current research was single point prevalence study as Cognitive tools were administered only one time (Figure 3).
Three validated patient assessment tools: the General Practitioner Assessment of Cognition (GPCOG) including Attendant Information tool was also used to seek details from the patient’s attendant/family members, the Memory Impairment Screen (MIS) and the Mini-Cog TM. Cognitive batteries were also translated to Hindi language along with the existing English language to enrol the patients at a faster pace.
The general practitioner assessment of cognition (GPCOG) [16]
The General Practitioner Assessment of Cognition (GPCOG) test comprised of two assessments. First step is patient assessment followed by the attendant information tool. The patient examination battery comprised of a name and address for subsequent recall test, time orientation and Clock drawing test for Visio-Spatial parameter assessment. Total score of the battery was 9 (nine). If patient scores 9, no significant cognitive impairment and further testing not necessary. In case score is 5-8, more information required for which Step 2, attendant informant assessment was performed. Scores between 0-4, cognitive impairment was indicated and subjects were advised to get further investigations done to rule out cause of Cognitive decline and possible Dementia (Table 1).
| GPCOG Score Range | No. of Patients | Battery Scoring Instructions | Cognitive Decline Status |
|---|---|---|---|
| Category 1 | |||
| Patient scores 9 | 0 | •If patient scores 9, no significant cognitive impairment and further testing not necessary. |
NA |
| Category 2 (Score) | |||
| Patient scores 5-8 | 13 | •5-8, more information required. Proceed with Step 2, informant section. |
•Administered the Informant/Attendant Interview. |
| Category 3 (Score) | |||
| Patient scores 0-4 | 17 | •If patient scores 0-4, cognitive impairment is indicated |
•Cognitive Impairment is indicated. •56.56% of the Patients were found to have Cognitive Decline when administered GPCOG test. |
Table 1: GPCOG score range vs. cognitive impairment in the diabetic group (n=30).
In the attendant information test, 6 questions (which will add to total score of 6) were asked from the patient’s relative comparing the current status with 5-10 years old scenario. To get a total score, the number of items answered ‘no’, ‘don’t know’ or ‘N/A’) were added. If patient scored 0-3, cognitive impairment is indicated and the subject was advised accordingly [16] (Table 2).
| GPCOG Score Range | No. of Patients | Battery Scoring Instructions | Cognitive Decline Status |
|---|---|---|---|
| Category (Score) | |||
| Patient scores 0-3 | 28 | •If patient scores 0-3, cognitive impairment is indicated |
•Cognitive Impairment is indicated. |
Table 2: Attendant score range vs. cognitive impairment in the diabetic group (n=30).
Memory impairment screen (MIS) [17]
In the MIS battery administration, patient was shown a sheet of paper with the 4 items to be recalled in 24-point or greater uppercase letters (on other side) and was asked to read the items aloud. Patient was told that each item belongs to a different category. A category cue was given to the patient and asked to indicate which of the words belongs in the stated category (e.g. “Which one is the game?”). Allow up to 5 attempts. Failure to complete this task indicated possible cognitive impairment. In case subject identified all 4 words, the sheet of paper was removed and patient was told to remember the words in a few minutes. Meanwhile the patient was engaged in distractor activity for 2 to 3 min, such as counting to 20 and back, counting back from 100 by 7, spelling WORLD backwards. After the distracting activity patient was asked to recall the 4 words with and without cues respectively. FREE RECALL was allotted 2 points per word and CUED RECALL-1 point per word (Table 3).
| GPCOG Score Range | No. of Patients | Battery Scoring Instructions | Cognitive Decline Status |
|---|---|---|---|
| Category 1 | |||
| Patient scores 5-8 | 1 | •If patient scores 5-8, no significant cognitive impairment. | No cognitive impairment. |
| Category 2 (Score) | |||
| Patient scores 0-4 | 29 | •If patient scores 0-4, cognitive impairment is indicated | •Cognitive Impairment is indicated. |
Table 3: MIS (memory impairment screen) vs. cognitive impairment in the diabetic group (n=30).
The maximum score for the MIS is 8.
• 5-8 No cognitive impairment
• ≤ 4 Possible cognitive impairment
Mini COGTM
Patient was provided 1 version of 6 versions of three (3) unrelated clinically validated words and was asked to remember the assigned three words. Then the subject was asked to repeat the words to ensure the learning was correct [18-21].
In the second step patient was asked to draw the face of a clock and advised to draw hands to read 10 minutes after 11:00 (or 20 min after 8:00). After this step was over, patient was asked to recall the three words from Step 1.
Scoring
In case of 3 recalled words, test was Negative for cognitive impairment. In case of 1-2 recalled words+normal CDT (Clock drawing test) test was Negative for cognitive impairment. For 1-2 recalled words+abnormal CDT and for 0 recalled words, positive for cognitive impairment (Table 4).
| MINI COG Score Range | No. of Patients | Battery Scoring Instructions | Cognitive Decline Status | ||
|---|---|---|---|---|---|
| Category 1 | |||||
| 3 recalled words | 2 | 3 recalled words - Negative for cognitive impairment | Negative for cognitive impairment | ||
| Category 2 (Score) normal CDT(Clock Drawing Test) | |||||
| 1-2 recalled words + | 4 | 1-2 recalled words + normal CDT- Negative for cognitive impairment | Negative for cognitive impairment | ||
| Category 3 (Score) | |||||
| 1-2 recalled words + abnormal CDT | 23 | 1-2 recalled words + abnormal CDT- Positive for cognitive impairment | Positive for cognitive impairment | ||
| Category 4 (Score) | |||||
| 0 recalled words | 1 | 0 recalled words - Positive for cognitive impairment | Positive for cognitive impairment | ||
Table 4: MINI COG test and cognitive impairment in the diabetic group (n = 30).
Statistical analysis
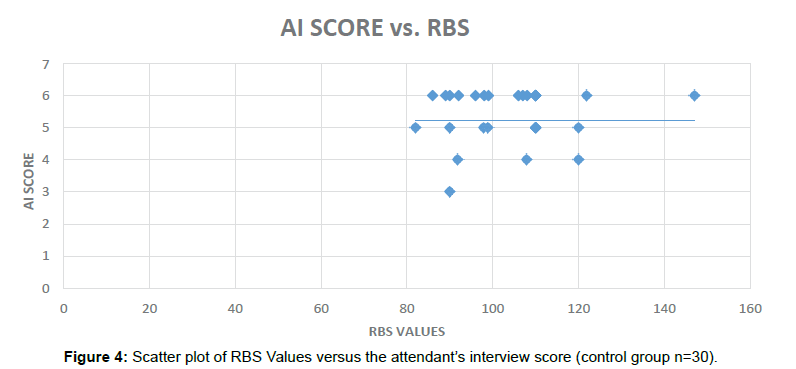
To assess associations between the Diabetes related Cognitive impairment, Pearson’s correlation coefficient (r) was calculated for various variables in the Diabetic patients and non-Diabetic subjects using IBM SPSS Statistics trial version (Figure 4).
Key focus was laid down on the parameters like duration of Diabetes, no of hypoglycemic episodes in the Diabetic group, HbA1C (Glycosylated Hemoglobin) values, age of subjects versus the individual battery score. Pearson’s correlation was calculated with the corresponding 2 tailed significance level. Similarly, the various parameters were correlated with the individual battery scores in the non-Diabetic group.
Results
In this prevalence study 60 subjects were enrolled. Out of these thirty (n=30) were type 2 Diabetic patients and rest were (n=30) non diabetics. The study was conducted in a hospital setting including both indoor and outdoor patients. In this study HbA1C values were compared with that of individual GPCOG, Attendant Interview, MIS and MINI COGTM. Mean and standard deviation of HbA1C were calculated and comparison was done with the corresponding Pearson’s Correlation values.
The mean values of HbA1C in the Diabetic group (n=30) was found to be 9.19 with a standard deviation of 1.58. The corresponding values of Pearson’s Correlation “r” in the diabetic group of the various Cognitive batteries were: GPCOG=-0.53, Attendent Interview=-0.43, MIS=-0.37, Negative values of the Pearson’s Correlation “r” indicates the negative correlation of HbA1C values with Cognitive batteries score. Lower the respective battery score, poorer is the cognitive function. Significant correlation was also found between HbA1C and duration of Diabetes, age and number of hypoglycemic episodes in the Diabetic group (Table 5a).
| HbA1C Levels | Duration of Diabetes (Years) | Ages (Years) | H/O No. of Hypoglycemic Episodes | ||
|---|---|---|---|---|---|
| GPCOG SCORE | Pearson’s Correlation | -0.520 | -0.426 | -0.563 | -0.547 |
| Significance (2-tailed) | 0.003(S) | 0.019(S) | 0.001(S) | 0.002(S) | |
| Attendant Information Score | Pearson’s Correlation | -0.429 | -0.572 | -0.565 | -0.360 |
| Significance (2- tailed) | 0.018(S) | 0.001(S) | 0.001(S) | 0.051(S) | |
| MIS Score | Pearson’s Correlation | -0.375 | -0.518 | -0.582 | -0.453 |
| Significance (2- tailed) | 0.041(S) | 0.003(S) | 0.001(S) | 0.012(S) | |
GPCOG score, Attendant Information Score and MIS Score were found to be Significant (S) 2 tailed with respect to the HbA1C levels (Diabetes control), Duration of Diabetes Mellitus in Years, Age in years and Number of Hypoglycemic Episodes.
Table 5a: Different variables, their correlation and corresponding level of significance in the diabetic group (n=30).
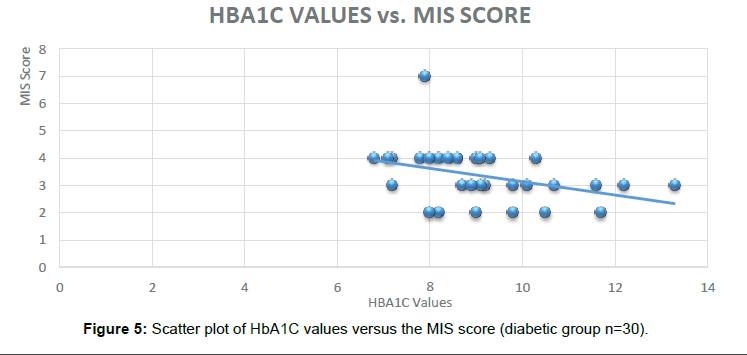
In the subjects who were administered the MINI Cog TM battery similar results were obtained indicating the HbA1C can be used as a Novel Biomarker to predict Dementia in Diabetic subjects. Out of the 30 subjects in the Diabetic group, 24 were found to be positive for the cognitive impairment (Figure 5).
In the Diabetic group, GPCOG score, Attendant Information Score and MIS Score were found to be Non-Significantly correlated 2 tailed (NS) with respect to the Weight in Kg, Kidney function test values (KFT). Duration of OHA (Oral Hypoglycemic Agents) treatment in years and Duration of OHA+Insulin Therapy in years (Table 5b).
| Weight in Kg | Kidney Function Test (Values) | OHA Treatment Duration in years | OHA+Insulin Treatment in Years | ||
|---|---|---|---|---|---|
| GPCOG SCORE | Pearson’s Correlation | -0.228 | -0.251 | 0.03 | -0.262 |
| Significance (2-tailed) | 0.225(NS) | 0.180(NS) | 0.986(NS) | 0.162(NS) | |
| Attendant Information | Pearson’s Correlation | -0.084 | -0.340 | -0.230 | -0.380 |
| Significance (2-tailed) | 0.657(NS) | 0.066(NS) | 0.904(NS) | 0.390(NS) | |
| MIS Score | Pearson’s Correlation | -0.001 | -0.354 | 0.001 | -0.342 |
| Significance (2- tailed) | 0.996(NS) | 0.06(NS) | 1.000(NS) | 0.065(NS) | |
Table 5b: Different variables, their correlation and corresponding level of significance in the diabetic group (n=30).
Similarly, in the non-Diabetic group (n=30), no significant Dementia/Cognitive Impairment was found when same group of Cognitive Batteries were administered.
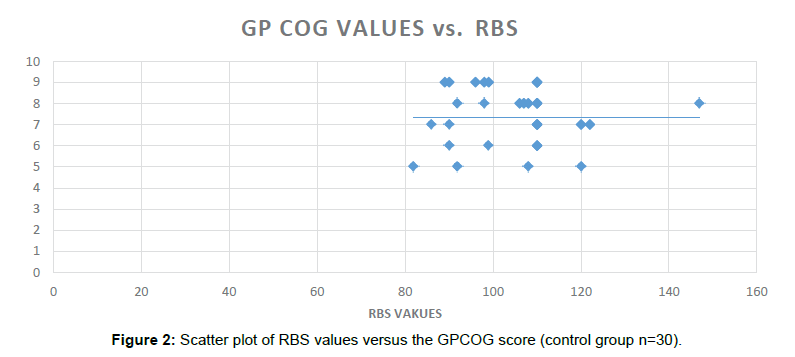
In the non-Diabetic group, GPCOG (Significance 2-tailed=0.864), AI score (Significance 2-tailed=0.520) and the MIS scores (Significance 2-tailed=0.685) were found to be non-significantly correlated with the RBS values (Table 6).
| RBS Values | ||
|---|---|---|
| GPCOG Score | Pearson’s Correlation | 0.033 |
| Significance (2-tailed) | 0.864 (NS) | |
| Attendant Information | Pearson’s Correlation | 0.122 |
| Significance (2-tailed) | 0.520 (NS) | |
| MIS Score | Pearson’s Correlation | 0.077 |
| Significance (2-tailed) | 0.685 (NS) | |
Table 6: RBS (random blood sugar) values, their correlation and corresponding level of significance in the non-diabetic group (n=30).
Discussion
Various studies have been provided evidence between the association of Diabetes Mellitus and Cognitive Impairment across the globe under different geographical conditions [21-35]. Studies suggest that out of multiple domains of the Cognitive Impairment, only limited are adversely impacted [21-35]. Type 2 Diabetes Mellitus is associated with faster brain aging [36,37].
Diabetes mellitus (DM) is globally one of the key disease burdens. It impacts 250 million people worldwide, with approximately 6 million new diagnosed patients every year. The prevalence of Diabetes varies for various age groups. In the people above 80years of age the prevalence is 15% as compared to 12% in population above 65-70 years.
Limited observational studies have been conducted in the North India in which Cognitive Impairment and Diabetes Mellitus have been investigated to bear a correlation. The current pilot study was conducted in the Delhi National Capital Region of North India to Determine Prevalence of Dementia and Cognitive Dysfunction in Type 2 Diabetic Subjects versus Non-Diabetic Control Group. The results are indicative of correlation between the Diabetes Mellitus and Cognitive decline. Clearly duration of Diabetes, poorly controlled Diabetes and number of hypoglycemic episodes were found to be strongly correlated with the Cognitive decline [38-40].
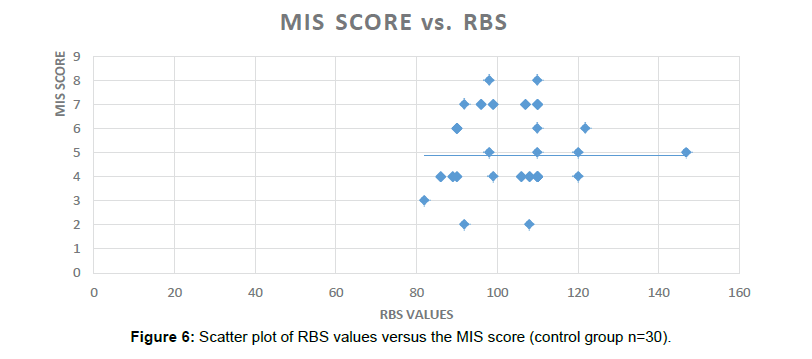
The current study highlights the usage of already existing Biomarkers like HbA1C in predicting/diagnosing the newer indication. HbA1C has been used to define the control of Diabetes Mellitus and we proposed the same for the predicting the Cognitive and Memory decline in this pilot study. In this study the HbA1C levels were found to be significantly correlated with the scores of different batteries: GPCOG (Significance 2-tailed=0.003), AI score (Significance 2-tailed=0.018) and the MIS scores (Significance 2-tailed=0.041) in the Diabetic group. Significant correlation was also found between HbA1C and duration of Diabetes, age and number of hypoglycemic episodes in the Diabetic group (Figure 6).
In the subjects who were administered the MINI Cog TM battery similar results were obtained indicating the HbA1C can be used as a Novel Biomarker to predict Dementia in Diabetic subjects. Out of the 30 subjects in the Diabetic group, 24 were found to be positive for the cognitive impairment. In the non-Diabetic group, GPCOG (Significance 2-tailed=0.864), AI score (Significance 2-tailed=0.520) and the MIS scores (Significance 2-tailed=0.685) were found to be nonsignificantly correlated with the RBS values.
HbA1C has been used widely in India as a biomarker for Diabetic diagnosis and an indicator of diabetes control in Clinical practice. With the benefits of testing requirement every 3 months, accurate results and cost effectiveness HbA1C has become more acceptable as compared to routine investigations like Random Blood Sugar, Post Prandial Blood Sugar, Glucose Tolerance test [40-47].
In the current study it was found that other parameters like duration of Diabetes, age of the subjects and number of hypoglycemia episodes, were also significantly correlated with the poor cognitive function and memory loss. This adds to the study limitation and asks for much larger study within the diabetic group to further evaluation of the Clinical application of HbA1C biomarker to predict Dementia and Cognitive decline.
On the basis of the significant results of this study HbA1C as a biomarker seem to have great clinical application to predict Dementia and Cognitive decline in Diabetic subjects. This can provide a platform for further investigating the poor Diabetes control and its correlation with the Cognitive decline and Dementia.
Conclusion and Perspective
The current pilot study evaluates the role of HbA1C as a biomarker to predict Dementia and Cognitive decline in Diabetic subjects. In the current study HbA1C was found to be significantly correlated with the multiple batteries used for Cognition and Memory screening. Saying that other parameters like duration of Diabetes, age of the subjects and history of hypoglycemia episodes were also significantly correlated with respect to cognitive decline.
Going forward, larger group studies are required focusing on the comparison between uncontrolled Diabetes with and without hypoglycemic episodes [48].
Further, Imaging techniques also like structural and functional MRI also possess a significant potential in the context of screening and diagnosis of various memory impairment disorders. Functional MRI evaluates the blood flow response and compares that with the brain activity. It was found that age did not impact the hemodynamic blood flow making Functional MRI an important radiological tool to diagnose disease related Dementia [49].
A team of Radiologists and Scientists from Harvard Medical School and universities reviewed multiple Neuroimaging markers for the diagnosis of Dementia of Alzheimer’s type. Team found that fMRI taskrelated abnormal increase in hippocampal in the preclinical and early stage of MCI (Mild Cognitive Decline) followed by decreased activity as the disease progress [50].
Functional MRI can be one of the key diagnostic tool to further strengthen the current study findings. We also stress upon the study to be conducted on a much larger population to get more significant results.
References
- Cole AR, Astell A, Green C, Sutherland C (2007) Molecular connexions between dementia and diabetes. Neurosci Biobehav Rev 31: 1046-1063.
- Wild S, Roglic G, Green A, Sicree R, King H (2004) Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 27: 1047-1053.
- Zhao Y, Ye W, Boye KS, Holcombe JH, Hall JA, et al. (2008) Prevalence of other diabetes-associated complications and comorbidities and its impact on health care charges among patients with diabetic neuropathy. J Diabetes Complications 24: 9-19.
- Thomas VS, Darvesh S, MacKnight C, Rockwood K (2001) Estimating the prevalence of dementia in elderly people: A comparison of the Canadian Study of Health and Aging and National Population Health Survey approaches. Int Psychogeriatr 13: 169-175.
- Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, et al. (1999) Diabetes mellitus and the risk of dementia: The Rotterdam study. Neurology 53: 1937-1942.
- Biessels GJ, Deary IJ, Ryan CM (2008) Cognition and diabetes: A lifespan perspective. Lancet Neurol 7: 184-190.
- Xu W, Qiu C, Gatz M, Pedersen NL, Johansson B, et al. (2009) Mid- and late-life diabetes in relation to the risk of dementia: A population-based twin study. Diabetes 58: 71-77.
- Schrijvers EM, Witteman JC, Sijbrands EJ, Hofman A, Koudstaal PJ, et al. (2010) Insulin metabolism and the risk of Alzheimer disease: The Rotterdam study. Neurology 75: 1982-1987.
- Akter K, Lanza EA, Martin SA, Myronyuk N, Rua M, et al. (2011) Diabetes mellitus and Alzheimer’s disease: Shared pathology and treatment. Br J ClinPharmacol 71: 365-376.
- Roriz-Filho SJ, Sá-Roriz TM, Rosset I, Camozzato AL, Santos AC, et al. (2009) (Pre)diabetes, brain aging and cognition. Biochimica et Biophysica Acta 1792: 432-443.
- Cosway R, Strachan MW, Dougall A, Frier BM, Deary IJ (2001) Cognitive function and information processing in type 2 diabetes. Diabet Med 18: 803-810.
- Burns JM, Honea RA, Vidoni ED, Hutfles LJ, Brooks WM, et al. (2012) Insulin is differentially related to cognitive decline and atrophy in Alzheimer’s disease and aging. Biochim Biophys Acta 1822: 333-339.
- Saczynski JS, Jónsdóttir MK, Garcia ME, Jonsson PV, Peila R, et al. (2008) Cognitive impairment: An increasingly important complication of type 2 diabetes: The age, gene/environment susceptibility--Reykjavik study. Am J Epidemiol 168: 1132-1139.
- http://www.dementiaresearch.org.au/dcrc-assessment-and-better-care.html
- Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A (2000) The mini-cog: A cognitive “vital signs†measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry 15: 1021-1027.
- Borson S, Scanlan JM, Chen P, Ganguli M (2003) The mini-Cog as a screen for dementia: Validation in a population- based sample. J Am Geriatr Soc 51: 1451-1454.
- McCarten JR, Anderson P, Kuskowski MA, McPherson SE, Borson S, et al. (2012) Finding dementia in primary care: The results of a clinical demonstration project. J Am Geritr Soc 60: 210-217.
- Brands AM, Biessels GJ, De Haan EH, Kappelle LJ, Kessels RP (2005) The effects of type 1 diabetes on cognitive performance: A meta-analysis. Diabetes Care 28: 726-735.
- Awad N, Gagnon M, Messier C (2004) The relationship between impaired glucose tolerance, type 2 diabetes and cognitive function. J Clin Exp Neuropsychol 26: 1044-1080.
- Stewart R, Liolitsa D (1999) Type 2 diabetes mellitus, cognitive impairment and dementia. Diabet Med 16: 93-112.
- Strachan MW, Deary IJ, Ewing FME, Frier BM (1997) Is type II diabetes associated with an increased risk of cognitive dysfunction? A critical review of published studies. Diabetes Care 20: 438-445.
- Allen KV, Frier BM, Strachan MW (2004) The relationship between type 2 diabetes and cognitive dysfunction: Longitudinal studies and their methodological limitations. Eur J Pharmacol 490: 169-175.
- Hassing LB, Hofer SM, Nilsson SE, Berg S, Pedersen NL, et al. (2004) Comorbid type 2 diabetes mellitus and hypertension exacerbates cognitive decline: Evidence from a longitudinal study. Age Ageing 33: 355-361.
- Kanaya AM, Barrett-Connor E, Gildengorin G, Yaffe K (2004) Change in cognitive function by glucose tolerance status in older adults: A 4-year prospective study of the Rancho Bernardo study cohort. Arch Intern Med 164: 1327-1333.
- Nielson KA, Nolan JH, Berchtold NC, Sandman CA, Mulnard RA, et al. (1996) Apolipoprotein-E genotyping of diabetic dementia patients: Is diabetes rare in Alzheimer’s disease? J Am Geriatr Soc 44: 897-904.
- Bucht G, Adolfsson R, Lithner F, Winblad B (1983) Changes in blood glucose and insulin secretion in patients with senile dementia of Alzheimer type. Acta Med Scand 213: 387-392.
- Wolf-Klein GP, Siverstone FA, Brod MS, Levy A, Foley CJ, et al. (1988) Are Alzheimer patients healthier? J Am Geriatr Soc 36: 219-224.
- Janson J, Laedtke T, Parisi JE, O'Brien P, Petersen RC, et al. (2004) Increased risk of type 2 diabetes in Alzheimer disease. Diabetes 53: 474-481.
- Watson GS, Craft S (2004) Modulation of memory by insulin and glucose: Neuropsychological observations in Alzheimer’s disease. Eur J Pharmacol 490: 97-113.
- Zimmet P, Alberti KG, Shaw J (2001) Global and societal implications of the diabetes epidemic. Nature 414: 782-787.
- Gispen WH, Biessels GJ (2000) Cognition and synaptic plasticity in diabetes mellitus. Trends Neurosci 23: 542-549.
- Biessels GJ, Kamal A, Ramakers GM, Urban IJ, Spruijt BM, et al. (1996) Place learning and hippocampal synaptic plasticity in streptozotocin-induced diabetic rats. Diabetes 45: 1259-1266.
- Brownlee M (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813-820.
- Kent S (1976) Is diabetes a form of accelerated aging? Geriatrics 31: 149-151.
- Perlmuter LC, Hakami MK, Hodgson-Harrington C, Ginsberg J, Katz J, et al. (1984) Decreased cognitive function in aging non-insulin-dependent diabetic patients. Am J Med 77: 1043-1048.
- Elias PK, Wilson PW, Elias MF, Silbershatz H, D'Agostino RB, et al. (1997) NIDDM and blood pressure as risk factors for poor cognitive performance. Diabetes Care 20: 1388-1395.
- Robertson-Tchabo EA, Arenberg D, Tobin JD, Plotz JB (1986) A longitudinal study of cognitive performance in noninsulin dependent (type II) diabetic men. Exp Gerontol 21: 459-467.
- Croxson SC, Jagger C (1995) Diabetes and cognitive impairment: A community-based study of elderly subjects. Age Ageing 24: 421-424.
- Yoon S, Cho H, Kim J, Lee DW, Kim GH, et al. (2017) Brain changes in overweight/obese and normal weight adults with type 2 diabetes mellitus. Diabetologia 60: 1207-1217.
- Palta P, Carlson MC, Crum RM, Colantuoni E, Sharrett AR, et al. (2017) Diabetes and cognitive decline in older adults: The Ginkgo Evaluation of Memory Study. J Gerontol A Biol Sci Med Sci 73: 123-130.
- Kadohara K, Sato I, Kawakami K (2017) Diabetes mellitus and risk of early-onset Alzheimer's disease: A population-based case-control study. Eur J Neurol 24: 944-949.
- Chomova M, Balazova M, Muchova J (2017) Diabetes induced abnormalities of mitochondrial function in rat brain cortex: The effect of n3 fatty acid diet. Mol Cell Biochem 435: 109-131.
- Eberhardt O, Topka H (2017) Neurological outcomes of anti-diabetic therapy: What the neurologist should know. Clin Neurol Neurosurg 158: 60-66.
- Ciudin A, Espinosa A, Simó-Servat O, Ruiz A, Alegret M, et al. (2017) Type 2 diabetes is an independent risk factor for dementia conversion in patients with mild cognitive impairment. J Diabetes Complications 31: 1272-1274.
- Ragy MM, Kamal NN (2017) Linking senile dementia to type 2 diabetes: Role of oxidative stress markers, C reactive protein and tumor necrosis factor-α. Neurol Res 39: 587-595.
- The International Expert Committee (2009) International Expert Committee Report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 32: 1327-1334.
- Grinband J, Steffener J, Razlighi QR, Stern Y (2017) BOLD neurovascular coupling does not change significantly with normal aging. Hum Brain Mapp.
- Ewers M, Sperling RA, Klunk WE, Weiner MW, Hampel H (2011) Neuroimaging markers for the prediction and early diagnosis of Alzheimer’s disease dementia. Trends Neurosci 34: 430-432.
Citation: Gupta A, Singh A, Deka RC, Gupta R, Jha R (2018) To Investigate Role of Glycosylated Hemoglobin (Hba1c) as a Biomarker for Prediction of Dementia and Cognitive Dysfunction in Type 2 Diabetic Patients. J Alzheimers Dis Parkinsonism 8: 437. DOI: 10.4172/2161-0460.1000437
Copyright: © 2018 Gupta A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 7666
- [From(publication date): 0-2018 - Dec 15, 2025]
- Breakdown by view type
- HTML page views: 6626
- PDF downloads: 1040