Toxicological Evaluation of the Median Lethal Concentration (LC50) of Aqueous Extract of Adenium obesum Stem Bark in African Catfish, Clarias gariepinus (Burchell 1822) Juveniles
Received: 01-Mar-2019 / Accepted Date: 03-May-2019 / Published Date: 13-May-2019 DOI: 10.4172/2476-2067.1000140
Abstract
The study evaluate the acute toxicity of aqueous extract of Adenium obesum stem bark to determine the median lethal concentration (LC50) in exposed Clarias gariepinus under laboratory conditions using static non-renewal bioassays over a 96-h exposure with continuous aeration. The fish (N=180, mean weight and length 21.48 ± 3.32 g and 11.37 ± 1.23 cm), were randomly distributed 10 (ten) fish per group in triplicates. There were six (6) experimental groups G1 (Control), G2 (6.5 mg/l), G3 (7.8 mg/l), G4 (8.5 mg/l), G5 (9.5 mg/l) and G6 (11.5 mg/l). Exposed fish showed clinical signs of changed behaviors with adaptive responses, respiratory distress and nervous compromise, including mortality in some of the exposed fish. The clinical signs observed and their severity was concentration and exposure period-dependent. The LC 50 value of 8.38 mg/l was established for the extract in the exposed fish where mean mortality was significantly (p<0.05) concentration and exposure period-dependent. The phytochemical constituents and LC 50 of aqueous extract Adenium obesum stem bark evaluated will assist toxicologists and aquatic researchers in determining the safety concentrations of Adenium obesum in exposed Clarias gariepinus juveniles and aquatic studies.
Keywords: Adenium obesum; Clarias gariepinus ; Toxicity; Median Lethal Concentration; Toxicology
Introduction
Adenium obesum (Forssk) Roem and Schult with synonyms: Adenium somalense Balf. f (1888); Adenium socotranum Vierh, (1904); Adenium arabicum Balf. f; Adenium coetaneum Stapf; Adenium honghel A. DC, Nerium obesum Forssk, belongs to the Family Apocynaceae [1-3]. The plant was discovered and described in Kenya in 1752 by a German Scientist, P. Forsskal. Adenium is an Arabic name of the plant, Oddaejn, which means Aden, which is the former name of Yemen [1] while obesum was derived from the swelling of the basal part of the plant stem [4] displayed in Figure 1.
However, A. obesum is known locally in Nigeria as “Kariya ” amongst the Hausa ethnic group [5,6] just as it is also called “Akpalataa” amongst the Igbo ethnic group of south-eastern zone of the country.
Aqueous extract of Adenium obesum stem bark have been reported to have effect against some bacteria such as Escherichia coli, Proteus mirabilis, Pseudomonas aeruginosa, Staphylococcus aureus [6].
Phytochemical constituents of Adenium obesum
Several cardiac glycosides have been reported in A. obesum [7]. The main cardiac glycoside in the plant is Oleandrigenin β-gentiobiosyl (1 →4) β-D- thevetoside [8,9]. In addition, Oleandrigenin-β-D-glucosyl (1 →4)-β-D- digitalose was isolated from the chloroform fraction of the plant [10]. Hoffman and Cole reported the presence of other active cardenolides (Somalin, hongheloside A, 16-acetylstrospeside and honghelin) and an active flavonol (3, 3-bis [o-methyl] quercetin) from the ethanol extract of A. obesum. Ethanol extract of A. obesum have been reported to contain an inactive triterpene (dihydroifflaionic acid) and an inactive flavonol 38 (3-0-methylkaempferol). The methanol extract of A. obesum stem bark has been reported to contain some alkaloids, flavonoids, saponins, tanins, glycosides, anthroquinones and steroids [11]. However, only saponins, tannins, steroids and glycosides were reported from the petroleum spirit extract of Adenium obesum stem bark [12]. Similarly, a triterpenoid named botulin (Lup-20 (29)- ene-3, 28-diol) was reportedly isolated from the stem bark of the plant [12]. Studies has shown the potential of Adenium obesum as a biological reducing agent and capping agent for the synthesis of Silver Nano particles
Adult Clarias gariepinus showed various signs of toxicity ranging from uncoordinated movements, repeated attempts to jump out of reconstituted extracts and excessive mucous secretions to increased opercula movements, exposed snouts, adoption of different postures and sudden darts when exposed to the ethanolic extract of Adenium obesum stem bark [13].
This study investigates the toxic effect of aqueous extract of Adenium obesum stem bark on Clarias gariepinus juveniles by determination of 96-hour LC 50 value using probit analysis in SPSS version 20.
Materials and Methods
Plant collection
The Adenium obesum stem bark was collected from Bassawa area within Zaria, Kaduna State Nigeria around November-December, 2016, and authenticated at the Herbarium section of the Department of Biological Sciences, A.B.U, Zaria, where a specimen was deposited and a voucher number 01386 was assigned. The leaves was picked and dried under shade until constant weight was obtained. The dried leaves were crushed into coarse powder using a pestle and mortar and stored for the extraction process.
Plant extraction
The stem bark of Adenium obesum was dried under shade until constant weight is obtained, stem bark were crushed into coarse powder using a pestle and mortar and stored for the extraction process. The fine powder was added into distilled water and shaken gently for ten minutes using a shaker to make a homogenous mixture. The mixture was left for 24 hours and then filtered. The filtrate was used for the study; the extraction process was carried out as described by Saravanan [14].
Preliminary phytochemical screenings was conducted on the aqueous extract of Adenium obesum stem bark in order to confirm the presence of phytochemical constituents following the methods described by [15,16].
Experimental animals
An ethical clearance approval was given by the Ahmadu Bello University Committee on Animal Use and Care (ABUCAUC) with approval number ABUCAUC/2017/014 for this study.
The live juvenile of the African catfish, C. gariepinus (N=180, average weight and weight of (21.48 ± 3.32) g and length of (11.37 ± 1.23) cm respectively were purchased from a commercial catfish farm of reputable standing and authenticated at the Fishery Section, Department of Biological Sciences, A.B.U., Zaria, Nigeria. Fish acclimatization lasted for 21 days under natural day and night photoperiods (12/12-h) with complete changing of pond water once in every three days. The fish were fed to their satisfaction (ad libitum) twice daily with 2 mm Coppens® fish feed for aquaculture (Coppens® International by Helmond, Holland). A range finding test to determine the five extract concentrations as described by Fafioye et al. [17] was performed. Mortality was used as an end point of toxicity and this was determined as described in the OECD guideline No. 423.
Toxicity bio-assay
After acclimatization experimental fishes were selected at random and were kept in a static system of water. The feeding was stopped one day prior to exposure to aqueous extract Adenium obesum stem bark and fishes were not fed throughout the test. The acute toxicity tests were performed according to the static nonrenewable bioassay procedure [18]. The experimental design consisted of a control and five concentrations (6.5, 7.8, 8.5, 9.5, and 11.5 mg/l) with 10 (ten) fish per group in triplicate. A glass aquarium of 40 litres capacity with aeration was used per each group in replicate. Each glass aquarium was covered with nylon mesh tied firmly with rubber strap to prevent the fish from jumping out. Fishes showing no respiratory movement and response to tactile stimuli were considered as dead and removed immediately. During the exposure in different concentrations of aqueous extract Adenium obesum stem bark, the behavioral changes of the fishes were also recorded. Daily physicochemical characteristics, temperature and pH of fish culture water were ascertained using a Hanna ‘‘Combo portable hand instrument (Hi 98129, Hanna Instrument, Mauritius) while their dissolved oxygen contents were similarly established using the modified Winkler-Azide method [19,20].
Statistical analyses
Data was expressed as mean ± SEM and then subjected to Two-way Analysis of Variance (ANOVA) for statistical significance at p<0.05. Tukey’s multiple comparison tests for means was used to compare differences between the various means using SPSS version 20.
Results
The extraction process of 3 kg Adenium obesum stem bark yielded a total of 207.53 g, Adenium obesum stem bark aqueous extract and an extractive yield of 6.91% w/w was obtained. The qualitative constituents of aqueous extract of Adenium obesum stem Bark is presented in Table 1.
| Constituents | Test | Qualitative analysis |
|---|---|---|
| Carbohydrates | Molisch test | + |
| Anthraquinones | Borntrager test | - |
| Glycosides | Fehling test | + |
| Cardiac glycosides | Kellar-Killant test | + |
| Saponins | Frothing test | + |
| Steroids and | Lieberman-Burchard | + |
| Triterpenes | ||
| Tanins | Ferric chloride test | + |
| Flavonoids | Shinoda test | + |
| Alkaloids | Dragendorff test | + |
Table 1: Phytochemical constituents of aqueous extract of Adenium obesum stem bark.
The physico-chemical parameters across the groups was nonsignificant (p>0.05) for oxygen and temperature, while there was a nonsignificant (p>0.05) increase between the control and the other groups for pH, there was a significant (p<0.05) increase in the total dissolved solids (TDS) and electric conductivity (μs/cm) is presented in Table 2.
| Group | PH | TEMP (°C) | DO (mg/l) | TDS (mg/l) | Electric Conductivity (µs/cm) |
|---|---|---|---|---|---|
| 1 | 7.01 ± 0.01a | 27.01 ± 0.01a | 4.87 ± 0.01a | 3.37 ± 0.01a | 35.02 ± 0.12a |
| 2 | 8.00 ± 1.01b | 27.02 ± 0.01a | 4.88 ± 0.03a | 46.01 ± 3.01b | 124.22 ± 1.42b |
| 3 | 8.01 ± 0.11b | 27.00 ± 0.00a | 4.79 ± 0.12a | 53.02 ± 3.22c | 231.10 ± 2.67c |
| 4 | 8.02 ± 0.01b | 27.00 ± 0.00a | 4.89 ± 0.11a | 77.13 ± 6.13d | 354.22 ± 4.11d |
| 5 | 8.07 ± 0.02b | 27.00 ± 0.00a | 4.91 ± 0.20a | 86.05 ± 7.55e | 397.11 ± 4.87e |
| 6 | 8.13 ± 0.01b | 27.01 ± 0.02a | 4.88 ± 0.03a | 107.02 ± 9.65f | 499.81 ± 6.31f |
Table 2: Physicochemical parameters of the different concentrations for acute toxicity test.
The behavioral display of the exposed fish is presented in Table 3.
| Variables | Extract | Exposure period | |||
|---|---|---|---|---|---|
| Clinical signs | (mg/l) | 24-h | 48-h | 72-h | 96-h |
| Exposed snout | G1 | - | - | - | - |
| G2 | - | + | ± | + | |
| G3 | - | + | ± | + | |
| G4 | - | + | ± | + | |
| G5 | - | + | - | + | |
| G6 | - | + | - | + | |
| Motionless | G1 | - | - | - | - |
| G2 | - | + | + | + | |
| G3 | - | + | + | + | |
| G4 | - | + | + | +++ | |
| G5 | - | + | + | +++ | |
| G6 | - | + | ++ | +++ | |
| Different postures (Vertical, Angular, Flat) | G1 | - | - | - | - |
| G2 | - | + | + | + | |
| G3 | - | + | + | ++ | |
| G4 | - | + | ++ | +++ | |
| G5 | - | + | ++ | +++ | |
| G6 | - | ++ | ++ | +++ | |
| Sudden dart | G1 | - | - | - | - |
| G2 | - | + | + | + | |
| G3 | - | + | + | ++ | |
| G4 | - | + | ++ | +++ | |
| G5 | - | + | ++ | +++ | |
| G6 | - | ++ | ++ | +++ | |
| Swirling and/or sluggish movements | G1 | - | - | - | - |
| G2 | - | + | + | + | |
| G3 | - | + | + | ++ | |
| G4 | - | + | ++ | ++ | |
| G5 | - | + | ++ | +++ | |
| G6 | - | ++ | ++ | +++ | |
| Loss of balance | G1 | - | - | - | - |
| G2 | + | + | + | + | |
| G3 | + | + | + | ++ | |
| G4 | + | + | ++ | +++ | |
| G5 | + | + | ++ | +++ | |
| G6 | ++ | ++ | ++ | +++ | |
Table 3: Behavioral display of exposed Clarias gariepinus (24 h – 96 h).
The analysis showing probit mortality responses and concentration is presented in Table 4.
| Group | Extract Conc (mg/l) | Log Conc | Number of Subjects | Observed Responses | Expected Responses | Residual | Probability |
|---|---|---|---|---|---|---|---|
| 1 | 0 | 0 | 30 | 0 | 0 | 0 | 0 |
| 2 | 6.5 | .813 | 30 | 10 | 9.813 | .187 | .327 |
| 3 | 7.8 | .892 | 30 | 14 | 15.601 | -1.601 | .520 |
| 4 | 8.5 | .929 | 30 | 22 | 19.478 | 2.522 | .649 |
| 5 | 9.5 | .978 | 30 | 23 | 24.225 | -1.225 | .808 |
| 6 | 11.5 | 1.061 | 30 | 29 | 28.865 | .135 | .962 |
Table 4: The mortality of Clarias gariepinus at 96 h after exposure to different concentrations of aqueous extract Adenium obesum stems bark.
The LC 50 value of 8.38 mg/l with a lower confidence limited (LCL) of 5.218 mg/l and an upper confidence limit (UCL) of 9.374 mg/l with 95% confidence limited determined by SPSS version 20 is presented in Table 5.
| Probability | 95% Confidence limits for concentration | 95% Confidence Limits for log (Concentration)a | ||||
|---|---|---|---|---|---|---|
| Estimate | Decrease Bound | Upper Bound | Estimate | Decrease Bound | Upper Bound | |
| LC1 | 5.355 | 1.183 | 7.035 | 0.729 | 0.073 | 0.847 |
| LC2 | 5.644 | 1.411 | 7.262 | 0.752 | 0.149 | 0.861 |
| LC3 | 5.836 | 1.577 | 7.411 | 0.766 | 0.198 | 0.870 |
| LC4 | 5.984 | 1.714 | 7.526 | 0.777 | 0.234 | 0.877 |
| LC5 | 6.108 | 1.835 | 7.620 | 0.786 | 0.264 | 0.882 |
| LC6 | 6.215 | 1.945 | 7.702 | 0.793 | 0.289 | 0.887 |
| LC7 | 6.310 | 2.046 | 7.775 | 0.800 | 0.311 | 0.891 |
| LC8 | 6.397 | 2.141 | 7.841 | 0.806 | 0.331 | 0.894 |
| LC9 | 6.477 | 2.231 | 7.901 | 0.811 | 0.349 | 0.898 |
| LC10 | 6.551 | 2.317 | 7.957 | 0.816 | 0.365 | 0.901 |
| LC15 | 6.868 | 2.711 | 8.196 | 0.837 | 0.433 | 0.914 |
| LC20 | 7.131 | 3.071 | 8.394 | 0.853 | 0.487 | 0.924 |
| LC25 | 7.365 | 3.415 | 8.371 | 0.867 | 0.533 | 0.933 |
| LC30 | 7.581 | 3.757 | 8.735 | 0.880 | 0.575 | 0.941 |
| LC35 | 7.788 | 4.102 | 8.893 | 0.891 | 0.613 | 0.949 |
| LC40 | 7.988 | 4.457 | 9.050 | 0.902 | 0.649 | 0.957 |
| LC45 | 8.188 | 4.827 | 9.209 | 0.913 | 0.684 | 0.964 |
| LC50 | 8.388 | 5.218 | 9.374 | 0.924 | 0.718 | 0.972 |
| LC55 | 8.394 | 5.636 | 9.50 | 0.934 | 0.751 | 0.980 |
| LC60 | 8.809 | 6.088 | 9.743 | 0.945 | 0.784 | 0.989 |
| LC65 | 9.036 | 6.581 | 9.966 | 0.956 | 0.818 | 0.999 |
Table 5: Probit analysis aqueous extract of Adenium obesum stem bark.
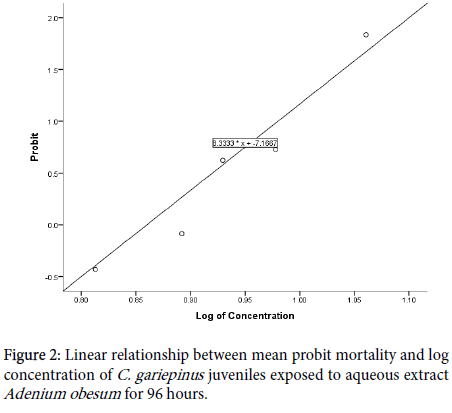
The relationship between the probit mortality to logarithm of concentration is presented in Figure 2.
Discussion
Acute bioassay of toxicants/botanicals is an important procedure in aquatic ecotoxicology and toxicopathological fields. The aim of such study is to determine and establish the lethal concentration of toxicants/botanicals or their mixtures that can be tolerated by aquatic organisms in an acute exposure and also assist in establishing/setting the sub lethal concentrations of such toxicants/botanicals in research studies. The phytochemical findings in this study revealed the presence of alkaloids, saponins, tannins, cardiac glycosides, glycosides and flavonoid similar finding was reported by Abalaka et al. [13] who reported that the ethanol extract and aqueous extract of Adenium obesum stem bark, respectively contained carbohydrates, glycosides, tannins, cardiac glycosides, saponins and flavonoids, steroids and triterpenes in addition to resin. Authors of Tijjani et al. [11,12,21] reported the presence of alkaloids, saponins, tannins, glycosides, anthraquinones and steroids in addition to triterpenoid, betulin from methanol, petroleum spirit and the petroleum ether extracts of A. obesum stem bark. The differences noticed in the phytochemical constituents of Adenium obesum extract extract among these authors, especially as it relates to the presence/absence of resins, botulin and anthraquinones may be due to the extraction methods used, the age and parts of the plants used, genetic variability between species, climatic conditions and the nature of the soil profile upon which the plant was cultivated [22].
Physico-chemical parameters such as temperature, pH, dissolved oxygen, pH, electric conductivity and total dissolved solids are important aquatic parameters that determine fish health, growth and reproduction. In this study, the TDS and the electric conductivity are different from the control except for temperature, pH and the DO. The pH of the water samples varied from concentration to concentration and the values obtained for the different treatments where lower than the standard of 6.5 to 8.5 [23], the increase in pH with time may be due to the production of basic products of metabolism. In the acute bioassay of Clarias gariepinus exposed to sponge plant fruit extract recorded a low pH. TDS and Electrical conductivity also increased across the different treatments, this may be due to the chemical composition of Adenium obesum. DO is one of the most important factor for all living organisms especially fish survive. The DO in this study did not decreased as was recorded but rather was steadily maintained throughout the study, this may be due to the continuous aeration provided by aerators within the system. The reduction in the DO content in a bioassay media has been reported to reduce as toxicant concentration increases which may be due to antioxidant property of the toxicant [24]. The physico-chemical parameters monitored in this study tend to have contributed little or none to the toxicity of Adenium obesum stem bark extract.
The behavioral responses noticed due to the toxicity of the aqueous extract of Adenium obesum in this study is similar to the findings of previous investigators on Clarias gariepinus juveniles exposed to different plant extracts [13,25-27]. Similarly, Ubahe et al. [28] observed irrational behavior in Clarias gariepinus exposed to Hypoestes forskalei extract, and these include vigorous movement, fast back stroke movement, restlessness, increased opercular movement and jumping. The erratic behaviour prior to death in the present and past studies may be associated with the impact of toxicants on fish. The excessive mucus secretions observed in the exposed fish in this study has also been reported [29,30]. Excessive mucus secretions are part of natural defense mechanisms by exposed fish to coat their body surfaces in order to prevent and/or reduce the absorption of the offending toxicant. However, such excessive mucus secretions are reported to reduce respiratory activity in fishes. This present observation might be due to bio-transformation of the active toxic constituents of the plant's extract over time, especially as the extract concentrations were not maintained daily throughout the exposure period [31,32]. The appearance and intensity of clinical signs in the contact phase of the fish toxicity study of juvenile Clarias gariepinus exposed to aqueous Adenium obesum extract was concentrationdependent as these increased with increasing extract concentrations. The initial agitations and restlessness characterized by erratic movements and repeated attempts of the fish to jump out of the culture water in the contact phase of the fish toxicity study were natural avoidance response to escape from the toxic aquatic environment. This was possibly to prevent the continuous absorption of the toxicant in the water environment. This is a normal adaptation response, which allows fish to survive in altered environment [33]. There was progressive respiratory distress, characterized by increased opercula movements, air gulping and exposed snouts above water surfaces. Respiratory impairment may arise from increasing gill cellular damage and/or increasing accumulation of elaborated mucous on gill surfaces [17,30,34]. Oreochromis niloticus juveniles treated with fresh root bark extract of Moringa oleifera also displayed these behavioral and clinical signs.
The established LC 50 value of 8.38 mg/l with a lower confidence limited (LCL) of 5.218 mg/l and an upper confidence limit (UCL) of 9.374 mg/l showed that the extract was very toxic to the exposed fish. There was strong correlation (0.949) between increase in concentrations of aqueous extract Adenium obesum stem bark and mortality of the exposed fish [13], reported LC 50 value of 7.15 mg/l in Adult Clarias gariepinus exposed to ethanoic extract of Adenium obesum stem bark, the difference in the LC 50 values of these studies may be due to extraction methods employed and range finding concentration values of the extract used to determine the concentrations for the definitive test. Studies have reported different LC 50 for various plant extracts. However, the toxicities of other plants’ extracts on fish having similar results have been reported such as Blighia sapida and Kigelia africana on C. gariepinus, Parkia biglobosa and Raphia vinifera on C. gariepinus and Tilapia [17], Tobacco on O. niloticus and C. gariepinus [35-47], Raphia hookeri on C. gariepinus.
Conclusion
The cause of mortality in this study was anoxia, as a result of the excessive mucus coating of the secondary lamellae which eventually leads to the suffocation of the fish due to the continuous assault of the toxicant on the exposed fish, respiration is compromised as shown by fish exposed snout with gradual degeneration to other clinical signs. From the results obtained, it can be concluded that the aqueous extract of Adenium obesum stem bark is toxic to exposed Clarias gariepinus juveniles.
Acknowledgement
The authors would like to acknowledge the contribution of Professor Alpha Raj Mekapogu of the Department of Veterinary Pharmacology and Toxicology, College of Veterinary Science, S.V. Veterinary University, Proddatur, Indian, who provided the guide for the experimental design of this study and Kabiru Ibrahim of the Department of Pharmacognosy and therapeutic, faculty of Pharmaceutical Sciences, A.B.U, Zaria, Kaduna State, for assisting with the phytochemical screening of Adenium obesum.
References
- McLaughlin J, Garofalo J (2002) The desert rose (Adenium obesum) Miami-Dade County/University of Florida Cooperative Extension Service, Pp: 66.
- Arbonnier M (2004) Trees, shrubs and lianas of West African dry zones: Margraf Publishers, Paris.
- Oyen L (2008) Adenium obesum (Forssk) Roem and Schult. Plant Resources of Tropical Africa. Medicinal Plant 11: 46-49.
- Plaizier A (1980) A revision of Adenium Roem and Schult and Diplorhynchus Welw. ex Fic. and Hiern (Apocynaceae). Medelelingen Landbouw hoge school, Wageningen Pp: 1-40.
- Dalziel JM (1956) Useful Plants of West Tropical Africa: Crown Agents for Overseas Governments, London, UK.
- Adamu HM, Abayeh OJ, Agbo MO, Abdullahi AL, Uba A, et al. (2005) An ethnobotanical survey of Bauchi State herbal plants and their antimicrobial activity. J ETHNOPHARMACOL 99: 1-4.
- Mettam R (1941) Plant poisoning in the domestic animals. Farm Forest 1: 58-60.
- Yamauchi T, Abe E (1990) Cardiac glycosides and pregnanes from Adenium obesum (Studies on the constituents of Adenium obesum). Chemistry and Pharmacology Bulletin 64: 669-672.
- Ahmad VU, BashaA (2007) Spectroscopic data of steroid Glycosides. Cardenolides and Pregnanes. 4:2612.
- Kiyohara H, Ichino C, Kawamura Y, Nagai T, Sato N, et al. (1970) Nicotine as a fish poison. Prog. Fish Culture 32 : 103–110.
- Tijjani A, Ndukwe IG, Ayo RG (2011) Studies on anti-bacterial activity of Adenium obesum (Apocynaceae) stem-bark. Continental Journal of Microbiology 5 : 12-17.
- Tijjani A, NdukweIG, Ayo RG (2012) Isolation and characterization of Lup-20 (29)-ene-3, 28-diol (Betulin) from the stem bark of Adenium obesum (Apocynaceae). Tropical Journal of Pharmaceutical Research 11 : 259-262.
- Abalaka SE, Fatihu MY, Ibrahim NDG, Ambali S (2015) Liver histopathological changes in Clarias gariepinus exposed to ethanol extract of Adenium obesum stem bark. J. Morphol 32: 22-28.
- Saravanan M, Vasantha Kumar D, Malarvizhi A, Ramesh M (2010) Biosafety of Azardirachta indica (A. Juss) leaves extracts on certain biochemical parameters in Labeo rohita. Journal of Biopesticides 3 : 227-231.
- Sofowora A (2008) Medicinal plants and traditional medicines in Africa. (3rd edn), Spectrum Books Limited, Ibadan, Nigeria.
- Evans W (2009) Trease and Evans Pharmacognos.: Saunders Elsevier (16th edn), Philadelphia.
- Fafioye OO, Adebisi AA, Fagaden SO (2004) Toxicity of Parkia biglobosa and Raphia vinifera extracts on Clarias gariepinus juveniles. Afr. J. Biotechnol 3: 627-630.
- Abalaka SE, Fatihu MY, Ibrahim NDG, Ambali S (2013) Exploitation of ethanol extract of Adenium obesum stem bark as a potent organic piscicide. Res J Biol Sci 8: 143-149.
- Apha (1985) Standard method for the examination of water and waste water: American Public Health Association (6th edn), Washington DC, USA.
- Tijjani A, Sallau MS, Sunusi I (2011) Synergistic activivty of methanol extract of Adenium obesum (Apocynaceae) stem bark and oxytetracycline against some clinical bacterial isolates. Bayero Journal of Pure and Applied Science 4 : 79-82.
- Sellapan S, Akoh CC, Krewer G (2002) Phenolic compounds and antioxidant capacity of Georgia-grown blueberries and blackberries. Journal of Agricultural and Food Chemistry 50: 2432–2438.
- World Health Organization (2003). Guidelines for safe recreational water environment. Coastal and Fresh Waters, Geneva.
- Prasad K, Laxdal VA, Ming YU, Raney BL (1995) Antioxidant activity of Allicin and active ingredient of Garlic. Molecular and Cellular Biochemistry 1489 : 183-189.
- Yekeen TA, Fawole OO (2011) Toxic effects of endosulfan on haematological and biochemical indices of Clarias gariepinus. African Journal of Biotechnology 10: 14090-14096.
- Adeboyejo OA, Fagbenro OA, Adeparusi E O, Clarke EO (2013) Acute toxicity of industrial effluents from Agbara Environs of Ologe Lagoon on early life stages of African-Sharp-Tooth catfish Clarias gariepinus. American J Res Commun 1: 50-59.
- Orji OU, Ibiam UA, Aja PM (2014) Acute toxicity studies of the lyophilized aqueous extract of Psychotria microphylla leaf on Clarias gariepinus juveniles. Int J Biol Sci 3: 38-44.
-  Ubahe GA, Idowu BA, Omoniyi IT (2012) Effects of Hypoestes forskalei Schult Roem leaf extract on the behavior of Clarias gariepinus. Nature and Science 10 :158 – 162.
- Jothivel N, Paul VI (2008) Evaluation of the acute toxicity of the seed of Anamirta cocculus (Linn.) and its piscicidal effect on three species of freshwater fish . Int. J Toxicol 5:1.
- Abalaka SE, Auta J (2010) Toxic Effects of aqueous and ethanol extracts of Parkia biglobosa Pods on Clarias gariepinus Adults. ‎World J Biol Res 3:9-11.
- Kela SL, Ogunsusi RA, OgboguVC, NwudeN (1989) Screening of some Nigerian plants for molluscicidal activity. Rev Elev Med Vet Pays Trop 42:195-202.
- Fafioye OO (2005) Plants with piscicidal activities in south Western Nigeria. Turk J Fish Aquat Sci 5: 91-97.
- Svecevieus G (2001) Avoidance response of rainbow trout, Oncorhynchus mykiss to heavy metal model mixtures. A comparison with acute toxicity tests. Bulletin of Environmental contamination and Toxicology 67 : 680-687.
- Sambasiva Rao KRS (1999) Pesticide impact on fish metabolism, Discovery publishing house, New Delhi.
- Agbon AO, Omoniyi IT, Teko AA (2002) Acute toxicity of tobacco (Nicotiana tobaccum) leaf dust on Oreochromis niloticus and haematological changes resulting from sublethal exposure. J. Aquat Sci 17: 5-8.
- Abdel-Sattar E (2012) In vitro antiinfluenza virus activity of a cardiotonic glycoside from Adenium obesum (Forssk.) Phytomedicine 19:111-114.
- Adesina BT, Omitoyin BO, Ajani EK, Adesina OA (2013) Acute-lethal toxicity (LC50) effect of Moringa oleifera (Lam.) Fresh Root Bark Extract on Oreochromis niloicus Juveniles Under Renewal Toxicity Exposure. Int J Agric Apic Res 9: 182-188.
- Audu BS, Adamu KM, Nonyelu ON (2014) Changes in haematological parameters of Clarias gariepinus exposed to Century Plant (Agave americana) leaf dust. Int J Apic Res 6: 54-65.
- Cagauan AG, Galaites MC, Fajardo LJ (2004) Evaluation of botanical piscicides on Nile Tilapia (Oreochromis niloticus L.) and mosquito fish (Gambusia affinis Baird and Girard). Proceedings of 6th International Symposium on Tilapia in Aquaculture.
- Fafioye OO, Adebisi AA, Fagade SO (2004) Toxicity of Parkia biglobosa and Raphia vinifera extracts on Clarias gariepinus juveniles. Afr J Biotechnol 3: 627–630.
- Faraha MA, Alib MA, Chenc SM, Lic Y, AlHemaidb FM, et al. (2016) Silver nanoparticles synthesized from Adenium obesum leaf extract induced DNA damage, apoptosis and autophagy via generation of reactive oxygen species. Colloids Surf B Biointerfaces 141:158–169.
- Finney DJ (1971) Probit Analysis, Cambridge University Press (3rd edn), London, England.
- Hoffman JJ, Cole JR (1977) Phytochemical investigation of Adenium obesum Forskal (Apocynaceae): Isolation and Identification of cytotoxic agents. J Pharm Sci 66: 1336-1338.
- Mahjoor AA, Loh R (2008) Some histopathological aspects of chlorine toxicity in rainbow trout (Oncorhynchus mykiss). Asian Journal of Animal and Veterinary Advances 3: 303-306.
- Omoniyi I, Agbon AO, Sodunke SA (2002) Effect of lethal and sublethal concentrations of tobacco (Nicotiana tobaccum) leaf dust extract on weight and heamatological changes in Clarias gariepinus (Burchell). J Applied Sci  Environ. Mgt. (JASEM) 6: 37-41.
- Abdullahi AL, Uba A, Dukku HU, Wufem BM (2005) An ethnobotanical survey of Bauchi State herbal plants and their antimicrobial activity. Journal of Ethnopharmacology 99: 1-4.
Citation: Muyiwa BO, Sambo JS, Oniye SO (2019) Toxicological Evaluation of The Median Lethal Concentration (LC 50) of Aqueous Extract of Adenium obesum Stem Bark in African Catfish, Clarias gariepinus (Burchell 1822) Juveniles. Toxicol Open Access 5: 140. DOI: 10.4172/2476-2067.1000140
Copyright: © 2019 Muyiwa BO, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 5057
- [From(publication date): 0-2019 - Dec 19, 2025]
- Breakdown by view type
- HTML page views: 4090
- PDF downloads: 967