Letter to Editor Open Access
Two New Immunoassays to Study the Binding Capacity of Staphylococcal Protein A (SpA) or Streptococcal Protein G (SpG) to Sera from Four Mammalian Species Including Wild and Domestic Animals
Angel Justiz-Vaillant1*, Norma McFarlane-Anderson2 Wayne Mohammed1 Sehlule Vuma1
1Pathology and Microbiology Unit, Department of Para-Clinical Sciences, Faculty of Medical Sciences, The University of the West Indies, St. Augustine, Trinidad and Tobago, West Indies
2Biochemistry Section, Department of Basic Medical Sciences, The University of the West Indies, Mona Campus, Jamaica, West Indies
- *Corresponding Author:
- Angel Justiz-Vaillant
Pathology and Microbiology Unit
Department of Para-Clinical Sciences
Faculty of Medical Sciences
The University of the West Indies
St. Augustine, Trinidad and Tobago
West Indies
Tel: +868-773-5914
E-mail: angel.vaillant@sta.uwi.edu
Received Date: February 16, 2015; Accepted Date: March 18, 2015; Published Date: March 25, 2015
Citation: Justiz-Vaillant A, McFarlane-Anderson N, Mohammed W, Vuma S (2015) Two New Immunoassays to Study the Binding Capacity of Staphylococcal Protein A (SpA) or Streptococcal Protein G (SpG) to Sera from Four Mammalian Species Including Wild and Domestic Animals. J Anal Bioanal Tech 6:235. doi: 10.4172/2155-9872.1000235
Copyright: ©2015 Justiz-Vaillant A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Description
The aim of this study was to report on the interactions between Staphylococcal Protein A (SpA) or Streptococcal Protein G (SpG) and sera from four mammalian species: raccoon, skunk, coyote, and mule using two novel techniques. SpA has a molecular weight of 42 kDa. It binds to the Fc fragment of IgG produced by several animal species. The native protein consists of five domains. Of these, four show high structural homology of about 58 aminoacids and they have binding capacity to immunoglobulins [1,2]. Streptococcal protein G, Type III bacterial Fc receptor, is a small globular protein produced by several streptococcal species and is composed of two or three nearly identical domains, each of 55 amminoacids. SpG is well-known for binding to IgG from many species [3,4].
Study of the SpA binding capacity to sera by a novel assay: Inhibition of the agglutination of SpA-bearing Staphylococcus aureus.
Most sera were commercially available (Sigma-Aldrich Co). Briefly serial dilutions of 25 μl of the various mammalian sera were added in duplicate to 96 wells micro-titer plates containing 25 μl of SpA-bearing S. aureus cells (Sigma-Aldrich Co) and incubated for 1 hour at RT. Inhibition of agglutination was seen in positive samples (containing antibodies that react with SpA) and agglutination at the bottoms of the wells was seen in negatives samples. In this test a donkey serum was used as a positive control and a chicken serum was used as a negative control.
Study of the SpG binding capacity to sera by a novel assay: Neutralization of the SpG inhibitory effect on the gel test.
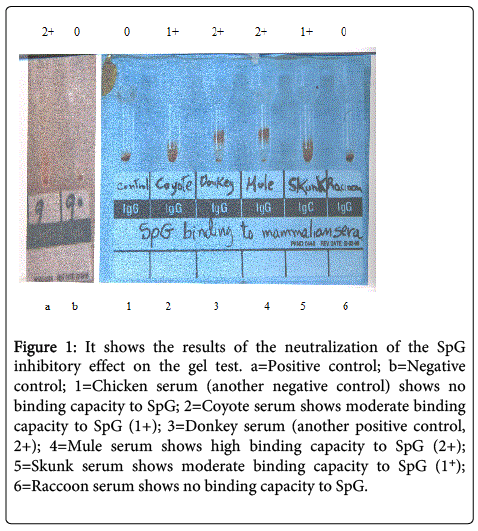
Following titrations to determine the optimal concentrations, 2 ng/μl of SpG was treated with 2 μl samples of sera from mule, coyote, skunk, raccoon, chicken and donkey and centrifuged at 13 000 rpm for 5 min. The mixture was resuspended and incubated with an equal volume of a 1% suspension of sensitized human O+ RBC in an antiglobulin gel test card for 15 min at 37°C. The card was centrifuged, visualized and photographed. After centrifugation positive reaction was graded from 0 to 4+. A 4+ reaction was indicated by a solid band of RBCs on the top of the gel. A 3+ reaction displayed agglutinated RBC in the upper half of the gel column. A 2+ reaction was characterized by RBC agglutinates that dispersed throughout the length of the column. A1+ reaction was indicated by RBC agglutination mainly in the lower half of the gel column with some non-agglutinated RBCs pellet at the bottom. Negative reactions had RBC pellets on the bottom of the microtube with no agglutination within the matrix of the gel column as shown in Figure 1.
Figure 1: It shows the results of the neutralization of the SpG inhibitory effect on the gel test. a=Positive control; b=Negative control; 1=Chicken serum (another negative control) shows no binding capacity to SpG; 2=Coyote serum shows moderate binding capacity to SpG (1+); 3=Donkey serum (another positive control, 2+); 4=Mule serum shows high binding capacity to SpG (2+); 5=Skunk serum shows moderate binding capacity to SpG (1+); 6=Raccoon serum shows no binding capacity to SpG.
ELISA
The results of the two new techniques were compared with a sensitive Enzyme-Linked Immunosorbent Assay (ELISA) to confirm the respective binding capacities of SpA and SpG with serum specimens: The 96 well polystyrene microplates (U-shaped bottom) were coated with 500 ng of SpA or SpG (Sigma-Aldrich) in coating buffer for 4 h at 37°C. The microplates were washed four times with PBS-Tween-20 and blocked with 3% non-fat milk in PBS, 25 μl/well, 1h, at Room Temperature (RT). The microplates were washed four times again. Samples were added in dilutions of 1:32. After incubation for 1h at RT the microplates were washed four times and 50 μl of peroxidase-labelled-SpLA or peroxidase-labelled-SpG diluted 1:3000 was added. The microplates were then incubated for 1h at RT, washed four times. Tetramethylbenzidine (TMB) solution (50 μl) was used. After a further incubation of 15 min in the dark, the reaction was stopped with 3M H2SO4 and read in a microplate reader at 450 nm. The cut-off point was 0.25 for SpA-ELISA and 0.31 for SpG-ELISA.
Both novel techniques: inhibition of the agglutination of SpA-bearing Staphylococcus aureus and neutralization of the SpG inhibitory effect on the gel test were effective in the study of SpA or SpG binding capacity to animal sera and the results were corroborated by the ELISA (as shown in Table 1). The SpA test demonstrated a high binding capacity to sera from skunk, coyote, raccoon and mule, and the SpG test showed high binding capacity to sera from mule, moderate binding capacity to sera from coyote and skunk and very low interaction with raccoon sera as shown in Figure 1. The study of these protein-protein interactions is important since SpA or SpG can be used as a “secondary antibody” conjugated to enzymes, gold, and other molecules in immunoassays for detection of specific antibodies, immunodiagnosis of infectious diseases and in the purification of immunoglobulins in the wild animal population that lacks commercially prepared antibodies for immunotesting. The results reported here are novel in nature.
| Serum samples | SpA-ELISA (Mean OD/SD) | SpG-ELISA (Mean OD/SD) |
|---|---|---|
| Skunk | 0.67/0.008 | 0.49/0.006 |
| Coyote | 0.71/0.005 | 0.45/0.007 |
| Raccoon | 0.69/0.01 | 0.22/0.003 |
| Mule | 0.57/0.008 | 0.6/0.01 |
| Donkey (+ control) | 0.62/0.006 | 0.63/0.004 |
| Chicken (- control) | 0.08/0.002 | 0.01/0.001 |
| Blank | 0.004/0.001 | 0.005/0.001 |
Table 1: Sandwich ELISA for detection of SpA or SpG binding capacity to sera from wild and domestic animals. The mean Optical Density (OD)
References
- Richman DD, Cleveland PH, Oxman MN, Johnson KM (1982) The binding of staphylococcal protein A by the sera of different animal species. J Immunol 128: 2300-2305.
- Langone JJ (1982) Protein A of Staphylococcus aureus and related immunoglobulin receptors produced by streptococci and pneumonococci. Adv Immunol 32: 157-252.
- Kronvall G,Jonsson K (1999) Receptins: a novel term for an expanding spectrum of natural and engineered microbial proteins with binding properties for mammalian proteins. J Mol Recognit 12: 38-44.
- Björck L, Kronvall G (1984) Purification and some properties of streptococcal protein G, a novel IgG-binding reagent. J Immunol 133: 969-974.
- Nakane P, Kawoi A (1974) Peroxidase-labelled anti- body: a new method of conjugation. J Histochem Cytochem 22: 1084-1091.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 15215
- [From(publication date):
April-2015 - Aug 29, 2025] - Breakdown by view type
- HTML page views : 10538
- PDF downloads : 4677