UNBS5162 Inhibits U251 Glioma Cells Proliferation and Metastasis by Promoting Apoptosis
Received: 11-Jul-2018 / Accepted Date: 10-Oct-2018 / Published Date: 10-Nov-2018
Abstract
Naphthalimides have potent anti-cancer activity against human cancer cells. UNBS5162, as a derivative of naphthalimide, is capable of removing primary amine group and reducing toxicity. Recently studies have discovered that UNBS5162 could significantly down-regulate pro-angiogenic chemokines in human prostate tissue, indicating it might have an anti-angiogenic effect. In this work, we intended to explore the functional role of UNBS5162 on glioma cells proliferation, migration and invasion. Cell viability, migration and invasion capabilities of U251 cells were examined by Cell Counting Kit-8 (CCK-8) and transwell assays. The cell apoptosis was tested by Annexin V-FITC/ PI and flow cytometry. Apoptosis-associated proteins and the related-markers of Phosphatidylinositol 3-kinase (PI3K) were examined by western blot analysis. The CCK-8 test suggested that UNBS5162 markedly suppressed the viability of glioma cell line U251. UNBS5162 markedly repressed glioma cell migration and invasion, which was shown from results of transwell assay. Further, UNBS5162 promoted glioma cell apoptosis rate, accompanied by reduced expression level of anti-apoptotic protein Bcl-2 and elevated expression level of pro-apoptotic protein Active Caspase-3 and Bax in U251 cell line administrated with UNBS5162. Additionally, UNBS5162 suppressed the Phosphatidylinositol 3-kinase (PI3K) signaling pathways in U251 cell line. Therefore, the current finding unveils that UNBS5162 can repress glioma cell viability and induce glioma cell apoptosis by regulating the PI3K signaling pathway, suggesting it might be a potentially promising drug target for clinical glioma therapy.
Keywords: UNBS5162; Glioma; Proliferation; Apoptosis
Introduction
Glioma is recognized as one of the commonest and the most deadly human intracranial tumors, and it accounts for more than 40% of primary central nervous system tumors [1,2]. The life expectancy of glioblastoma (GBM) patients is 6 to 12 months. Even in the most advantageous factors, the patients will die within 2 years [3]. According to the World Health Organization’s neuropathological classification, glioma is divided into astrocytoma, GBM, oligodendrocytoma and choroid papilloma and so on. The degree of malignancy of glioma is divided into four levels [4,5]. The most serious astrocytoma is GBM, belonging to grade IV gliomas. GBM, as the most intractable tumor, has a poor response to chemotherapy and radiotherapy [6]. Although the advancement of GBM treatment have achieved in recent years, the conditions of prognosis of it is still poor, with the five-year survival rate lower than 10% [7] Therefore, unearthing novel valuable drugs for GBM therapy appears to be particularly important.
Naphthalimides, a kind of compounds that bound to DNA by intercalation, have relatively strong anti-tumor action against a variety of cancer cells [8,9]. However, due to dose limitation of bone marrow toxicity, these naphthalimides failed to enter phase III, leading to thrombocytopenia, anemia and leukopenia [10]. Since the primary amine group in naphthalimides has side effects, it has been optimized and UNBS5162 is one of the derivatives. Studies have found that UNBS5162 is a G protein coupled receptor antagonist [11]. UNBS5162 can reduce CXCL chemokine expression in experimental prostate cancer, which has a good inhibitory effect on the growth of tumor cells (Mijatovic et al., 2008). In addition, UNBS5162 can avoid blood toxicity, involve in cell cycle regulation, and relate to angiogenesis. Researches on UNBS5162 anti-tumor were less. Studies have found that UNBS5162 has a good therapeutic effect in the treatment of prostate cancer [12]. Other researches have suggested that UNBS5162 exhibits inhibitory roles in human melanoma cell, retinoblastoma cell, A549 non-smallcell lung cancer and esophageal cancer squamous cell [13-16]. In our study, we found UNBS5162 treatment could impair viability and metastasis of glioma U251 cells, which might be modulated by PI3K signaling inhibition induced apoptosis.
Materials and Methods
Cell culture
Human glioma cell line U251 and normal brain glial cell line HEB were purchased from the Chinese Academy of Science (Shanghai, China). Cells were cultivated in Dulbecco’s modified eagle media (DMEM) medium (HYCLONE, USA) containing with 10% FBS (Gibco, USA) at a 37 °C incubator with 5% CO2.
Cell counting Kit-8 (CCK8) proliferation test
CCK8 was used to detect cell viability following the producer’s instructions. In brief, 1 × 103 cells per well was plated in 96-well plates. NC group was treated with 0.1% DMSO, the experimental group was treated with series of concentrations (0.1, 1, 10, 100 μM) UNBS5162 (MedChemExpress, Monmouth Junction, NJ, USA). Next, cell viability was measured once every 24 h. For detecting cell viability, 10 μl of CCK8 reagent (Beyotime Institute of Biotechnology, Shanghai, China) was added to each well and kept in 37°C incubator for 1 h. The Optical density (OD) value was tested by microplate reader (Bio-Rad, Berkeley, CA, USA) at a wave-length of 450 nm.
Transwell assay
After cell treated with UNBS5162 for 24 h, cell migration and invasion were tested using the transwell assay in accordance with producer’s instructions. For examining cell migration, 300 μl serumfree medium containing with 1 × 105 cell was plated into the top of transwell chamber (Millipore Corp., Billerica, MA, USA) and 500 μl medium with 10% FBS was plated into the low-storied chamber. After cultivation of 12 h, cells on the upper chamber were eliminated using cotton swabs and cells on the low-storied chamber were fixed in 4% paraformaldehyde, then stained with 0.1% crystal violet. Next, five random fields were chosen under microscope, photographed and counted. For examining cell invasion, Matrigel (BD Biosciences, San Diego, CA, USA) dissolved with serum-free DMEM diluted at 1:6 overnight in advance and paved to the upper chamber of transwell, the remaining steps resembled migration assay [17].
Western blot assay
Cell proteins were extracted with RIPA lysis buffer and inhibitors specific to protease and phosphatase (Beyotime). Proteins concentrations were tested by bicinchoninic acid (BCA) (Beyotime ) kit. Then, protein with equal amount (20 μg) was separated in 10% sodium dodecyl sulfate -polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride (PVDF) membranes. After blocking with 5% BSA for 1.5 h, membranes were cultivated with primary antibodies at 4°C overnight and then incubated with secondary antibodies in dark place at room temperature for 2 h. An enhanced-chemiluminescence (ECL, Beyotime) detection system was applied to visualize the bands. The gray value was scanned by Quantity One (Bio-Rad). GAPDH was used as the internal control. Membranes were incubated with the antibodies as follows: primary antibody rabbit anti-human, AKT, p-AKT, mTOR, p-mTOR, p-P70S6K, Bcl-2, Bax, Active Caspase-3 (at dilute of 1 : 1000, Cell Signaling Technology, Danvers, MA, USA), GAPDH (at dilute of 1:5000, Proteintech Group, Inc, Rosemont, IL, USA); corresponding horseradish peroxidase (HRP)-labelled secondary antibody (at dilute of 1:5000, Proteintech Group, Inc).
Cell apoptosis assay
After 10 μM UNBS5162 treating U251 cells for 24 h, cells apoptosis percentage was tested by Annexin V-FITC/Propidium Iodide (PI) and flow cytometry analyses in light of producer’s manuals. After cell were collected, centrifuged and resuspended, 1 × binding buffer were added to resuspend cells, and cells density were adjusted to 3 × 106/ml. Cells were stained with 5 μl Annexin V-FITC and 10 μl PI (Beyotime) in dark place for 20 min. Finally, cells were detected and analyzed using a FACS Calibur instrument (BD Biosciences).
Statistical analysis
SPSS18.0 statistical analysis software (SPSS Inc., Chicago, IL) and GraphPad Prism 6.0 (GraphPad Software, Inc., LaJolla, CA, USA) were utilized to analyze the experimental data. The data are exhibited as the mean ± standard deviation (SD). The Student’s t test, ANOVA and post hoc Dunnett were used to analyze statistical correlation of data. p<0.05 indicated statistically remarkable difference.
Results
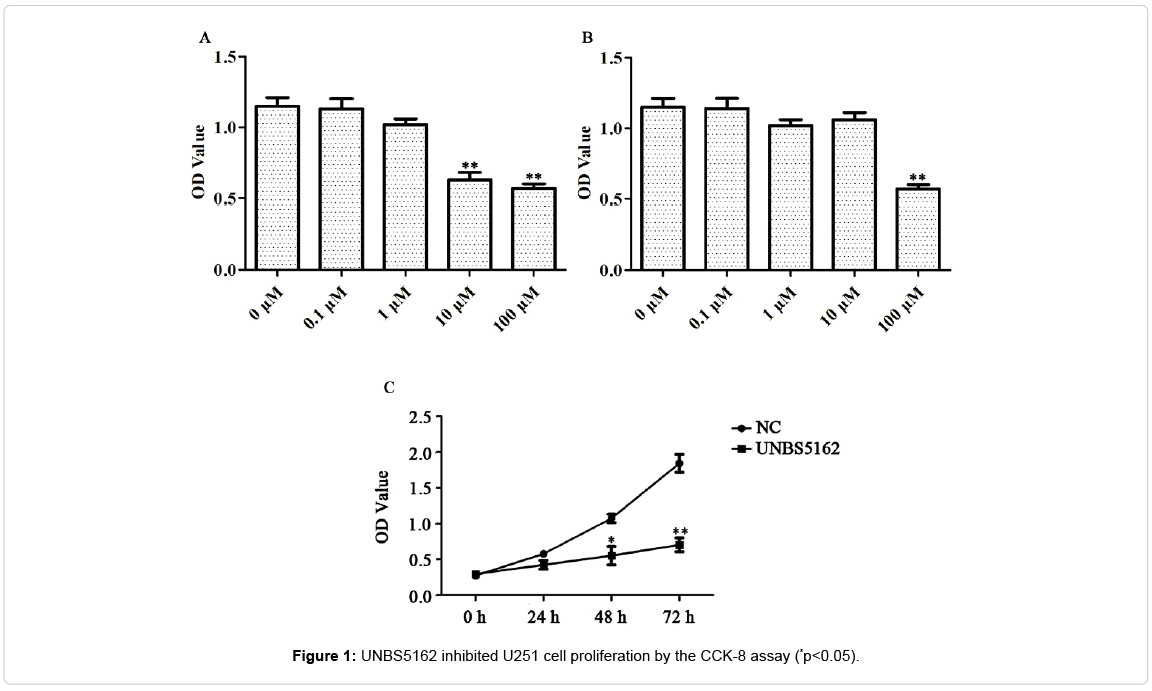
UNBS5162 inhibits glioma cell U251 viability
To assess the impact of UNBS5162 on GBM, we chose glioma cell U251 and the normal cell HEB using CCK-8 assay. 10 μM and 100 μM UNBS5162 treatment were effective on U251 cells (Figure 1A, p<0.01). The viability of HEB cells was repressed only in 100 μM UNBS5162 treated group (Figure 1B, p<0.01). So in the following experiments, 10 μM UNBS5162, a highly-effective and low-toxicity dose, was used. And the results also show that UNBS5162 obviously decreased the OD value of U251 cells in a time-dependent mode (Figure 1C, p<0.05, p<0.01). These data indicated that UNBS5162 hampered glioma cell U251 viability effectively.
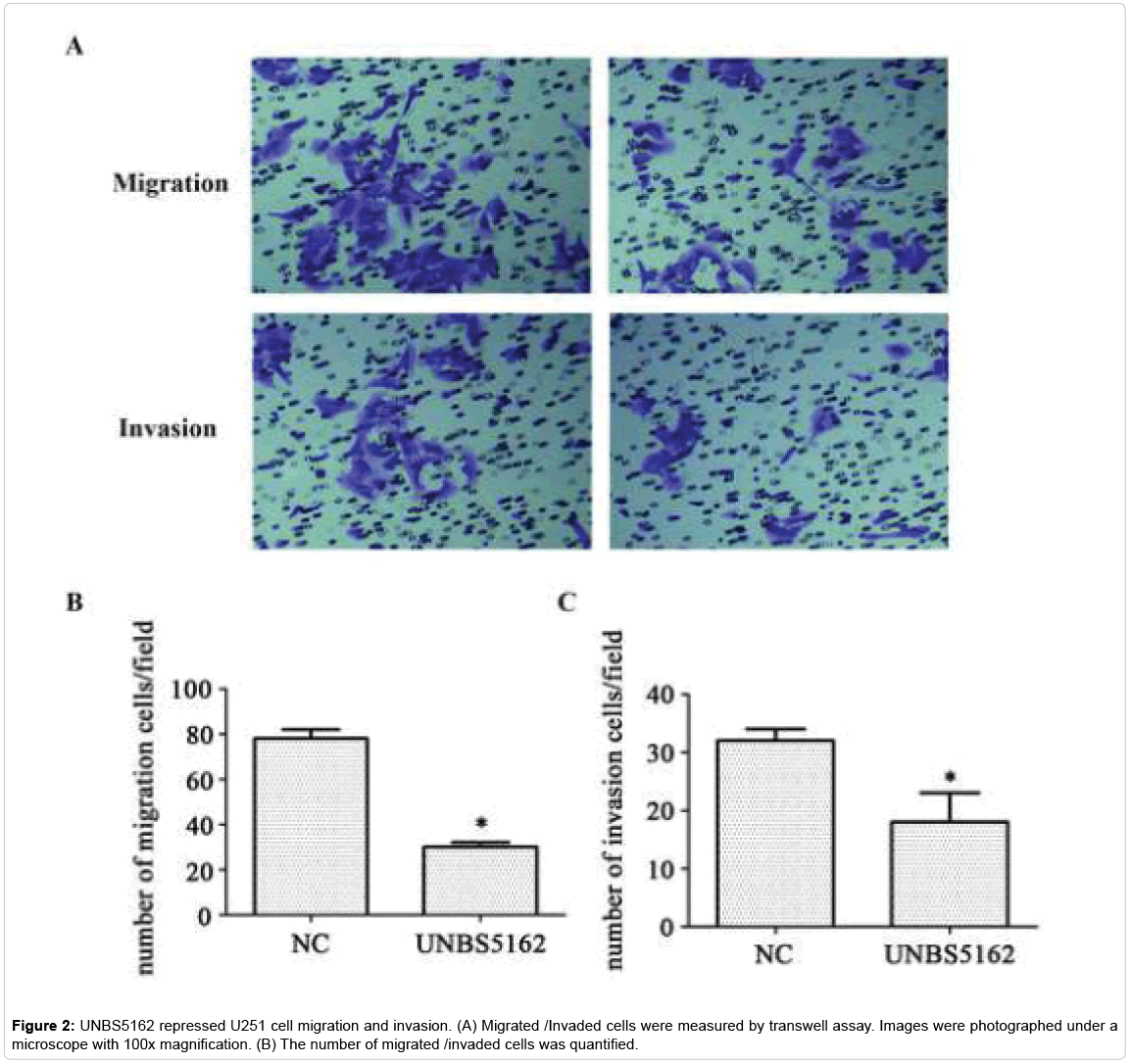
UNBS5162 inhibits glioma cell U251 migration and invasion
Then, we assessed the effect of UNBS5162 on migration and invasion capabilities of glioma cell U251 using transwell assays. In the experiment of migration, the number of positive cells staining with crystal violet was decreased by UNBS5162. Similarly, in the experiment of invasion, the cells were also reduced. These results suggest that UNBS5162 can inhibit migration and invasion of glioma cells.
The number of migrated cells in UNBS5162 treating group was less than that of control group (18 ± 5 vs. 32 ± 2), indicating a functional role of UNBS5162 on down-regulating the migration capability of U251 cells (Figures 2A and 2B, p<0.05). The capability of invasion was also inhibited (30 ± 2 vs. 78 ± 4) (Figures 2A and 2C, p<0.05), indicating UNBS5162 inhibiting U251 cells migration and invasion.
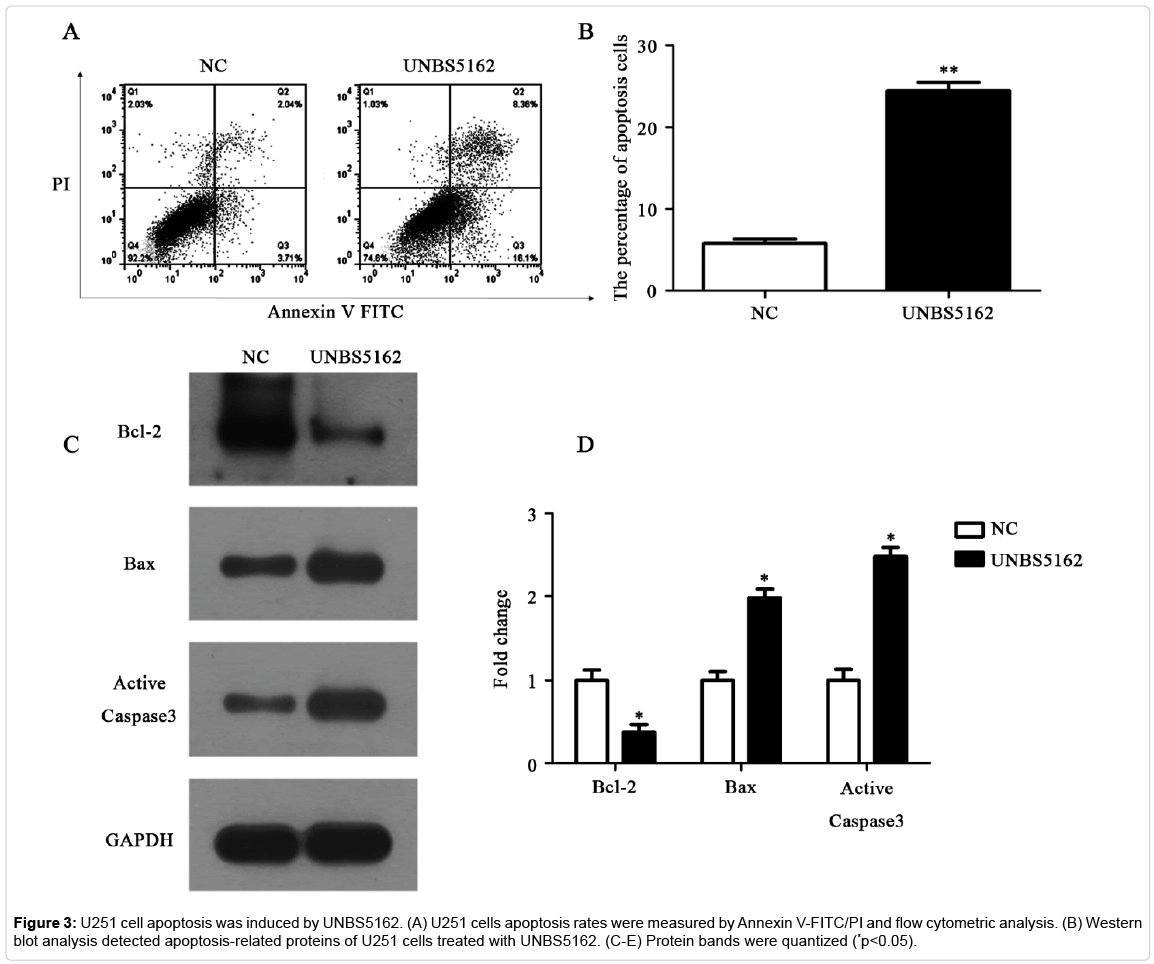
UNBS5162 promotes glioma cell U251 apoptosis
The function of UNBS5162 on U251 cells apoptosis was tested by an Annexin V-FITC/PI double staining analysis. The apoptosis percentage in UNBS5162 group was 24.46% while the apoptosis percentage in control group was 5.75% (Figures 3A and 3B, p<0.05). These data illustrated that UNBS5162 could induce glioma cell apoptosis. Furthermore, the apoptosis hallmarks such as anti-apoptotic protein Bcl-2, pro-apoptotic protein Bax and Active Caspase-3, were measured using western blot assay. Corresponding to the result of flow cytometric analysis, the expression of anti-apoptotic protein Bcl-2 was found to be decreased, and the expression of pro-apoptotic protein Active Caspase-3 and Bax were observed to be elevated in the UNBS5162 group on comparing the control group (Figure 3C and 3D, p<0.05). All of these data indicated that UNBS5162 promoted U251 cell apoptosis.
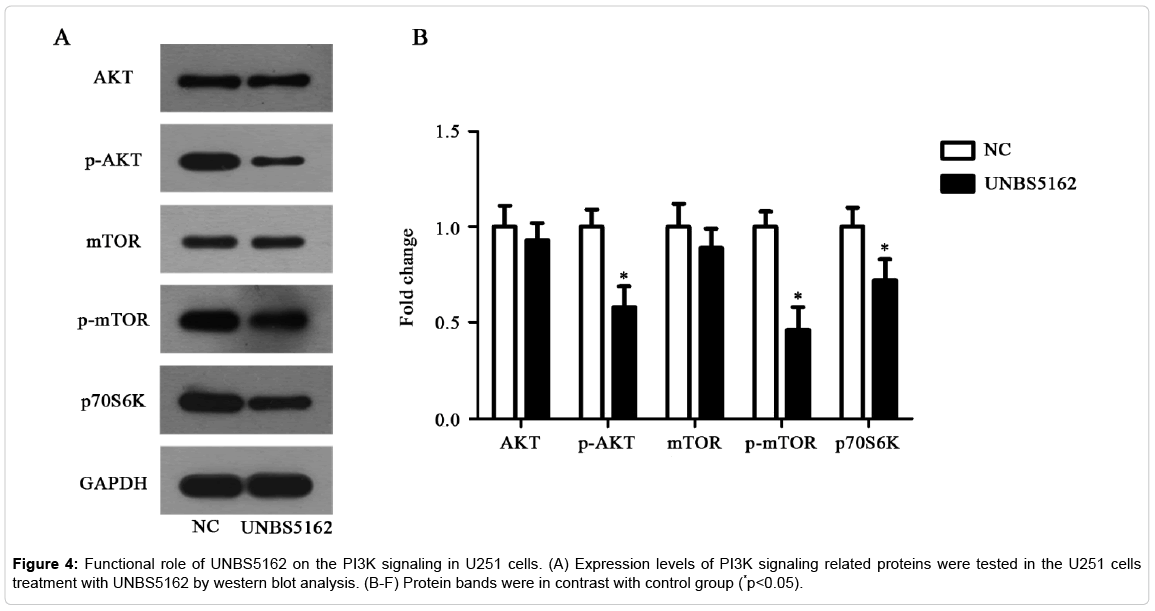
PI3K signaling pathway mediates the inhibitory effect of UNBS5162 on glioma cell U251
It is well-known that PI3K signaling is an extremely vital pathway in numerous of tumors. We selected key molecules of this pathway, including AKT, mTOR, p-AKT, p-mTOR, and p-P70S6K proteins to assess the activity of PI3K signaling. The result of western blot assay showed that expressions of p-AKT and p-mTOR were significantly decreased in UNBS5162 treating U251 cells. Further, the expression of p-P70S6K was decreased after UNBS5162 treatment (Figure 4, p<0.05). These results revealed U251 cell growth inhibition resulted from UNBS5162 treatment was regulated by PI3K pathway.
Discussion
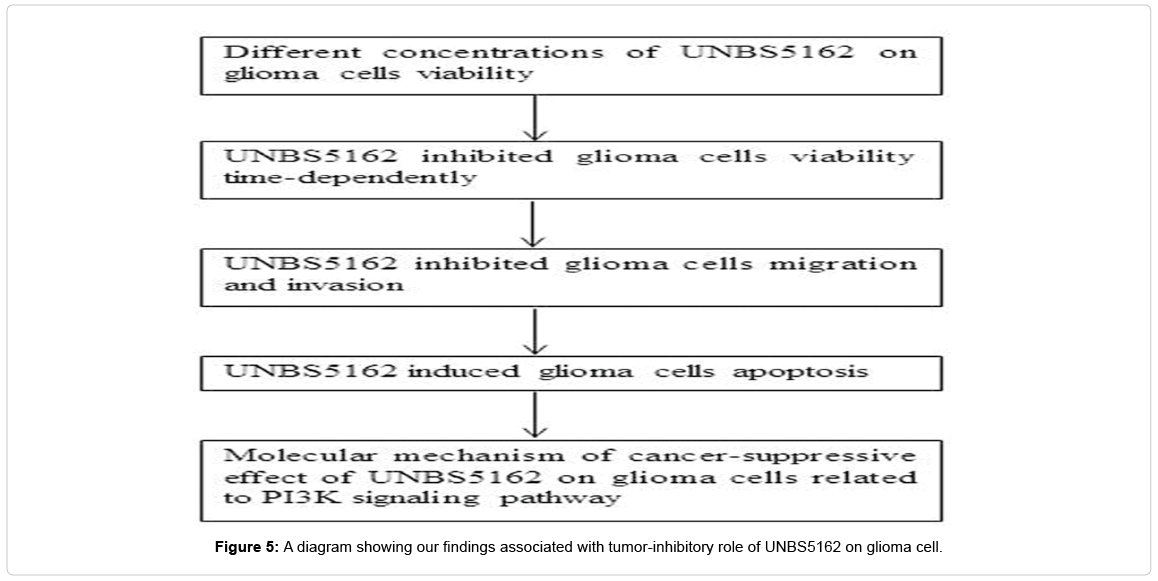
In this study, UNBS5162, a special derivative of naphthalimide, were executed a preliminary exploration in anti-cancer effect and underlying molecular mechanism of action. Notably, we uncovered that UNBS5162 repressed the U251 cell proliferation, migration and invasion. Further, cell apoptosis was observed to be efficiently induced by UNBS5162. Ultimately, it was found that the effects of UNBS5162 on U251 might be closely related to the inhibition of PI3K signal pathway. A diagram (Figure 5) was used to explain our findings.
DNA is recognized as an ideal target for anti-tumor drugs and DNA intercalators which are widely used in cancer treatment. Naphthalimide compounds have good embedding properties to DNA, and are often used as DNA intercalators, which has become a hotspot in recent years. The Spanish Brana team first synthesized a series of naphthalimide compounds. The anti-tumor activities of these compounds were tested in vitro and in vivo experiments. It has proved that some of these compounds have a good anti-tumor activity. The naphthalimide has been a hot topic in the investigation of anti-tumor drugs [18]. These first synthesized compounds usually contain only one naphthoyl structure, and these compounds are commonly referred to as mononaphthalimide. Amonneide, elinafide and bisnafide were three successful naphthalimide compounds in clinical trials, which were recognized as potential anti-cancer agents by adding different stages of the drug. Studies have reported that a novel naphthalimide derivative, UNBS3157, can avoid the clinical metabolism of blood toxicity of amonafide [19].
Studies have reported that UNBS3157, rapidly hydrolysis of saline into a single product UNBS5162, has anti-cancer activity [20]. UNBS5162 is a novel naphthalimide that is used to avoid specific metabolites of amonafide which induces blood toxicity. Studies have reported that UNBS5162 significantly attenuates the cell cycle progression of human cancer cells by significantly increasing the duration of its G2 phase. Researches have shown that UNBS5162 can significantly downregulate pro-angiogenic chemokines in human prostate tissue and the esophageal, showing it has an effect on anti-angiogenesis. Thymus, bone marrow, gastrointestinal tract and so on have been determined as targets for UNBS5162. Meanwhile, researches have suggested that in vitro incubation of UNBS5162 with human cancer cells obviously reduced the expression of the proangiogenic chemokines (e.g. CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, and CXCL8). Because of these features, UNBS5162, as an antitumor agent, has considerable prospects to provide a new drug target for clinical cancer treatment and research. Researches have shown that UNBS5162 treatment can obviously reduce vascular surface area in human prostate cancer xenografts in vivo. But the functional action of UNBS5162 in other cancers remains unknown. In this work, we performed western blot assay, CCK8 proliferation test, transwell invasion/migration analyses and flow cytometry detection with U251 cell lines under different administration conditions. Our results suggested that UNBS5162 significantly repressed cell proliferation, migration/invasion capabilities and promoted cell apoptosis. In the future, UNBS5162 may become a potential molecular marker for therapy of glioma.
In our report, transwell assay showed that UNBS5162 inhibited glioma cell U251 invasion and migration. Furthermore, UNBS5162 could promote cell apoptosis and upregulate pro-apoptotic protein Active Caspase-3 and Bax, as well as downregulate anti-apoptotic protein Bcl-2. Bax/Bcl-2 and activation of Caspase-3, identified as a pivotal mechanism of action of anti-tumoral drug, are involved in cell death and apoptosis of tumor cells, which is developed as an important determining factor of chemotherapy effectiveness. Studies have shown that changes in tumor cell apoptosis are mainly due to abnormal expression of apoptosis-related factors and abnormal of cell apoptotic signal transduction pathways [21]. PI3K, known as a family of lipid kinases, plays an essential part in cell growth by inhibiting apoptosis in various cancers [22]. Our results showed that U251 cell apoptosis was associated with the PI3K pathway. In addition, PI3K pathway was involved in the development of cancer through AKT, mTOR and p-P70S6K, which suggests PI3K pathway to be the action pathways of UNBS5162 affecting on cancer. The hallmarks p-AKT and p-mTOR in the pathway exert an important action in the proliferation and metastasis of tumor cells. Activation of Akt antagonizes angiogenesis, tumor metastasis and invasion and phosphorylates protein kinase mTOR [23]. Researchers have suggested that PI3K/AKT/mTOR pathway facilitates the resistant phenotype in glioma. Thus, the PI3K/ AKT/mTOR signaling is considered as the most commonly used route for drug intervention in glioma. P70S6K is a serine/threonine kinase, worked as a downstream factor of the PI3K/AKT/mTOR pathway, and is tightly linked with cell proliferation. At present, mTOR is the clearest kinase that activates P70S6K by phosphorylating threonine 389. The mTOR/P70S6K signaling pathway has been activated frequently in multiple human cancer [24-31]. Consistent with these reports, we suggested that UNBS5162 inhibited the PI3K pathway in U251 cells, reduced p-AKT and its downstream effectors mTOR and p-P70S6K. Collectively, the down-regulation of the PI3K pathway may contribute to suppressive effect of UNBS5162 on U251 cells.
Conclusion
In conclusion, we identified that UNBS5162 could repress glioma cell proliferation, migration and invasion and promote cell apoptosis, which might be regulated by PI3K signaling. Our finding provides a foundation for further preclinical and clinical studies of UNBS5162 in glioma therapy strategy.
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ Contributions
LL and PG conceived the study, analyzed the data and drafted the manuscript. DHW and GPL performed the experiments, analyzed the data and revised the manuscript. All authors read and approved the final manuscript.
Consent for Publication
All authors agree to the publication.
Competing Interests
The authors declare that they have no competing interests.
References
- Bush N.A, Chang, S.M & Berger M.S Current and future strategies for treatment of glioma. Neurosurgical Review, 2016. 40: p. 1-14.
- Wang, X., Jia, Y., Wang, P., Liu, Q. & Zheng, H. Current status and future perspectives of sonodynamic therapy in glioma treatment. Ultrasonics Sonochemistry, 2017b. 37: 592-599.
- Khajanchi S & Banerjee, S. Quantifying the role of immunotherapeutic drug T11 target structure in progression of malignant gliomas: mathematical modeling and dynamical perspective. Mathematical Biosciences. 2017.
- Louis, D. N., Ohgaki, H., Wiestler, O. D., Cavenee, W. K., Burger, P. C., Jouvet, A., et al.The 2007 WHO Classification of Tumours of the Central Nervous System. Acta Neuropathologica, 2007. 114: p. 547-547.
- Weller, M., Bent, M. V. D., Hopkins, K., Tonn, J. C., Stupp, R., Falini, A., et al. EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncology, 2014. 15: e395–e403.
- Hao, J., Liang, C. & Jiao, B Eukaryotic translation initiation factor 3, subunit C is overexpressed and promotes cell proliferation in human glioma U-87 MG cells. Oncology letters, 2015. 9: p. 2525-2533.
- Stupp, R., Hegi, M. E., Mason, W. P., Van den Bent, M. J., Taphoorn, M. J., Janzer, R. C., et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. The Lancet Oncology, 2009. 10: 459-466.
- Ingrassia, L., Lefranc, F., Kiss, R. & Mijatovic, TNaphthalimides and azonafides as promising anti-cancer agents. Current Medicinal Chemistry, 2009. 16: p. 1192-1213.
- Zhao, L., Li, J., Li, Y., Liu, J., Wirth, T. & Li, Z. Selenium-containing naphthalimides as anticancer agents: Design, synthesis and bioactivity. Bioorganic & Medicinal Chemistry, 2012. 20: 2558-2563.
- El-Azab, A. S., Alanazi, A. M., Abdel-Aziz, N. I., Al-Suwaidan, I. A., El-Sayed, M. A. A., El-Sherbeny, M. A., et al.Synthesis, molecular modeling study, preliminary antibacterial, and antitumor evaluation of N-substituted naphthalimides and their structural analogues. Medicinal Chemistry Research, 2013. 22: p. 2360-2375.
- Mijatovic, T., Mahieu, T., Bruyère, C., De, N. N., Dewelle, J., Simon, G., et al. UNBS5162, a novel naphthalimide that decreases CXCL chemokine expression in experimental prostate cancers. Neoplasia, 2008. 10: 573.
- Mahadevan, D., Northfelt, D. W., Chalasani, P., Rensvold, D., Kurtin, S., Hoff, D. D. V., et al. Phase I trial of UNBS5162, a novel naphthalimide in patients with advanced solid tumors or lymphoma. International Journal of Clinical Oncology, 2013. 18: p. 934-941.
- He D & Zhang, S.UNBS5162 inhibits the proliferation of esophageal cancer squamous cells via the PI3K/AKT signaling pathway. Mol Med Rep, 2018. 17: p. 549-555.
- Liu, C., Xing, J. & Gao, Y.UNBS5162 inhibits the proliferation of human A549 non-small-cell lung cancer cells by promoting apoptosis. Thorac Cancer, 2018. 9: p. 105-111.
- Shi, X., Yang, L., Xie, J., Zhao, Y., Cong, J., Li, Z., et al. UNBS5162 inhibits proliferation of human melanoma cells by inducing apoptosis via the PI3K/Akt pathway. Mol Med Rep, 2018. 18:Â 3382-3388.
- Wang, B., Shen, J. & Wang, J. UNBS5162 inhibits proliferation of human retinoblastoma cells by promoting cell apoptosis. Onco Targets Ther, 2017a. 10: p. 5303-5309.
- Cheng H, Zou T, Xu Y, Wang Y, Wu A, Dai J, et al.Investigations on the interactions between naphthalimideâ€based antiâ€tumor drugs and human serum albumin by spectroscopic and molecular modeling methods. Luminescence, 2015. 31: p. 88-95.
- Brana F.A & Ramos, M. Naphthalimides as Anticancer Agents: Synthesis and Biological Activity. Springer Netherlands, 2010.
- Van, Q. E., Mahieu, T., Dumont, P., Dewelle, J., Ribaucour, F., Simon, G., et al. 2,2,2-Trichloro-N-({2-[2-(dimethylamino)ethyl]-1,3-dioxo-2,3-dihydro-1H-benzo[de]isoquinolin- 5-yl}carbamoyl)acetamide (UNBS3157), a novel nonhematotoxic naphthalimide derivative with potent antitumor activity. Journal of Medicinal Chemistry, 2007. 50: p. 4122-34.
- Zhu, H., Huang, M., Yang, F., Chen, Y., Miao, Z. H., Qian, X. H., et al.R16, a novel amonafide analogue, induces apoptosis and G2-M arrest via poisoning topoisomerase II. Molecular Cancer Therapeutics, 2007. 6: p. 484.
- Tovilovic, G., Ristic, B., Siljic, M., Nikolic, V., Kravic-Stevovic, T., Dulovic, M., et al. mTOR-independent autophagy counteracts apoptosis in herpes simplex virus type 1-infected U251 glioma cells. Microbes & Infection, 2013. 15: p. 615-624.
- Thorpe, L. M., Yuzugullu, H. & Zhao, J. J. PI3K in cancer: divergent roles of isoforms, modes of activation, and therapeutic targeting. Nature Reviews Cancer, 2014.15: p. 7-24.
- Zhang, L., Huo, X., Liao, Y., Yang, F., Gao, L. & Cao, L. Zeylenone, a naturally occurring cyclohexene oxide, inhibits proliferation and induces apoptosis in cervical carcinoma cells via PI3K/AKT/mTOR and MAPK/ERK pathways. Scientific Reports, 2017. 7.
- Ren, H. Y., Wang, J., Yang, F., Zhang, X. L., Wang, A. L., Sun, L. L., et al. Cytoplasmic TRAF4 contributes to the activation of p70s6k signaling pathway in breast cancer. Oncotarget, 2015. 6: p. 4080-96.
- Bush N.A, Chang, S.M & Berger M.S. Current and future strategies for treatment of glioma. Neurosurgical Review, 2016. 40: p. 1-14.
- Cheng H, Zou T, Xu Y, Wang Y, Wu A, Dai J, et al.Investigations on the interactions between naphthalimideâ€based antiâ€tumor drugs and human serum albumin by spectroscopic and molecular modeling methods. Luminescence, 2015. 31: p. 88-95.
- Choi, E. J., Cho, B. J., Lee, D. J., Hwang, Y. H., Sun, H. C., Kim, H. H., et al.Enhanced cytotoxic effect of radiation and temozolomide in malignant glioma cells: targeting PI3K-AKT-mTOR signaling, HSP90 and histone deacetylases. BMC Cancer, 2014. 14: p. 7-17.
- Halacli S & O Dogan AL. FOXP1 regulation via the PI3K/Akt/p70S6K signaling pathway in breast cancer cells. Oncology Letters, 2015. 9: p. 1482-1488.
- Li, S., Zhong, W., Li, Z. & Meng, X. Unprecedented synthesis, in vitro and in vivo anti-cancer evaluation of novel triazolonaphthalimide derivatives. European Journal of Medicinal Chemistry, 2012. 47: p. 546-552.
- Li, X., Lin, Y., Wang, Q., Yuan, Y., Zhang, H. & Qian, X. ChemInform Abstract: The novel anti-tumor agents of 4-triazol-1,8-naphthalimides: Synthesis, cytotoxicity, DNA intercalation and photocleavage. European Journal of Medicinal Chemistry, 2011. 42: p. 1274-79.
- Liu, L. B., Xie, H., Xue, Y. X., Liu, Y. H., Li, Z. & Wang, P. Endothelial-monocyte-activating polypeptide II induces rat C6 glioma cell apoptosis via the mitochondrial pathway. Biochemical & Biophysical Research Communications, 2015. 457: p. 595.
Citation: Li L, Guo P, Wan DH, Liu GP (2018) UNBS5162 Inhibits U251 Glioma Cells Proliferation and Metastasis by Promoting Apoptosis. Cell Mol Biol 64: 152.
Copyright: © 2018 Li L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 3203
- [From(publication date): 0-2018 - Nov 09, 2025]
- Breakdown by view type
- HTML page views: 2322
- PDF downloads: 881