Research Article Open Access
Utilisation of Bovine Bone Pellet as a Matrix-Matched Reference Material for Calcified Tissues in LA-ICP-MS Application
Pingping Han1,2, Yinghong Zhou1, Shifeier Lu1, Tain Lloyd3, Thor Friis1, Karine Moromizato4, Charlotte Allen4* and Yin Xiao1*1Institute of Health and Biomedical Innovation, Queensland University of Technology, Brisbane, Australia
2Tissue Engineering and Microfluidic Laboratory, Australian Institute for Bioengineering and Nanotechnology, The University of Queensland, Australia
3School of Biomedical Sciences, The University of Queensland, Australia
4Institute for Future Environments, Queensland University of Technology, Brisbane, Australia
- *Corresponding Author:
- Yin Xiao
Institute of Health and Biomedical Innovation
Queensland University of Technology, Brisbane
QLD, 4059, Australia
Tel: +61-7- 31386240
Fax: +61-7-31386030
E-mail: yin.xiao@qut.edu.au
Charlotte Allen
Institute for Future Environments
Queensland University of Technology
Brisbane, QLD, 4000, Australia
Tel: +61-7-3138 0177
E-mail: cm.allen@qut.edu.au
Received date: October 18, 2015; Accepted date: November 09, 2015; Published date: November 16, 2015
Citation: Han P, Zhou Y, Lu S, Lloyd T, Friis T, et al. (2015) Utilisation of Bovine Bone Pellet as a Matrix-Matched Reference Material for Calcified Tissues in LAICP- MS Application. J Anal Bioanal Tech S13:006. doi:10.4172/2155-9872.S13-006
Copyright: © 2015 Han P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
One of the most challenging aspects of interpreting quantitative information of biological samples from laser inductively coupled plasma mass spectrometry (LA-ICP-MS) is a lack of appropriate matrix-matched internal standards that is needed for calibrations. There are standards available; however, most certified reference standard materials are suboptimal, due to the high variability and complexity of biological materials, especially for calcified tissues. In the present study, we described an approach in which bovine bone pellets are used as reliable matrixmatched standards for quantitative analysis of bone samples. Bovine tibial bones, sourced from a local butcher shop, were treated with or without autoclave sterilization. The samples were lyophilized over a 24 hour period, after which the elemental distributions in autoclaved, non-autoclaved bone pellets and naïve bone fragments were investigated using inductively coupled plasma optical emission spectrometry (ICP-OES) and LA-ICP-MS methods, in addition to homogeneity analysis of non-autoclaved bone pellets. The results demonstrated that non-autoclaved and autoclaved bone pellets shared similar average elemental concentrations after correcting for background signal; natural bone fragments, on the other hand, showed large sample variations. Factors such as low cost and ease of manufacture, “home-made” non-autoclaved bone pellets are the preferred option and these were subjected to further investigations. The homogeneity analysis revealed that non-autoclaved bone pellets had a higher degree of homogeneity, with minimal standard deviations and a uniform particle size of less than 100 μm. These results show that non-autoclaved bovine bone pellets are reliable and easy-to-make alternative to matrix-matched reference material with which to analyse calcified tissues by LA-ICP-MS.
Keywords
Bovine bone; Autoclave sterilization; Lyophilisation; LAICP- MS
Introduction
The atomic distribution and concentration of beneficial or toxic metals and non-metals in biological tissues is of great interest. Over the past decade, laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) has become as a powerful analytical tool for the purposes of qualitative imaging or mapping of element concentrations [1]. LA-ICP-MS can depict analytic distributions in biological tissue sections with a spatial resolution ranging from 10-100 μm [2] and offers a detection limit from parts per million to as low as parts per billion [3]. These are benefits that make LA-ICP-MS a versatile tool in mapping the elements making up soft and hard tissues, thereby contributing better understanding of biological processes especially where the quantifications of these trace elements are concerned [1,4,5].
During the past decade LA-ICP-MS has been applied to a number of applications to investigate biological structures from ranging from human and animal tissues [6,7], plants tissues [2] and other biological samples such as hair [8] or synovial fluid samples [9]. In human tissues this method has been used with biopsy sections from breast [10], lymph node [11], brain [12] and eye lenses [13], and of particular relevance for the present study, ICP-MS analyses have been reported for mineralised tissues [14-16].
For quantitative analyses with LA-ICP-MS, reproducibility at 5% and better can be achieved if two conditions are satisfied: 1) an internal standard element can be quantified or estimated accurately, and 2) a matrix-matched Certified Reference Material (CRM), is available. The reasons that these conditions are necessary related to the fact that all materials ablate somewhat differently, with different yields of material over a constant time of ablation at the same analytical settings. With an internal standard these ablation differences can be “divided out”. Ideally, a suitable matrix-matched standard should reflect the in vivo situation as close as possible in order to overcome differences in physio-chemical aspects between biological samples and the calibration standard [17,18]. In addition, an optimum matrix-matched standard should have a matrix composition as similar to the sample as possible in order to ensure that data obtained from different experiments are consistent and comparable, as well as be homogeneously distributed within samples and standard matrices [19]. Therefore, the optimum calibration standard is one that contains an element composition that resides naturally within the matrix instead of being added to the sample or in the carrier gas stream.
Ultimate accuracy is dependent on how well matched the reference material is to the sample being analysed. Recent studies have used pelletized NIST SRM 1486 Bone Meal or SRM 1400 Bone Ash (National Institute of Standards and Technology Gaithersburg, MD) as an ‘in house’ control material [20,21]. However, both of these SRMs are expensive and their physical properties were significantly altered by either boiling or incineration compared to the unknown samples. In published data, the selection of reference materials for the purposes of quantification (“a standard”) has only received scant attention, especially in the case of quantification experiments of mineralised tissues using LA-ICP-MS. We have come to the view that it is necessary to develop matrix-matched reference materials of mineralised tissues that are treated as similarly as the specimens.
The principal objective of this study was to establish a bone material, homogenized by grinding to a fine powder, to be used as a reference material for quantification of bone samples. The material must have a homogeneity and matrix composition that matches the unknowns. In the first test, the reference materials were analysed by LA-ICP-MS and calibrated against NIST SRM 610 and 612 in order to evaluate the homogeneity of the ground and pelletized bone. In the second test, the relative abundance of elements in bovine bones ground to fine powders were determined independently by inductive coupled plasma atomic emission spectrometry (ICP-AES), The calibration materials described here are a major improvement on the commercially available standards and this is as far as we know the first study to report a multi-point calibration strategy for quantitative analysis of mineralised tissues.
Materials and Methods
Materials
In this study, we used bovine tibia bone sourced from a local butcher shop (Kelvin Grove, Brisbane, Australia). All samples were free from inflammation and any obvious developmental defects. Standard Reference Materials (SRM) 610 and SRM 612 were purchased from the National Institute of Standards and Technology (Gaithersburg, MD, USA) and used to demonstrate accuracy and precision of the LA-ICPMS method for determining of elemental mass fractions in pelletized bovine bone samples.
Sample preparation
The bovine tibia bones (n=8) were fixed in 4% paraformaldehyde at 4°C overnight. After rinsing with phosphate buffered saline (PBS) buffer, all soft tissues were thoroughly removed. The samples were divided into two treatment groups: (a) bones subjected to autoclave sterilisation at 121°C for 20 mins (n=4) and; (b) non-autoclaved bones (n=4). To avoid moisture influencing the quantitative analysis, the samples were all freeze-dried for 24 hrs with a -80°C freeze-drier to preserve perishable bone materials (Christ Alpha 1-2 LD Freeze Dryer, SciQuip Ltd, UK). After this, the bones were grounded to powders using a powderiser machine (CMI Ltd) and the powders were compressed into pellets in 6 mm die using a stainless steel manual hydraulic pellet pressing machine at approximately 2 ton pressure for 30 seconds. Pre-ablation of the surface was performed using the laser conditions described in Table 1 to reduce the chance of contamination arising from the press.
| Parameters | Values |
|---|---|
| RF power | 1550W |
| Sampling depth | 5mm |
| Sample (argon) flow | 0.85 L/min |
| Isotopes measured | 12C,13C,23Na,29Si,31P, 39K,43Ca,47Ti, 56Fe, 59Co, 66Zn, 77Se, 88Sr, 137Ba, 208Pb |
| Dwell time per isotope | 0.02s |
Table 1: ICP-MS operating conditions.
LA-ICP-MS instrumentation and cross-calibration
The bone pellets were analysed against the SRM 612 and 610 external calibration standards using Ca as the internal standard element. The assured Ca concentration in NIST 612 was 50 ppm. An Agilent 8800 single collector, quadrupole ICP-MS (Agilent Technologies Inc., Santa Clara, California, USA) attached to a 193 nm wavelength excimer laser and 2-volume Trueline ablation cell from ESI New Wave Research (Bozeman, Montana, USA) was employed to investigate the element distributions in this study. Parameters such as gas flows and repetition rates were optimized for the subsequent laser ablation analysis. The pellets and NIST standards were sampled in the laser sample chamber, which used helium as carrier gas and was repeatedly evacuated and back-filled with He to omit a changing gas (air+He) from the introduction system. The output He from the ablation cell was connected to Ar flow to the ICP via a Y-piece. Helium flow was set to 450 ml/min in the cell, and 100 ml/min in the cup stationed above the ablation site. The laser was set to a modest energy output generating a fluence of 3.0 J/cm2, and a pulse rate of 10 Hz. A masked rectangular beam 15 by 150 microns was moved parallel to the short dimension at 5 microns/second to create a track approximately 750 microns long and 15 microns deep in the tissues. The ICP-MS operating parameters are summarized in Table 1. The time to cycle through the 9 selected isotopes was 0.12 s. Scans of varying duration were bracketed by 50 seconds of background (laser-off) data acquisition. Data were analysed and displayed using IOLITE [22].
After completing sample preparation and placement in the ablation chamber, the whole system was purged with He carrier gas at 0.5 L min-1 for 3 hrs before starting the analysis session in order to minimize any effect on the carbon signal stemming from air in the cell.
Ionic concentration analysis for non-autoclaved bovine bone powders
Further quantification of elements in bovine bone was performed on the bone powders. The powders were dissolved in 1% (v/v) HNO3 and held at 95°C for 2 hrs and further equilibrated with multi-element standard solutions to arrive at a final concentration range of 0.1-100 μg g-1 of the powders. The absolute concentration in the pellets was quantified by inductive coupled plasma atomic emission spectrometry (ICP-AES, Perkin-Elmer Optima 7000DV, USA).
Data analysis
The data were processed using the SPSS software (SPSS Inc., Chicago, IL, USA). The elemental concentrations determined for each bone sample were displayed with mean ± SD (standard deviation) values.
Results and Discussion
LA-ICP-MS analysis of the pelletised bone samples
Signal intensities obtained during LA-ICP-MS analysis were sufficient to detect all of the selected isotopes above the gas blank for autoclaved, non-autoclaved bone pellets, naïve bone fragments and NIST SRM 610 and SRM 612 (Table 2). Autoclaved and non-autoclaved bone samples had comparable signal intensities when corrected for background with the elements 29Si, 44Ca, 47Ti, 56Fe, 59Co, 66Zn, 88Sr and 208Pb being above the gas background. The relative standard deviations were lower in the bone pellets compared to the naïve bone fragments groups, suggesting that these elements were not homogenously distributed in the natural bone fragments.
| Methods | Sample | 24Mg ppm | 29Si ppm | 44Ca ppm | 47Ti ppm | 56Fe ppm | 59Co ppm | 66Zn ppm | 88Sr ppm | 208Pb ppm |
|---|---|---|---|---|---|---|---|---|---|---|
| ICP-MS | Autoclaved bone pellets | 150.1 ± 2.8 | 6.19 ± 1.92 | 9807.2 ± 83.6 | 0.68 ± 0.13 | 2.9 ± 0.57 | 0.0016 ± 0.00028 | 2.95 ± 0.18 | 10.54 ± 1.38 | 0.0092 ± 0.0004 |
| Non-autoclaved bone pellets | 156.9 ± 3.2 | 6.89 ± 1.99 | 9828.4 ± 66.4 | 0.62 ± 0.12 | 2.64 ± 0.5 | 0.031 ± 0.0016 | 3.14 ± 0.28 | 19.48 ± 0.92 | 0.0081 ± 0.0002 | |
| Naïve bone fragments | 536.9 ± 394 | 274.3 ± 271.15 | 9619.3 ± 189.8 | 8.84 ± 9.25 | 33.88 ± 28.48 | 12.78 ± 10.86 | 29.9 ± 17.34 | 10.03 ± 0.5 | 0.06 ± 0.061 | |
| ICP-AES | Non-autoclaved bone powders | 247.4 ± 25.7 | 1.13 ± 0.17 | 13105 ± 1123.5 | -0.44 ± 0.041 | 2.78 ± 0.324 | 0.82 ± 0.027 | 5.1 ± 0.19 | 27.2 ± 0.91 | -0.76 ± 0.102 |
*Note: ± values are standard deviations calculated from 25 replicates for each group
Table 2: Elemental contents (ppm) for bovine bone samples.
As for 59Co, the concentration was higher in non-autoclaved bone samples compared to autoclaved bone samples, suggesting an effect from autoclaving that results in greater variability of the 59Co distribution within samples and variability in ICP-MS measurement. As a whole we judged both procedures as equally suitable for the preparation of bone standards. The non-autoclaved bone pellets were marginally better as a matrix-matched reference material for mineralised tissues by being more economic and faster to manufacture.
Ion dissolution analysis for non-autoclaved bone powders
The elemental concentrations of magnesium, calcium, iron, silicon cobalt, strontium and lead released from non-autoclaved bone powders were shown in Table 2. When compared to the data from ICP-AES, it demonstrated that ICP-MS is a more powerful tool which is sensitive with lower limit for some of the elements, such as Si, Ti and Pb. On the other hand, ICP-AES showed the higher concentration for Ca, Zn and Sr when compared to that of ICP-MS data.
LA-ICP-MS measurement of natural bone fragments
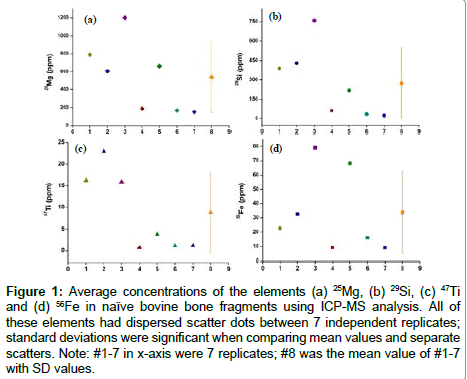
A biogenic signal is expected to produce a constant concentration of the elemental composition of a given region and is therefore an important parameter to assess natural bone fragments. Cross-sections of seven replicates were analysed to obtain concentration profiles of elements such as Mg, Si, Ti, and Fe (Figure 1 and Table 2). Regions 1-7 from Figure 1 exhibited large variation in the concentration of all the elements studied compared to their mean value, suggesting an unstable signal in these bone fragments. Similar results were observed in the other four bone samples analysed under the same conditions by LAICP- MS. The data shown in Table 2 reveals a degree of variation in the signal of all the elements as well as a significantly higher standard deviation resulting from seven replicates, and suggests that the elements in natural bone fragments are not homogenously distributed. Natural bone fragments are therefore of limited utility as internal standards.
Figure 1: Average concentrations of the elements (a) 25Mg, (b) 29Si, (c) 47Ti and (d) 56Fe in naïve bovine bone fragments using ICP-MS analysis. All of these elements had dispersed scatter dots between 7 independent replicates; standard deviations were significant when comparing mean values and separate scatters. Note: #1-7 in x-axis were 7 replicates; #8 was the mean value of #1-7 with SD values.
Homogenous analysis for non-autoclaved bone pellets
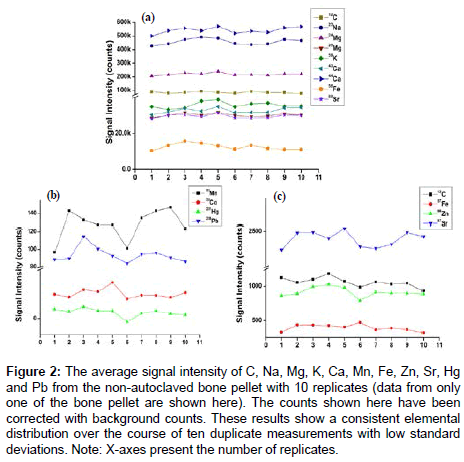
The homogeneity of non-autoclaved bone samples (n=4 donors, 10 replicates for each donor) were analysed by LA-ICP-MS for elemental distribution of C, Na, Mg, K, Ca, Mn, Fe, Zn, Sr, Hg and Pb (Figure 2). The signal intensity exhibited only a minimal standard deviation across the replicates and suggests that lyophilisation is the optimum way of drying the samples prior to ICP-MS analyses since it appears not to result in any loss of elements.
Figure 2: The average signal intensity of C, Na, Mg, K, Ca, Mn, Fe, Zn, Sr, Hg and Pb from the non-autoclaved bone pellet with 10 replicates (data from only one of the bone pellet are shown here). The counts shown here have been corrected with background counts. These results show a consistent elemental distribution over the course of ten duplicate measurements with low standard deviations. Note: X-axes present the number of replicates.
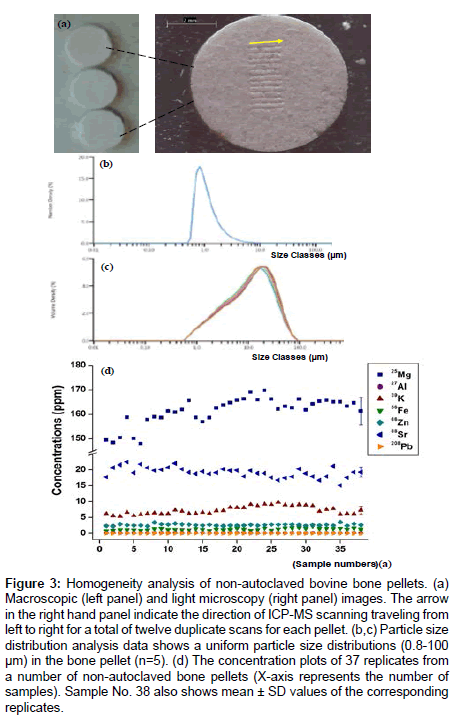
A further test of homogeneity of non-autoclaved bone pellets was shown with macroscopic and light microscopy images, which demonstrated the particle size was less than 100 um and distributed evenly on the surface. After normalization of non-autoclaved bone pellets against SRMs, the data from 37 replicates for each donor (4 donors) show that the individual scatter plots cluster close to their mean value with high precision for all the elements (Figure 3).
Figure 3: Homogeneity analysis of non-autoclaved bovine bone pellets. (a) Macroscopic (left panel) and light microscopy (right panel) images. The arrow in the right hand panel indicate the direction of ICP-MS scanning traveling from left to right for a total of twelve duplicate scans for each pellet. (b,c) Particle size distribution analysis data shows a uniform particle size distributions (0.8-100 μm) in the bone pellet (n=5). (d) The concentration plots of 37 replicates from a number of non-autoclaved bone pellets (X-axis represents the number of samples). Sample No. 38 also shows mean ± SD values of the corresponding replicates.
The lyophilisation technique described here is an ideal method for quantitative assessment of any mineralised tissues including teeth and bones and only requires a timeframe of approximately 24 h using a single standard. With such a matrix-matched standard, LA-ICPMS methods can be applied in the study of mineralized tissues with metal isotopes revealing not only the intensity but also the sources of metal exposures in at-risk communities. However, the feasibility of its application as suitable standard require further evaluation by analysing and comparing the results of a real bone or tooth sample analysis from using NIST SRM 610/SRM 612 and the non-autoclaved bovine bone pellets.
Conclusion
In this study, non-autoclaved bone pellets were shown to be suitable as internal standards for quantification experiments of samples with similar biological properties, such as bovine tibial bone. We showed that this standard yielded high accuracy of elemental quantification and was simple and fast to produce. Freeze-dried bovine bone pellets as standards are matrix-matched reference materials that are reliable, exact and quantitative, and therefore suitable for a wide range of elements.
Acknowledgements
The work was supported by the facilities at the Institute of Health and Biomedical Innovation (IHBI) and the Institute for Future Environments (IFE) of Queensland University of Technology. The authors would like to thank the assistance from Mr. Edward Ren and IFE staff during the LA-ICP-MS investigations and data analysis.
References
- Kindness A, Sekaran CN, Feldmann J (2003) Two-dimensional mapping of copper and zinc in liver sections by laser ablation-inductively coupled plasma mass spectrometry. Clin Chem 49: 1916-1923.
- Wu B, Becker JS (2012) Imaging techniques for elements and element species in plant science. Metallomics 4: 403-416.
- Becker JS, Matusch A, Wu B (2014) Bioimaging mass spectrometry of trace elements - recent advance and applications of LA-ICP-MS: A review. Anal Chim Acta 835: 1-18.
- Becker JS, Zoriy M, Matusch A, Wu B, Salber D, et al. (2010) Bioimaging of metals by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS). Mass Spectrom Rev 29: 156-175.
- Pornwilard MM, Weiskirchen R, Gassler N, Bosserhoff AK, Becker JS (2013) Novel bioimaging techniques of metals by laser ablation inductively coupled plasma mass spectrometry for diagnosis of fibrotic and cirrhotic liver disorders. PLoS One 8: e58702
- Becker JS, Matusch A, Palm C, Salber D, Morton KA, et al. (2010) Bioimaging of metals in brain tissue by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) and metallomics. Metallomics 2: 104-111.
- Bourassa MW, Miller LM (2012) Metal imaging in neurodegenerative diseases. Metallomics 4: 721-738.
- Dressler VL, Pozebon D, Mesko MF, Matusch A, Kumtabtim U, et al. (2010) Biomonitoring of essential and toxic metals in single hair using on-line solution-based calibration in laser ablation inductively coupled plasma mass spectrometry. Talanta 82: 1770-1777.
- Austin C, Hare D, Rozelle AL, Robinson WH, Grimm R, et al. (2009) Elemental bio-imaging of calcium phosphate crystal deposits in knee samples from arthritic patients. Metallomics 1: 142-147.
- Seuma J, Bunch J, Cox A, McLeod C, Bell J, et al. (2008) Combination of immunohistochemistry and laser ablation ICP mass spectrometry for imaging of cancer biomarkers. Proteomics 8: 3775-3784.
- Hare D, Burger F, Austin C, Fryer F, Grimm R, et al. (2009) Elemental bio-imaging of melanoma in lymph node biopsies. Analyst 134: 450-453.
- Dobrowolska J, Dehnhardt M, Matusch A, Zoriya M, Palomero-Gallagherb N, et al. (2008) Quantitative imaging of zinc, copper and lead in three distinct regions of the human brain by laser ablation inductively coupled plasma mass spectrometry. Talanta 74: 717-723.
- Konz I, Fernández B, Fernández ML, Pereiro R, González-Iglesias H, et al. (2014) Quantitative bioimaging of trace elements in the human lens by LA-ICP-MS. Anal Bioanal Chem 406: 2343-2348.
- Austin C, Smith TM, Bradman A, Hinde K, Joannes-Boyau R, et al. (2013) Barium distributions in teeth reveal early-life dietary transitions in primates. Nature 498: 216-219.
- Hare D, Austin C, Doble P, Arora M (2011) Elemental bio-imaging of trace elements in teeth using laser ablation-inductively coupled plasma-mass spectrometry. J Dent 39: 397-403.
- Dolphin AE, Goodman AH, Amarasiriwardena DD (2005) Variation in elemental intensities among teeth and between pre- and postnatal regions of enamel. Am J Phys Anthropol 128: 878-888.
- Becker JS, Matusch A, Depboylu C, Dobrowolska J, Zoriy MV (2007) Quantitative imaging of selenium, copper, and zinc in thin sections of biological tissues (slugs-genus arion) measured by laser ablation inductively coupled plasma mass spectrometry. Anal Chem 79: p. 6074-6080.
- Austin C, Fryer F, Lear J, Bishop D, Hare D (2011) Factors affecting internal standard selection for quantitative elemental bio-imaging of soft tissues by LA-ICP-MS. Journal of Analytical Atomic Spectrometry 26: 1494-1501.
- Durrant SF (1999) Laser ablation inductively coupled plasma mass spectrometry: achievements, problems, prospects. Journal of Analytical Atomic Spectrometry 14: 1385-1403.
- Bellis DJ, Hetter KM, Jones J, Amarasiriwardena D, Parsons PJ (2006) Calibration of laser ablation inductively coupled plasma mass spectrometry for quantitative measurements of lead in bone. J Anal At Spectrom 21: 948-954.
- Vasinova Galiova M, Nyvltova Fisakova M, Kynicky J, Prokes L, Neff H, et al. (2013) Elemental mapping in fossil tooth root section of Ursus arctos by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS). Talanta 105: 235-243.
- Hellstrom J (2008) Iolite: software for spatially resolved LA-(quad and MC)-ICP-MS analysis. Mineral Association of Canada.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 10995
- [From(publication date):
specialissue-2015 - Aug 25, 2025] - Breakdown by view type
- HTML page views : 10041
- PDF downloads : 954