Research Article Open Access
Harmonized Collaborative Validation of a Simultaneous and Multiple Determination Method for Nivalenol, Deoxynivalenol, T-2 Toxin, HT-2 Toxin, and Zearalenone in Wheat and Barley by Liquid Chromatography Coupled to Tandem Mass Spectrometry (LC-MS/MS)
Hiroyuki Nakagawa1*, Shigehiro Naito1, Yuusuke Kitani2, Yoshinao Ito2, Yoshikazu Aoyama3, Matsuhisa Koyama3, Yuusuke Hiejima4,Keisuke Nakamura4, Hiroshi Miyazaki5, Horie-Ishiguro Morita5, Masayoshi Tamura6, Naoki Mochizuki6, Masaru Nakamura7, Yuusuke Seki8, Hisae Kadokura9, Hidaka Ikeda10, Tomoko Horie-Ishiguro11, Yoriko Saito12, Miyoko Tajima12, Yohsuke Shigemasu13, Kikuko Kasama14, Yasuyo Oguma14, Yuki Sago1, Tetsuhisa Goto15 and Kazuyuki Hirayae161National Agriculture and Food Research Organization (NARO), National Food Research Institute (NFRI), 2-1-12 Kannon-dai, Tsukuba-shi, Ibaraki 305-8642, Japan
2Food and Agricultural Materials Inspection Center (FAMIC) Headquarters, Saitama, Japan
3Food and Agricultural Materials Inspection Center (FAMIC) Nagoya Regional Center, Aichi, Japan
4Food and Agricultural Materials Inspection Center (FAMIC) Kobe Regional Center, Hyogo, Japan
5Japan Grain Inspection Association, Tokyo Research Laboratory, Tokyo, Japan
6Asahi Group Holdings, Ltd., Research Laboratories for Food Safety Chemistry, Ibaraki, Japan
7Showa Sangyo Co., Ltd., Research & Development Center, Chiba, Japan
8Nisshin Seifun Group Inc., QE (quality exam.) Center, Saitama, Japan
9Kagome Co., Ltd., Research & Development Division Tochigi, Japan
10S&B Foods, Inc., Itabashi Spice Center, Tokyo, Japan
11NH Foods Ltd., R&D Center, Ibaraki, Japan
12Co-op net Business Association, Quality Control Department, Saitama, Japan
13National Federation of Agricultural Co-operative Associations, Central Research Institute for Feed and Livestock, Ibaraki, Japan
14Food and Drug Safety Center (FDSC), Hatano Research Institute (HRI), Kanagawa, Japan
15Shinshu University, Faculty of Agriculture, Nagano, Japan
16National Agriculture and Food Research Organization (NARO), National Agricultural Research Center for Kyushu Okinawa Region, Kumamoto, Japan
- *Corresponding Author:
- Hiroyuki Nakagawa
National Agriculture and Food Research Organization (NARO)
National Food Research Institute (NFRI)
2-1-12 Kannon-dai, Tsukuba-shi
Ibaraki 305-8642, Japan
Tel: +81-29-838-8085
Fax:+81-29-838-7996
E-mail: hironkgw@affrc.go.jp
Received date: April 26, 2014; Accepted date: June 04, 2014; Published date: June 06, 2014
Citation: Nakagawa H, Naito S, Kitani Y, Ito Y, Aoyama Y, et al. (2014) Harmonized Collaborative Validation of a Simultaneous and Multiple Determination Method for Nivalenol, Deoxynivalenol, T-2 Toxin, HT-2 Toxin, and Zearalenone in Wheat and Barley by Liquid Chromatography Coupled to Tandem Mass Spectrometry (LCMS/ MS). J Anal Bioanal Techniques S6:002 doi: 10.4172/2155-9872.S6-002
Copyright: ©2014 Nakagawa H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Harmonized collaborative validation of a simultaneous and multiple determination method for nivalenol, deoxynivalenol, T-2 toxin, HT-2 toxin, and zearalenone in wheat and barley by liquid chromatography tandem mass spectrometry (LC-MS/MS) was conducted by participants from 12 laboratories. The fortified samples of wheat and barley at three different levels and one naturally contaminated wheat sample were extracted, consecutively purified through a Presep C18 (ODS) solid phase extraction column and a Bond Elut Mycotoxin multifunctional column, and were analyzed by LC-MS/MS. The employment of internal standards (verrucarol and zearalanone) was apparently effective to ensure repeatability and reproducibility with sufficient recovery of each mycotoxin. This is the first report of the harmonized collaborative validation study of a simultaneous and multiple determination method for both type A and B trichothecenes along with zearalenone by LC-MS/MS. The validated method should be practical for monitoring of the major Fusarium mycotoxins contained in wheat and barley.
Keywords
LC-MS/MS; Harmonized collaborative validation; Mycotoxin; Fusarium; Wheat; Barley
Introduction
Fusarium fungi are known plant pathogens that infect major cereals consumed as food and feed, and some produce mycotoxins such as trichothecenes, zearalenone (ZEA), and fumonisins [1]. Among the Fusarium mycotoxins, deoxynivalenol (DON), which belongs to type B trichothecenes, is the most important [2]. In Japan, Fusarium fungi infection of wheat and barley is serious, since they are widely planted and they frequently grow through the rainy season. Although the co-occurrence of these toxins is a considerable concern for food safety, fumonisin contamination is less frequent in wheat [3]. Therefore, trichothecenes and ZEA were selected as the analysis targets of this study. Many countries set regulation values for DON, a major type B trichothecene that is predominantly found in cereal and cereal-based products [4]. In Asia, nivalenol (NIV) contamination is as predominantly reported as DON [5,6], and NIV is also detected in cereals collected from various countries [7]. Among type A trichothecenes, T-2 toxin (T-2) and HT-2 toxin (HT-2) are receiving the most attention, due to their higher prevalence in crops, and the European Food Safety Authority (EFSA) Panel on Contaminants in the Food Chain (CONTAM) established a group tolerable daily intake (TDI) of 100 ng/kg body weight for the sum of T-2 and HT-2 [8]. From these circumstances, harmonized collaborative validation of the simultaneous detection method for the major Fusarium mycotoxins (NIV, DON, T-2, HT-2, and ZEA) in wheat and barley by LC-MS/ MS was carried out by 12 participating laboratories. Although there had been many reports on simultaneous and multiple detection of mycotoxins by LC-MS or LC-MS/MS [9-18], only a few of them were demonstrated to be fit for the purpose through an inter-laboratory validation [16-18]. Matrix effects were often reported as the major problem with LC-MS/MS analysis [12,14,15]. Several components derived from the matrix (foods and feeds) are concomitantly extracted with the target analytes (mycotoxins), and occasionally accompany them throughout the purification steps. Some of these components are even eluted simultaneously through the HPLC column, and enhance or suppress ionization of the target analytes [12]. These effects cause overor under-estimation of the target analytes, and are thus called “matrix effects.” The effects are likely to be significant when the calibration standard solutions are only prepared with pure chemicals, since such standards do not reflect the ionization of the analytes in the presence of matrix components. In this study, we employed verrucarol (VEL) and zearalanone (ZAN) as internal standards to ensure the repeatability and reproducibility, and to correct the recovery of each mycotoxin. As far as we know, this is the first report of a full-validation study on the simultaneous detection of trichothecenes (both type A and B) and zearalenone by LC-MS/MS.
Materials and Methods
Chemicals
NIV, DON, and T-2 were purchased from Wako pure chemical Industries Ltd. (Osaka, Japan), and HT-2, ZEA, VEL and ZAN from Sigma-Aldrich Co. (St. Louis, MO, USA). Acetonitrile (LC-MS grade) was purchased from Wako, distilled water (LC-MS grade) from Kanto Chemical (Tokyo, Japan), ammonium acetate (chemically pure grade) from Kanto, and acetic acid (>99.9% of chemically pure grade, not glacial) from Wako. All other chemicals used were commercially available and of a chemically pure grade.
Preparation of mycotoxin solutions
Mycotoxin solutions for stock, fortification, and calibration were prepared at NFRI as described below. NIV, DON, T-2, HT-2, ZEA, VEL, and ZAN obtained in the crystalline form were accurately weighed, individually dissolved in acetonitrile, and the volumes of these solvents were adjusted so that their concentrations were 100-200 mg/L. These stock solutions were stored in amber glass containers at 4°C (NIV, DON, T-2, and HT-2) or at -20°C (ZEA, VEL, and ZAN) to prevent photo-degradation and evaporation of the mycotoxins. The reference solutions for fortification were prepared by mixing stock solutions, excluding VEL and ZAN, at three different levels (concentration of each mycotoxin was adjusted as shown in Table 1), and a mixture of internal standards (VEL and ZAN at the concentrations of 2 mg/L and 1 mg/L, respectively) was prepared in acetonitrile. For the working solutions, each stock solution was taken, dried under a stream of nitrogen gas, and re-dissolved by dilution in acetonitrile/water/acetic acid (5/94/1, v/v/v). All of these prepared solutions were divided into amber glass bottles at the proper volume (described below), transferred to FDSC, and stored at 4°C after blind labeling.
| Fortification level | Low | Middle | High |
|---|---|---|---|
| NIV (mgL-1) | 0.4 | 1 | 10 |
| DON (mgL-1) | 0.4 | 1 | 10 |
| T-2 (mgL-1) | 0.08 | 0.2 | 2 |
| HT-2 (mgL-1) | 0.08 | 0.2 | 2 |
| ZEA (mgL-1) | 0.08 | 0.2 | 10 |
The solutions were prepared in acetonitrile, and divided in amber glass bottles (1.4mL each) with blinded labels
Table 1: Composition of spiking solutions.
Wheat and barley powder samples
Grains of wheat (Norin 61) and barley (six-rowed hulled barley) without Fusarium fungi infection were supplied by NARO Institute of Crop Science (NICS). These grains (3 kg of each) were finely ground at FDSC, and 20×10 g samples (both wheat and barley) were put in glass containers and sent to NFRI to be analyzed according to the procedure described below. After the absence of NIV, DON, T-2, HT-2, and ZEA was confirmed, the rest of the wheat and barley powder was used as blank samples. Alternatively, 1 kg of certified reference material of wheat powder naturally contaminated with T-2 and HT-2 (batch number TW-974) was purchased from Trilogy Co. Ltd (Washington, MO, USA), and stored at -20°C in the dark before use. The manufacturerlabeled concentrations of the toxins were 111.2 ± 13.8 μg/kg (T-2) and 308.5 ± 49.0 μg/kg (HT-2), respectively. These samples (both blank and naturally contaminated) were packed in aluminum bags with pouch sealing at FDSC, and were ready for delivery to the participants.
Materials delivered to participants
Each participating laboratory received the following: (a) blank powder samples of wheat and barley (70 g each in the aluminum bag, marked as “sample A” and “sample B,” respectively) for the fortification test without indicating which sample corresponded to wheat and which to barley; (b) 12 bottles of reference solution (1.4 mL each) at 3 different levels in duplicate (the concentration of each mycotoxin was shown in Table 1) that were delivered with blinded labels with random 3-figure numbers attached to “A” and “B,” such as A-XXX and B-YYY; (c) two bags (15 g each) of wheat powder (Trilogy) naturally contaminated with T-2 and HT-2 that were marked as “sample C” with blinded labels of random 3-figure numbers such as C-XXX and C-YYY; (d) a series of working solutions for calibration (11 bottles, the concentration of each mycotoxin is shown in Table 2); and (e) a bottle with an 8 mL mixture of internal standards (VEL and ZAN). All of these samples and materials were delivered to the participants from FDSC under the direction of NFRI, and stored in a refrigerator at each laboratory.
| Bottle No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NIV (mgL-1) | 1 | 2.5 | 5 | 10 | 25 | 50 | 100 | 250 | 500 | 1000 | 1500 |
| DON (mgL-1) | 1 | 2.5 | 5 | 10 | 25 | 50 | 100 | 250 | 500 | 1000 | 1500 |
| T-2 (mgL-1) | 0.2 | 0.5 | 1 | 2 | 5 | 10 | 20 | 50 | 100 | 200 | 300 |
| HT-2 (mgL-1) | 0.2 | 0.5 | 1 | 2 | 5 | 10 | 20 | 50 | 100 | 200 | 300 |
| ZEA (mgL-1) | 1 | 2.5 | 5 | 10 | 25 | 50 | 100 | 250 | 500 | 1000 | 1500 |
| VEL (internal standard) (mgL-1) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| ZAN (internal standard) (mgL-1) | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 |
All solutions were prepared in acetonitrile/water/acetic acid (5/94/1, v/v/v)
Table 2: Composition of working solutions delivered to the participant laboratories.
Fortification procedure
All of the delivered samples and materials, except the working solution (described above), were removed from the refrigerator, and left at room temperature for approximately 30 min. Then 10.0 ± 0.2 g samples were weighed and transferred from sample A (blank wheat powder) or sample B (blank barley powder) into glass containers (6 samples were prepared in parallel for both wheat and barley, respectively, and it was not specified whether they had to be put in flasks or bottles, as long as they were put in the same type of glass container). With a precision “Microman” model M1000 (Gilson S.A.S., France) micro liter pipette, an aliquot of 1.0 mL was accurately withdrawn from the 1.4 mL reference solutions (see Materials delivered to participants), and added to the above blank samples. For instance, if a bottle with a reference solution was labeled as A-XXX, then 1.0 mL was removed and added to sample A (10.0 ± 0.2 g) in a glass container. Then, 0.5 mL of the internal standard mixture (VEL and ZAN) was withdrawn with a “Microman” M1000, and added to each sample. After mixing it by patting the bottom of the glass container, it was covered with a piece of aluminum foil on the top, and kept in a freezer (-20°C) or a refrigerator (4°C) for longer than 12 hrs (shorter than 14 days). In the case of sample C (naturally contaminated wheat), fortification with the reference solution was omitted and only internal standard mixture was added after 10.0 ± 0.2 g was weighed. The fortification level of each mycotoxin was set in reference to the predominant regulation value (1.0 mg/kg) in the world as well as the provisional regulation value in Japan (1.1 mg/kg) for DON, and for ZEA (1 mg/kg) by the Ministry of Agriculture, Forestry and Fisheries of Japan (MAFF) for feeds (imported). NIV was regarded as dominant as DON. In case of T-2 and HT-2, a group TDI value of 100 ng/kg body weight for the sum of them (T-2 and HT-2) is set by EFSA [8], which is almost one-tenth the provisional maximum tolerable daily intake (PMTDI) value for DON by JECFA [2]. Therefore, their fortification was set at around one-tenth (for the lower level) and twice (for the higher level) of the EFSA’s TDI value.
Extraction and purification of mycotoxins
Extraction and purification of mycotoxins were performed using a procedure established through modification of previous reports [14,15]. We initially conducted purification though a Bond Elut Mycotoxin column (Agilent Technologies, Santa Clara, CA, USA, Part No. 12165001B) alone, yet considerable noize suggested to be derived from matrix components was observed in some samples. Hence, we made modification of the purification step to use a a Presep C18 solid phase extraction (SPE) column (ODS) (2 g/15 mL) (Wako, Part No. 296-34091) prior to the Bond Elut Mycotoxin. The samples fortified with internal standards were removed from the freezer or refrigerator, and left at room temperature for approximately 30 min. Thereafter, 40 mL of acetonitrile/water (80/20, v/v) and 0.4 mL of acetic acid (>99.9%) were added, and the mixture was homogenized for 5 min (or vigorously shaken for 30 min). The obtained slurry was centrifuged at 2,000 × g for 10 min, and a portion of the supernatant (15 mL) was loaded on a Presep C18 column. The resulting eluate was consecutively loaded on a multifunctional Bond Elut Mycotoxin column Bond Elut Mycotoxin column. After discarding the initial 3 mL of the solvent coming off the column, a 1.6 mL aliquot was removed from the following eluent, and dried under a nitrogen gas stream at 40°C. The residue was re-dissolved in 0.4 mL of acetonitrile/water/acetic acid (5/94/1, v/v/v), filtered with a hydrophilic PTFE disposable syringe filter unit DISMIC-13HP (pore size 0.20 μm) (Toyo Roshi Kaisha, Ltd., Tokyo, Grade 13HP020AN), and the filtrate was subjected to LC-MS/MS analysis.
LC-MS/MS analysis
Detection and quantification were performed by LC-MS/MS coupled with an HPLC system including a binary pump, an auto injector and an MS/MS detector mounted in each participant’s laboratory as summarized in Table 3. Basically, chromatographic separation was performed using a ZORBAX Eclispse XDB-C18 solvent saver column (250 × 3 mm i.d., 5 μm particle size) (Agilent, Part No.990967-302), maintained at 40°C with a column heater. The column was used at room temperature in case of difficulty with a column heater due to limited space. The carrier solvent was composed of water/acetic acid (99.9/0.1, v/v) containing 0.5 mM ammonium acetate (eluent A) and acetonitrile/acetic acid (99.9/0.1, v/v) (eluent B).Each component was prepared with chemicals of LC-MS grade (water, acetonitrile) or chemically pure grade (acetic acid). Sample injection was conducted at a volume between 2-20 μL, and properly adjusted in each laboratory so that the linearity of the calibration curve was sufficiently maintained (as described below). Elution was conducted at the flow rate of 0.3 mLmin-1 with a linear gradient of acetonitrile. After keeping the portion of B at 10% for 1 min, it was linearly increased to 90% within 14 min, followed by a hold time of 4 min at 90%. Thereafter, the portion of B was decreased to 10% within 1 min, and kept at 10% for 9 min prior to the next sample injection. Ionization was conducted with an electro spray ionization (ESI) probe in negative (recommended for NIV, DON, ZEA, VEL, and ZAN) or positive (recommended for T-2, HT-2, and VEL) polarity, depending on the target compounds, whereas some of them (HT-2, ZEA, and VEL) were detected in both polarities. Data acquisition was performed in two separate (positive and negative polarities) chromatographic runs under the selected reaction monitoring (SRM) mode of LC-MS/MS, and the monitor ions used for the detection of the respective mycotoxins by each laboratory as shown in Table 3.
| Laboratory | A | B | C | D | E | F | G | H | I | J | K | L | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LC | 2795 (Waters) | Acquity UPLC (Waters) | Acquity UPLC (Waters) | 1200 Series (Agilent) | Nexera (Shimadzu) | Alliance 2695 (Waters) | 2795 (Waters) | Acquity UPLC (Waters) | 1100 Series (Agilent) | 1100 Series (Agilent) | Acquity UPLC (Waters) | Prominence (Shimadzu) | |
| MS/MS | Quattro Premier XE (Waters) | TQD (Waters) | Quattro Premier XE (Waters) | QTRAP 3200 (ABsciex) | API 4000 (ABsciex) | Quattro Micro (Waters) | Quattro Premier XE (Waters) | TQD (Waters) | QTRAP 4000 (ABsciex) | API 3000 (ABsciex) | Quattro Premier XE (Waters) | TSQ Quantum Discovery MAX (Thermofisher) | |
| NIV | Precursor ion (m/z) | 371 [M+CH3COO]- | |||||||||||
| Product ion (m/z)1 | 281 | 281 | 281 | 59 | 281 | 281 | 281 | 311 | 59 | 59 | 281 | 281 | |
| DON | Precursor ion (m/z) | 355 [M+CH3COO]- | |||||||||||
| Product ion (m/z)2 | 59 | 295 | 265 | 295, 59 | 265, 59 | 59 | 265 | 59 | 59 | 59 | 265 | 295 | |
| ZEA | Precursor ion (m/z) | 317 [M-H]- | |||||||||||
| Product ion (m/z)3 | 131 | 131 | 175 | 131 | 175 | 175 | 175 | 131 | 131 | 131 | 175 | 131 | |
| T-2 | Precursor ion (m/z) | 484 [M+NH4]+ | |||||||||||
| Product ion (m/z)4 | 215 | 305 | 305 | 215, 185 | 305 | 305 | 305 | 305 | 215 | 215 | 305 | 305 | |
| HT-2 | Precursor ion (m/z) | 442 [M+NH4]+ | |||||||||||
| Product ion (m/z)5 | 263 | 263 | 263, 215 | 263 | 215 | 263 | 263 | 263 | 263 | 263 | 215 | 263 | |
| VEL | Precursor ion (m/z) | 325 [M+CH3COO]- | |||||||||||
| Product ion (m/z) | 59 | ||||||||||||
| Precursor ion (m/z) | 284 [M+NH4]+ | ||||||||||||
| Product ion (m/z) | 249, 231 | ||||||||||||
| ZAN | Precursor ion (m/z) | 319 [M-H]- | |||||||||||
| Product ion (m/z) | 205, 275 | ||||||||||||
1In addition to the product ion selected in each laboratory for quantification, any of the other product ions (59, 281, and 311) was used as the qualifier ion properly
2 In addition to the product ion selected in each laboratory for quantification, any of the other product ions (59, 265, and 295) was used as the qualifier ion properly
3 In addition to the product ion selected in each laboratory for quantification, any of the other product ions 131 and 175) was used as the qualifier ion properly
4In addition to the product ion selected in each laboratory for quantification, any of the other product ions (185, 215, and 305) was used as the qualifier ion properly
5In addition to the product ion selected in each laboratory for quantification, any of the other product ions (215 and 263) was used as the qualifier ion properly
Table 3: LC-MS/MS instruments and parameters used in each laboratory
Calibration curve
Working solutions containing NIV, DON, T-2, HT-2, and ZEA at concentrations between 0.2-1,500 μg/L with fixed concentrations of VEL (100 μg/L) and ZAN (50 μg/L) (Table 2) were prepared in acetonitrile/water/acetic acid (5/94/1, v/v/v), and used for calibration. For the correction of data on the quantitative analysis with LC-MS/ MS, VEL and ZAN were used as the internal standards to compensate for the matrix effects (ion suppression or enhancement) caused by co-existing components in each sample. The concentration ratio (X) (each trichothecene/VEL or ZEA/ZAN) and corresponding peak area ratio (Y) were plotted for the 11 bottles of working solution. A linear regression line was created with 1/X weighting. How the created equation describes the data (the ‘fit’) was expressed as a determination coefficient r2 (r-squared). The closer r2was to 1.00, the better the fit was. Therefore, at least 5 points covering the concentration level of the sample analyte were chosen from the 11 standards’ data to create a linear regression line so that an r2 value between 0.995-1.000 was obtained. The concentration of the analyte was calculated from the corresponding Y value with the linear regression line.
Harmonized collaborative validation
Harmonized collaborative validation was performed by the 12 participating laboratories in Japan in reference to the AOAC guideline. Due to the limited availability of certified reference materials of wheat or barley containing the targeted mycotoxins, collaborative validation was mainly designed based on the spike and recovery tests on two matrixes (wheat and barley). To minimize the effects of instrumental differences, preliminary test samples (wheat and barley powder samples obtained from other origin (not NICS) were spiked with the 5 mycotoxins and internal standards, extracted, purified, and re-dissolved in NFRI) were delivered to the candidate laboratories to check the LCMS/ MS conditions. The operational conditions were optimized in each laboratory (as shown in Table 3) so that the height of the signal peaks of NIV (10 μgL-1), DON (10 μgL-1), T-2 (2 μgL-1), HT-2 (2 μgL-1), and ZEA (1 μgL-1) in all the working solution bottles (No. 1 or 4 in Table 2, for instance) were sufficiently (more than 10 times, for instance) larger than the background noise level. When the LC-MS/MS conditions were confirmed to be suitable for the quantitative analysis, secondary test samples (wheat and barley powder samples from other origin were fortified by NFRI at two different levels from that used in Table 1) were delivered to check the skillfulness of operators at each laboratory. After it was confirmed that the operators’ skill was proper, the laboratory was requested to participate in harmonized collaborative validation.
Results
Statistics
The data obtained by participating laboratories were initially evaluated for evidence of outliers using statistical Cochran (between duplicates) and Grubbs single and Grubbs pair value tests (between laboratory means) [19]. The relative standard deviations for repeatability (RSDr) and reproducibility (RSDR), and the HorRat value calculated as the ratio of RSDR to the predicted RSDR were obtained using analysis of variance according to the AOAC guideline [20]. The predicted RSDR value was calculated according to the Thompson report [21]. The criteria for analytical methods mentioned in Commission Regulation (EC) No.401/2006 [22] were also used for evaluation of these parameters.
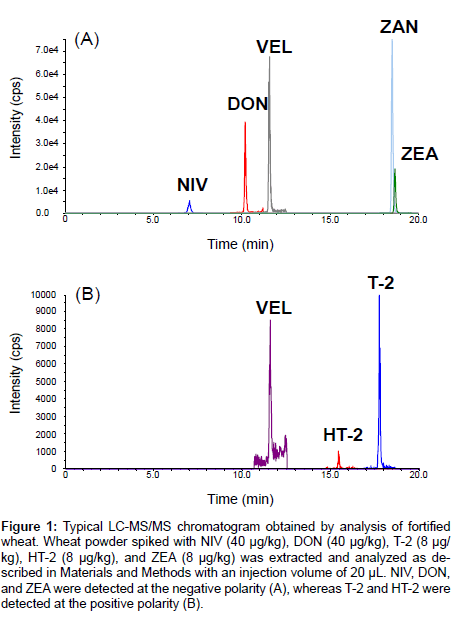
The typical LC-MS/MS chromatogram obtained through analysis of the fortified wheat was shown in Figure 1. NIV, DON, and ZEA were detected at negative polarity, whereas T-2 and HT-2 were detected at positive polarity. VEL and ZAN were also detected, and used to correct the variance through analysis. Results obtained from the harmonized collaborative validation are shown in Tables 4 and 5. Extraction of mycotoxins was performed by homogenization (5 min) by most of the participants, but at one laboratory (laboratory H) it was conducted by vigorous shaking (30 min). Values evaluated as outliers were represented in bold numbers. In Table 5, the calculated RSDr and RSDR values are indicated, and HorRat values were also obtained to evaluate the reproducibility of the presented method.
| Laboratory | A | B | C | D | E | F | G | H | I | J | K | L | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Analyte | Matrix and fortification level | Concentration (mg/kg) | Results (mg/kg) | Results (mg/kg) | Results (mg/kg) | Results (mg/kg) | Results (mg/kg) | Results (mg/kg) | Results (mg/kg) | Results (mg/kg) | Results (mg/kg) | Results (mg/kg) | Results (mg/kg) | Results (mg/kg) | ||||||||||||
| NIV | Wheat (low) | 40 | 56.4 | 56.7 | 35.9 | 36.6 | 43.7 | 41.6 | 45 | 39.1 | 41.4 | 38.1 | 40.7 | 42.2 | 93.7b | 106.1b | 47.8 | 59.1 | 44.3 | 47.7 | 33.6 | 40 | 33 | 35.5 | 47.9 | 47.7 |
| Wheat (middle) | 100 | 159.5 | 163.4 | 103.1 | 96 | 100.4 | 105.2 | 78.7 | 90.2 | 95.5 | 91.8 | 108.3 | 101.1 | 244.9a | 207.9a | 145.7 | 143 | 118.7 | 123.4 | 96.1 | 108.9 | 86.5 | 86.5 | 127.1 | 113.6 | |
| Wheat (high) | 1000 | 92.9 | 893.6 | 876 | 885.1 | 1004.9 | 1079.2 | 693 | 811 | 972.2 | 1010.9 | 952.6 | 974.9 | 999.2 | 1024 | 1107.3 | 1167.5 | 1210.5 | 1160.3 | 1040.5 | 1110.7 | 826.5 | 825 | 1409.3 | 1322.8 | |
| Barley (low) | 40 | 64.2 | 65.6 | 41.2 | 46 | 46.4 | 42.5 | 49.2 | 64.5 | 49.2 | 44.9 | 50.4 | 45.8 | 119.1b | 105.5b | 241.3b | 237.1b | 69.1 | 51.6 | 51.3 | 53.1 | 32 | 34.5 | 52.9 | 61.7 | |
| Barley (middle) | 100 | 143.7 | 140.5 | 108.8 | 108.1 | 102.9 | 105.1 | 107 | 112 | 102.4 | 120.4 | 103.8 | 107.4 | 286.2b | 289.9b | 373.2b | 397.5b | 153.1 | 138.3 | 127.2 | 137.1 | 81 | 83 | 149.8 | 143 | |
| Barley (high) | 1000 | 969.5 | 998.3 | 1048.1 | 1068.2 | 997 | 945.8 | 960 | 985 | 1030 | 908.4 | 926.3 | 948.3 | 1076.9 | 897.7 | 1483.0b | 1613.9b | 1124.1 | 1125.9 | 1190.1 | 1280.1 | 738 | 964.5 | 1480.0c | 1563.3c | |
| DON | Wheat (low) | 40 | 37.7 | 38.1 | 35.1 | 35.5 | 28.8 | 25.4 | 30.4 | 34.8 | 36 | 35.2 | 45.9 | 48 | 38.8 | 42.6 | 32.1 | 33.6 | 58.1 | 45.4 | 39.9 | 51.9 | 31.5 | 35 | 30.4 | 28.2 |
| Wheat (middle) | 100 | 99.3 | 107.5 | 93.9 | 92.8 | 75.9 | 70 | 69 | 71.4 | 93.6 | 92 | 102.9 | 101.3 | 88.2 | 67.4 | 103.4 | 107.5 | 119 | 113.4 | 109.1 | 123 | 84.5 | 85 | 70.9 | 76.3 | |
| Wheat (high) | 1000 | 1075.6 | 1027.2 | 922 | 850.8 | 745.1 | 792.6 | 705 | 817 | 1115.2 | 1127.4 | 917.9 | 928.7 | 819.9 | 820.6 | 966.3 | 911.7 | 998.2 | 1172.2 | 1120.9 | 1160.4 | 911 | 923 | 1151.6 | 948.4 | |
| Barley (low) | 40 | 40.1 | 42.8 | 41.7 | 42.2 | 30.9 | 30.1 | 30.6 | 44.8 | 38.7 | 37.6 | 47.3 | 43.6 | 45.2 | 43.9 | 32.1 | 40.4 | 88.8a | 58.5a | 143.7b | 106.4b | 34.5 | 39.5 | 42.8 | 38 | |
| Barley (middle) | 100 | 104.8 | 99.7 | 100.4 | 104.9 | 65.6 | 87.1 | 110 | 94.2 | 103 | 96.7 | 95.1 | 83.2 | 108.4 | 124.5 | 83.6 | 75.3 | 146.3 | 122.5 | 184.5b | 181.7b | 79.5 | 84.5 | 69.3 | 88.5 | |
| Barley (high) | 1000 | 1105.5 | 982.4 | 1026.6 | 1049.2 | 818.9 | 813.1 | 924 | 840 | 1118.3 | 1064 | 922.2 | 950.7 | 968.9 | 693.1 | 915.9 | 1073.3 | 993.2 | 1095.6 | 1180.9 | 1230.5 | 988 | 803 | 1180.6 | 1204.7 | |
| ZEA | Wheat (low) | 8 | 6.9 | 7.1 | 9.4 | 8.7 | 7.2 | 6.6 | 7.1 | 7.2 | 8 | 7.4 | 8.5 | 9.2 | 8.5 | 8 | 9.1 | 10.7 | 9 | 9.9 | 10.1 | 10.6 | 9 | 10 | 7.5 | 8.7 |
| Wheat (middle) | 20 | 17.4 | 18.1 | 23.4 | 24.9 | 19.4 | 19.8 | 17.6 | 18.4 | 20.5 | 20.3 | 22.8 | 21.6 | 21.5 | 21.3 | 25.8 | 25.9 | 25.8 | 24.1 | 26 | 29.1 | 23.5 | 23 | 22.9 | 21.5 | |
| Wheat (high) | 1000 | 993 | 971 | 971.4 | 886.2 | 971.1 | 838.4 | 864 | 780 | 1163.7 | 1093.2 | 1072.5 | 1066.4 | 1026.4 | 1029.5 | 1083.2 | 1212.2 | 901.6 | 923.5 | 1030.1 | 1089.1 | 1108 | 978.5 | 1088.4 | 1271.9 | |
| Barley (low) | 8 | 7.2 | 7.6 | 8.6 | 9.3 | 7.8 | 7.2 | 7 | 7.1 | 7.7 | 7.5 | 8.4 | 7.8 | 7.6 | 6.4 | 8.5 | 7.1 | 7.4 | 7.5 | 12.4b | 11.9b | 9.5 | 10 | 6.6 | 5.5 | |
| Barley (middle) | 20 | 18.8 | 17.5 | 22.8 | 26.5 | 19.7 | 15.1 | 18.3 | 17.7 | 18.8 | 19.8 | 20.7 | 22 | 20.2 | 20.7 | 21.8 | 18.7 | 19 | 17.1 | 31.2b | 30.2b | 23 | 23 | 21.4 | 17.6 | |
| Barley (high) | 1000 | 1078.5 | 1020.8 | 870.9 | 967.5 | 973.6 | 929.5 | 1020 | 970 | 1228.7 | 1244.6 | 1161.5 | 1193.4 | 950.0a | 526.8a | 833.8 | 1035.5 | 989.7 | 981.7 | 1080.1 | 1200.2 | 1124.5 | 1148.5 | 1281.9 | 1284.1 | |
| HT-2 | Wheat (low) | 8 | 11.2 | 9.4 | 9.9 | 11.8 | 8.1 | 7.7 | 9.7 | 7.9 | 7.8 | 7 | 9.1 | 8.8 | 10.6 | 10.6 | 8.3 | 7.9 | 4.7 | 5.1 | 9.6 | 9.3 | 3.2 | 2.5 | n.dd | n.dd |
| Wheat (middle) | 20 | 24.3 | 26.6 | 29.2 | 27.5 | 14.5 | 15.3 | 17.8 | 22.6 | 18.9 | 19.1 | 19.9 | 23 | 28.8 | 24.3 | 20.6 | 18 | 11 | 13.8 | 21.8 | 22.9 | 4.7 | 6.4 | 31.7 | 34.3 | |
| Wheat (high) | 200 | 277.3 | 270.4 | 258.3 | 276.3 | 217.7 | 197.2 | 202 | 155 | 211 | 204.1 | 186.6 | 192.4 | 232.6 | 233 | 179.5 | 196.7 | 117.1 | 116.4 | 189.5 | 213.1 | 64.7 | 63.8 | 619.9a | 213.3a | |
| Barley (low) | 8 | 7.1 | 8.2 | 10.6 | 9.5 | 5.8 | 4.7 | 5.4 | 7.2 | 5.3 | 4.3 | 8.1 | 8.1 | 11.1 | 9.8 | 8.6 | 8.6 | 5.6 | 4.5 | 6 | 8.1 | 4.5 | 3.3 | n.dd | n.dd | |
| Barley (middle) | 20 | 17.8 | 20.7 | 24.3 | 23.3 | 14.9 | 13.4 | 18.1 | 16.6 | 11.5 | 13.3 | 20.4 | 20.2 | 24.3 | 25.3 | 20.2 | 23.4 | 15.1 | 9.6 | 16.2 | 18.9 | 12.2 | 11.7 | 17 | 17.2 | |
| Barley (high) | 200 | 181.9 | 182.5 | 242.7 | 231.3 | 122.8 | 113.5 | 152 | 133 | 113 | 151 | 192.3 | 200.4 | 270.5a | 176.1a | 193.5 | 199.1 | 114.1 | 129.5 | 185.5 | 196.7 | 135.8 | 173.4 | 157.3 | 131.7 | |
| T-2 | Wheat (low) | 8 | 7.8 | 6.9 | 12 | 10.9 | 8.8 | 10.6 | 7.5 | 8.4 | 6 | 6.1 | 7.8 | 6.4 | 9 | 9.6 | 9 | 8.1 | 5.7 | 8 | 10.6 | 9.6 | 6.9 | 7.1 | 6.7 | 8.7 |
| Wheat (middle) | 20 | 16.2 | 19 | 29.7 | 30.9 | 22.6 | 20.1 | 21.3 | 20 | 17 | 17.8 | 18.2 | 18.2 | 21.6 | 21 | 21 | 19.2 | 14.2 | 14 | 27.4 | 26.5 | 13.2 | 16.5 | 17.2 | 18.2 | |
| Wheat (high) | 200 | 180.5 | 163.9 | 241.3 | 278.7 | 245.3 | 202.9 | 128 | 209 | 182.6 | 165.7 | 199.7 | 187.7 | 207.1 | 194.7 | 197.4 | 184.1 | 162 | 209.8 | 209.3 | 289.3 | 112.8 | 120.9 | 322 | 165.3 | |
| Barley (low) | 8 | 5.3 | 4.9 | 9.4 | 7.6 | 9.7 | 7.8 | 7.7 | 6.7 | 4.2 | 2.8 | 8.4 | 8.3 | 8.9 | 6.9 | 11.1 | 10.9 | 5 | 3.4 | 10.9 | 11.7 | 5.2 | 7 | 5.1 | 7.4 | |
| Barley (middle) | 20 | 12.9 | 10.4 | 19.2 | 15 | 24.1 | 21.8 | 25.4 | 18.1 | 7.7 | 10.6 | 20.8 | 19 | 17 | 17.3 | 17.2 | 29.6 | 12.3 | 9.1 | 25.1 | 27.3 | 18.2 | 15.9 | 15.6 | 13.9 | |
| Barley (high) | 200 | 134 | 126.4 | 162.2 | 194.3 | 193.6 | 183.8 | 157 | 157 | 110.9 | 132.4 | 163.5 | 169 | 131.9 | 142.8 | 258.2 | 222.5 | 101.6 | 108.3 | 275.1 | 261.6 | 324.4 | 316.4 | 147.9 | 129.2 | |
| T-2 | Wheat (naturally contaminated) | 111 | 111.3 | 106 | 163.4 | 181.4 | 92.6 | 89.8 | 133 | 121 | 111.8 | 115 | 67.9 | 64.4 | 158.4 | 153.9 | 174 | 158.7 | 63.1a | 106.1a | 174.5 | 166.5 | 125 | 139 | n.dd | n.dd |
Values evaluated as outliers are represented in bold numbers
aOutlier of Cochran test
bOutlier of single Grubbs test
cOutlier of paired Grubbs test
dNot reported
Table 4: Individual analytical results of LC-MS/MS determination of NIV, DON, ZEA, HT-2, and T-2 in wheat and barley.
| Analyte | Matrix (mg/kg) | No. of laboratories Valid/Outliers | Mean (mg/kg) | Mean recovery (%) | Repatability SD [Sr] | Repatability relative SD [RSDr, %] | Reproducibility SD [SR] | Reproducibility relative SD [RSDR, %] | HorRat |
|---|---|---|---|---|---|---|---|---|---|
| NIV | Wheat (40) | 11/1 | 43.4 | 108.5 | 3.3 | 7.6 | 5.5 | 17.1 | 0.8 |
| Wheat (100) | 11/1 | 111.0 | 111.0 | 5.5 | 4.9 | 24.3 | 21.9 | 1.0 | |
| Wheat (1000) | 12/0 | 1014.6 | 101.5 | 45.9 | 4.5 | 169.1 | 16.7 | 1.0 | |
| Barley (40) | 10/2 | 50.8 | 127.0 | 5.9 | 11.7 | 10.3 | 20.2 | 0.9 | |
| Barley (100) | 10/2 | 118.7 | 118.7 | 6.1 | 5.1 | 22.0 | 18.6 | 0.8 | |
| Barley (1000) | 10/2 | 1009.1 | 100.9 | 74.6 | 7.4 | 119.0 | 11.8 | 0.7 | |
| DON | Wheat (40) | 12/0 | 37.4 | 93.5 | 4.0 | 10.6 | 8.0 | 21.4 | 1.0 |
| Wheat (100) | 12/0 | 92.4 | 92.4 | 5.8 | 6.3 | 17.0 | 18.4 | 0.8 | |
| Wheat (1000) | 12/0 | 955.4 | 95.5 | 64.1 | 6.7 | 140.7 | 14.7 | 0.9 | |
| Barley (40) | 10/2 | 39.3 | 98.3 | 4.2 | 10.6 | 5.3 | 13.4 | 0.6 | |
| Barley (100) | 11/1 | 96.7 | 96.7 | 10.1 | 10.4 | 19.3 | 20.0 | 0.9 | |
| Barley (1000) | 12/0 | 997.6 | 99.8 | 85.4 | 8.6 | 142.8 | 14.3 | 0.9 | |
| ZEA | Wheat (8) | 12/0 | 8.5 | 106.3 | 0.6 | 6.8 | 1.2 | 14.6 | 0.7 |
| Wheat (20) | 12/0 | 22.3 | 111.5 | 0.9 | 4.1 | 3.1 | 14.0 | 0.6 | |
| Wheat (1000) | 12/0 | 1017.2 | 101.7 | 67.2 | 6.6 | 119.4 | 11.7 | 0.7 | |
| Barley (8) | 11/1 | 7.7 | 96.3 | 0.5 | 6.9 | 1.1 | 13.7 | 0.6 | |
| Barley (20) | 11/1 | 20.0 | 100.0 | 1.8 | 8.8 | 2.6 | 12.9 | 0.6 | |
| Barley (1000) | 11/1 | 1073.6 | 107.4 | 58.0 | 5.4 | 133.8 | 12.5 | 0.8 | |
| HT-2 | Wheat (8) | 11/0* | 8.2 | 102.5 | 0.7 | 9.0 | 2.5 | 30.5 | 1.4 |
| Wheat (20) | 12/0 | 20.7 | 103.5 | 1.9 | 9.2 | 7.5 | 36.5 | 1.7 | |
| Wheat (200) | 11/1 | 193.4 | 96.7 | 13.4 | 6.9 | 61.4 | 31.7 | 1.6 | |
| Barley (8) | 11/0* | 7.0 | 87.5 | 0.9 | 12.4 | 2.3 | 32.2 | 1.5 | |
| Barley (20) | 12/0 | 17.7 | 88.5 | 1.7 | 9.4 | 4.6 | 25.9 | 1.2 | |
| Barley (200) | 11/1 | 165.1 | 82.6 | 14.4 | 8.7 | 39.2 | 23.7 | 1.1 | |
| T-2 | Wheat (8) | 12/0 | 8.3 | 103.8 | 0.9 | 10.9 | 1.7 | 20.8 | 0.9 |
| Wheat (20) | 12/0 | 20.0 | 100.0 | 1.2 | 6.0 | 4.8 | 23.7 | 1.1 | |
| Wheat (200) | 12/0 | 198.3 | 99.2 | 42.9 | 21.6 | 50.7 | 25.6 | 1.3 | |
| Barley (8) | 12/0 | 7.3 | 91.3 | 1.0 | 14.1 | 2.6 | 34.8 | 1.6 | |
| Barley (20) | 12/0 | 17.6 | 88.0 | 3.4 | 19.0 | 5.9 | 33.5 | 1.5 | |
| Barley (200) | 12/0 | 179.3 | 89.7 | 12.4 | 6.9 | 66.0 | 36.8 | 1.8 | |
| T-2 | Naturally contaminated wheat (111) | 11/0* | 130.4 | 117.5 | 7.2 | 5.5 | 36.5 | 28.0 | 1.3 |
*One participant did not report the data
Table 5: Harmonized collaborative validation results of LC-MS/MS determination of NIV, DON, ZEA, HT-2, and T-2 in wheat and barley.
Figure 1: Typical LC-MS/MS chromatogram obtained by analysis of fortified wheat. Wheat powder spiked with NIV (40 μg/kg), DON (40 μg/kg), T-2 (8 μg/ kg), HT-2 (8 μg/kg), and ZEA (8 μg/kg) was extracted and analyzed as described in Materials and Methods with an injection volume of 20 μL. NIV, DON, and ZEA were detected at the negative polarity (A), whereas T-2 and HT-2 were detected at the positive polarity (B).
Nivalenol
One and two outliers were observed with wheat (40 and 100 μg/kg) and barley (40, 100, and 1000 μg/kg), respectively (Table 4). In the case of barley spiked with 40 μg/kg, the mean recovery was 127.0% (Table 5). The recovery values (111.0% and 118.7%) obtained for the fortification of 100 μg/kg were slightly higher than the criteria suggested by EU for DON (60-110% recovery at a concentration range of 100-500 μg/kg) [22]. On the other hand, the recommended recovery at a concentration range of 50-250 μg/kg was 60-130% for T-2 [22]. Considering the structure similarity between NIV and T-2, the recovery values obtained for NIV still seem to be acceptable. In addition, NIV recovery values under the other conditions were satisfactory (100.9-108.5%),and obtained RSDr (4.5-11.7%) and RSDr (11.8-21.9%) values were acceptable as compared with the performance criteria (RSDr ≤ 20% and RSDR ≤ 40%) suggested by EU for DON [22] (Table 5). In comparison with the AOAC guideline [20], a HorRat value between 0.5-1.5 was confirmed at all the spiked levels, indicating that the presented method was reproducible for the determination of NIV contained both in wheat and barley at a concentration between 40-1000 μg/kg.
Deoxynivalenol
One and two outliers were observed with barley at the fortification levels of 100 and 40 μg/kg, respectively (Table 4). Except for these, the recovery values (92.4-99.8%) were fine, and the obtained RSDr (6.3- 10.6%) and RSDR (13.4-21.4%) values were acceptable as compared with the performance criteria (recovery 60-110% or 70-120%, RSDr ≤ 20%, and RSDR ≤ 40%, respectively) suggested by EU for DON [22] (Table 5). A HorRat value within 0.5-1.5 was confirmed at all the spiked concentrations, indicating that the presented method was reproducible for the determination of DON contained both in wheat and barley at a concentration between 40-1000 μg/kg.
Zearalenone
One outlier was observed with barley at fortification levels of 8, 20, and 1000 μg/kg, respectively (Table 4). Except for this, the recovery values (96.3-111.5%) were fine, and the obtained RSDr (4.1-8.8%) and RSDR (11.7-14.6%) values were acceptable as compared with the performance criteria (recovery 60-120% or 70-120%, RSDr ≤ 25 or 40%, and RSDR ≤ 40 or 50%, respectively) suggested by EU for ZEA [22] (Table 5). A HorRat value within 0.5-1.5 was confirmed at all the spiked concentrations, indicating that the presented method was reproducible for the determination of ZEA contained both in wheat and barley at a concentration between 8-1000 μg/kg.
HT-2 Toxin
One of the participants did not report HT-2 data at a fortification level of 8 μg/kg due to insufficient sensitivity of the LC-MS/MS instrument (Table 4). One outlier was observed at a fortification level of 200 μg/kg for both wheat and barley. Meanwhile, the recovery values (82.6-103.5%) were fine, and the obtained RSDr (6.9-12.4%) and RSDR (23.7-36.5%) values were acceptable as compared with the performance criteria (recovery 60-130%, RSDr ≤ 30 or 40%, and RSDR ≤ 50 or 60%, respectively) suggested by EU for HT-2 [22] (Table 5). In comparison with the AOAC guideline [20], a HorRat value >1.5 was observed for wheat at fortification levels of 20 and 200 μg/kg. Probable lower intensity of HT-2 by LC-MS/MS (as shown in Figure 1B) resulted in its susceptibility to matrix noise. In addition, VEL peak intensity at the positive polarity was lower than that at the negative polarity (Figure 1A and 1B). Therefore, VEL seemed to be more susceptible to matrix noise at the positive polarity. Overall, the HorRat value was less than 2, indicating that the presented method was reproducible for the determination of HT-2 contained both in wheat and barley at a concentration between 8-200 μg/kg (Table 5). With regard to naturally contaminated wheat (batch number TW-974), the manufacturerlabeled concentrations of HT-2 (308.5 ± 49.0 μg/kg) was outside the calibration curve, therefore this concentration was not included as part of the method validation in this study.
T-2 Toxin
In the case of the fortified samples, the recovery values (88.0- 103.8%) were fine, and the obtained RSDr (6.9-21.6%) and RSDR (20.8- 36.8%) values were acceptable, as compared with the performance criteria (recovery 60-130%, RSDr ≤ 30 or 40%, and RSDR ≤ 50 or 60%, respectively) suggested by EU for T-2 [22] (Tables 4 and 5). Although no outliers were found, a HorRat value >1.5 was observed for barley at the fortification levels of 8 and 200 μg/kg. This was probably due to the lower VEL peak with its susceptibility to the matrix noise, as described above. Even so, the HorRat value was overall less than 2, indicating that the presented method was reproducible for the determination of T-2 contained both in wheat and barley at a concentration between 8-200 μg/kg. In the case of naturally contaminated wheat, one participant did not report any data due to the improper shape of the VEL peak under significant matrix effects (bottom in Table 4). After the removal of 1 outlier, a fine recovery value (117.5%), and acceptable RSDr (5.5%) and RSDR (28.0%) values were obtained (bottom in Table 5). Therefore, it is suggested that the present method is applicable not only for fortified samples, but also for the naturally contaminated samples.
Discussion
In spite of the recent progress in mass spectrometry as tools for the detection as well as quantification of various chemicals including pesticides, only a few studies on the inter-laboratory validation conducted on the multiple analysis of mycotoxins by LC-MS or LCMS/ MS with a sufficient number of participating laboratories have been reported [16-18]. The difficulty of performing method validation by LC-MS/MS through an inter-laboratory study may be appreciated based on the difficulty to gather a sufficient number of participants. However, there seemed to be fundamental reasons considering the properties of LC-MS/MS instruments. We postulated that the crucial factors could be “matrix effects,” “variance within the laboratory,” and “instrumental differences”. To compensate for the matrix effects, the use of internal standards or a matrix-matched calibration curve was often recommended. Klötzel et al. [14] used both of these in their first study on the simultaneous determination of 12 type A and B trichothecenes in cereals by LC-MS/MS. They adopted external calibration (standard solutions in the mobile phase; not matrix assisted) and correction using internal standards (de-epoxy-DON for trichothecenes, and ZAN for ZEA) with the Bond Elut Mycotoxin purification column in the successive study [15]. Therefore, we basically followed their procedure, except that we employed VEL as the internal standard for trichothecenes in place of de-epoxy-DON. Since VEL was binary ionized forming both [M+NH4]+ and [M+CH3COO]- adducts under the positive and negative polarities in the presence of ammonium acetate, it seemed more useful than de-epoxy-DON. The variance within the laboratory is possibly occurring with repeatability or reproducibility within each individual laboratory. Even if the samples are analyzed according to the same protocol with the same calibration standards by an identical LC-MS/MS instrument, extraction efficiency, purification efficiency, and ionization efficiency may differ within the laboratory depending on the time (between morning and evening, for instance) or between different days. Different operators and SPE columns of different lots may also be concerned if the analysis is repeated at several intervals of weeks or months. To minimize such variance within the laboratory, the use of internal standards also seemed to be effective. Klötzel et al. [14,15] added the internal standards (de-epoxy-DON and ZAN) after extraction, therefore the variance in the extraction efficiency among these samples was not adjusted during their procedure. In contrast, we added the internal standards (VEL and ZAN) prior to the extraction, so that the variance at the extraction step possibly occurring within the laboratory was properly corrected. The instrumental difference is the characteristic variance among the LC-MS/MS instruments especially concerning the behavior of the mass spectrometer during ionization and fragmentation. As shown in Table 3, participating laboratories used various LC-MS/MS instruments of several manufacturers. These instrumental differences may result in a variety of optimized MS/MS parameters such as abundant SRM transitions (Table 3). To minimize the effects of this instrumental difference, preliminary test samples (described above) were delivered to the participants, and SRM transitions subject to the matrix effects were eliminated from the optimized MS/MS parameters. In this way, satisfactory results were obtained through the harmonized collaborative validation.
In the previous study, Aoyama et al. [17] reported an interlaboratory study involving 11 laboratories on the determination of DON and NIV in wheat based on LC-UV and LC-MS(/MS) instruments. In their study, the data obtained by LC-MS/MS were not differentiated from those obtained by LC-MS, and were evaluated with the same validation criteria. From the standpoint of instrumental properties, LC-MS/MS is different from LC-MS, since the former usually isolates the precursor ions and further obtains corresponding fragment ions produced in the collision cell. Recently, Yoshinari et al. [18] reported another inter-laboratory study of the analysis of DON and its acetylated derivatives in wheat by LC-MS/MS. The validation study was conducted by 9 laboratories and involved three mycotoxins belonging to type B trichothecenes (DON, 3-acetyl-DON, and 15-acetyl-DON), but not the other major Fusarium mycotoxins such as NIV and ZEA. In Asia, including Japan, NIV contamination has been as predominantly reported as DON [5,6]. Although the results of these two studies were similar [17,18], it is not reasonable to compare them, because the concentrations of the fortified samples were quite different as described in the discussion of them [18].
Thus far, it has been suggested that the employment of internal standards for mycotoxin analysis by LC-MS(/MS) corrects for variance during the steps of extraction and clean-up, and that it compensates for the matrix effects [23]. Stable isotope dilution assays have often been regarded as effective, especially when carbon-13-labeled standards were used. Recent studies indicated that the use of isotope-labeled surrogates seemed to be effective to secure analytical values with the mycotoxin analysis by LC-MS/MS [24,25]. However, such isotopelabeled chemicals are very expensive, and it therefore seems difficult to adopt them for monitoring a large number of samples. The present method seems to be cost-effective since VEL and ZAN (neither of them are reported to be detected in naturally contaminated wheat and barley) are used as the internal standards for trichothecenes and zearalenone in place of carbon-13-labeled chemicals.
In conclusion, our report is the first report of harmonized collaborative validation of the multiple detection method for trichothecenes and zearalenone in wheat and barley by LC-MS/MS. The validated method is applicable both to wheat and barley within the reliable ranges of NIV (40- 1000 μg/kg), DON (40-1000 μg/kg), T-2 (8-200 μg/kg), HT-2 (8-200 μg/kg), and ZEA (8-1,000 μg/kg), and it is therefore suggested as a practical tool for monitoring these Fusarium mycotoxins (trichothecenes and zearalenone) in wheat and barley.
Acknowledgements
This research was supported by a grant from the Ministry of Agriculture, Forestry and Fisheries of Japan (Research project ensuring food safety from farm to table MT-3218). We are also very grateful to Dr. Takashi Yanagisawa and Dr.Chikako Kiribuchi-Otobe of NARO, NICS for their kindness to have supplied us with the wheat and barley grains without any Fusarium sp. fungi infection.
References
- Desjardins AE (2006) Fusarium mycotoxins, Chemistry, Genetics, and Biology. APS Press, St. Paul, Minnesota.
- Joint FAO/WHO Expert Committee on Food Additives (JECFA) (2001) WHO Food Additives series 47: Safety evaluation of certain mycotoxins in food. World Health Organization, Geneva 419-555.
- Kushiro M, Zheng Y, Nagata R, Nakagawa H, Nagashima H (2009) Limited surveillance of fumonisins in brown rice and wheat harvested in Japan. J Food Prot 72: 1327-1331.
- Food and nutrition papers 81: Worldwide regulations for mycotoxins in food and feed in 2003 (2003) Food and Agriculture Organization of the United Nations (FAO). Rome.
- Kim JC, Kang HJ, Lee DH, Lee YW, Yoshizawa T (1993) Natural occurrence of Fusarium mycotoxins (trichothecenes and zearalenone) in barley and corn in Korea. Appl Environ Microbiol 59: 3798-3802.
- Yoshizawa T, Jin YZ (1995) Natural occurrence of acetylated derivatives of deoxynivalenol and nivalenol in wheat and barley in Japan. Food Addit Contam 12: 689-694.
- Tanaka T, Hasegawa A, Yamamoto S, Lee U-S, Sugiura Y, et al. (1988) Worldwide contamination of cereals by the Fusarium mycotoxins nivalenol, deoxynivalenol, and zearalenone. 1. Survey of 19 countries. J Agric Food Chem 36: 979-983.
- EFSA Panel on Contaminants in the Food Chain (CONTAM) (2011) Scientific Opinion on the risks for animal and public health related to the presence of T-2 and HT-2 toxin in food and feed. EFSA Journal 9: 2481.
- De Boevre M, Di Mavungu JD, Maene P, Audenaert K, Deforce D, et al. (2012) Development and validation of an LC-MS/MS method for the simultaneous determination of deoxynivalenol, zearalenone, T-2-toxin and some masked metabolites in different cereals and cereal-derived food. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 29: 819-835.
- Berthiller F, Sulyok M, Krska R, Schuhmacher R (2007) Chromatographic methods for the simultaneous determination of mycotoxins and their conjugates in cereals. Int J Food Microbiol 119: 33-37.
- Åberg AT, Solyakov A, Bondesson U (2013) Development and in-house validation of an LC-MS/MS method for the quantification of the mycotoxins deoxynivalenol, zearalenone, T-2 and HT-2 toxin, ochratoxin A and fumonisin B1 and B2 in vegetable animal feed. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 30: 541-549.
- Sulyok M, Berthiller F, Krska R, Schuhmacher R (2006) Development and validation of a liquid chromatography/tandem mass spectrometric method for the determination of 39 mycotoxins in wheat and maize. Rapid Commun Mass Spectrom 20: 2649-2659.
- Lattanzio VM, Gatta SD, Suman M, Visconti A (2011) Development and in-house validation of a robust and sensitive solid-phase extraction liquid chromatography/tandem mass spectrometry method for the quantitative determination of aflatoxins B1, B2, G1, G2, ochratoxin A, deoxynivalenol, zearalenone, T-2 and HT-2 toxins in cereal-based foods. Rapid Commun Mass Spectrom 25: 1869-1880.
- Klötzel M, Gutsche B, Lauber U, Humpf HU (2005) Determination of 12 type A and B trichothecenes in cereals by liquid chromatography-electrospray ionization tandem mass spectrometry. J Agric Food Chem 53: 8904-8910.
- Klötzel M, Lauber U, Humpf HU (2006) A new solid phase extraction clean-up method for the determination of 12 type A and B trichothecenes in cereals and cereal-based food by LC-MS/MS. Mol Nutr Food Res 50: 261-269.
- Senyuva HZ, Gilbert J, Stroka J, Biselli S, De Girolamo A, et al. (2010) Determination of fumonisins B1 and B2 in corn by LC/MS with immunoaffinity column cleanup: interlaboratory study. J AOAC Int 93: 611-621.
- Aoyama K, Akashi H, Mochizuki N, Ito Y, Miyashita T, et al. (2012) Interlaboratory study of LC-UV and LC-MS methods for the simultaneous determination of deoxynivalenol and nivalenol in wheat. Shokuhin Eiseigaku Zasshi 53: 152-156.
- Yoshinari T, Tanaka T, Ishikuro E, Horie M, Nagayama T, et al. (2013) Inter-laboratory study of an LC-MS/MS method for simultaneous determination of deoxynivalenol and its acetylated derivatives, 3-acetyl-deoxynivalenol and 15-acetyl-deoxynivalenol in wheat. Shokuhin Eiseigaku Zasshi 54: 75-82.
- Horwitz W (1995) Protocol for the Design, Conduct and Interpretation of Method-Performance Studies. Pure Appl Chem 67: 331-343.
- Ciolino LA, Fraser DB, Yi TY, Turner JA, Barnett DY, et al. (1999) Reversed phase ion-pair liquid chromatographic determination of nicotine in commercial tobacco products. 2. Cigarettes. J Agric Food Chem 47: 3713-3717.
- Thompson M (2000) Recent trends in inter-laboratory precision at ppb and sub-ppb concentrations in relation to fitness for purpose criteria in proficiency testing. Analyst 125: 385-386.
- Commission of the European Communities (2006) Official Journal of the European Union: L70/12-34 (Commission Regulation No.401/2006).
- Rychlik M, Asam S (2008) Stable isotope dilution assays in mycotoxin analysis. Anal Bioanal Chem 390: 617-628.
- Varga E, Glauner T, Köppen R, Mayer K, Sulyok M, et al. (2012) Stable isotope dilution assay for the accurate determination of mycotoxins in maize by UHPLC-MS/MS. Anal Bioanal Chem 402: 2675-2686.
- Al-Taher F, Banaszewski K, Jackson L, Zweigenbaum J, Ryu D, et al. (2013) Rapid method for the determination of multiple mycotoxins in wines and beers by LC-MS/MS using a stable isotope dilution assay. J Agric Food Chem 61: 2378-2384.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 16496
- [From(publication date):
specialissue-2014 - Aug 29, 2025] - Breakdown by view type
- HTML page views : 11751
- PDF downloads : 4745