Make the best use of Scientific Research and information from our 700+ peer reviewed, Open Access Journals that operates with the help of 50,000+ Editorial Board Members and esteemed reviewers and 1000+ Scientific associations in Medical, Clinical, Pharmaceutical, Engineering, Technology and Management Fields.
Meet Inspiring Speakers and Experts at our 3000+ Global Conferenceseries Events with over 600+ Conferences, 1200+ Symposiums and 1200+ Workshops on Medical, Pharma, Engineering, Science, Technology and Business
Review Article Open Access
Poly (N-Isopropylacrylamide) Microgel-Based Etalons for Optical Sensing
| Liang Hu and Michael J. Serpe* | ||
| Department of Chemistry, University of Alberta, Edmonton, AB, T6G2G2 Canada | ||
| Corresponding Author : | Michael J. Serpe Department of Chemistry, University of Alberta Edmonton, AB, T6G2G2 Canada E-mail: michael.serpe@ualberta.ca |
|
| Received April 07, 2012; Accepted May 05, 2012; Published May 09, 2012 | ||
| Citation: Hu L, Serpe MJ (2012) Poly (N-Isopropylacrylamide) Microgel-Based Etalons for Optical Sensing. J Anal Bioanal Tech 3:132. doi: 10.4172/2155-9872.1000132 | ||
| Copyright: © 2012 Hu L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Poly (N-isopropylacrylamide) (pNIPAm) is one of the most completely studied “smart” polymers due to its unique reversible thermoresponsivity. That is, when pNIPAm in water is heated > ~31°C, it transits from a random coil to a globule conformation; this transition is reversed when T < ~31°C. This conformational change is accompanied by water exchange process. When pNIPAm undergoes the coil to globule transition, water is expelled, while water is “sorbed” when the polymer undergoes the opposite process.
\| Introduction | |
| Poly (N-isopropylacrylamide) (pNIPAm) is one of the most completely studied “smart” polymers due to its unique reversible thermoresponsivity. That is, when pNIPAm in water is heated > ~31°C, it transits from a random coil to a globule conformation; this transition is reversed when T < ~31°C. This conformational change is accompanied by water exchange process. When pNIPAm undergoes the coil to globule transition, water is expelled, while water is “sorbed” when the polymer undergoes the opposite process. Researchers have synthesized various structures from pNIPAm, e.g., block [1], random [2], and star [3] polymers, as well as crosslinked hydrogels, and ultimately colloidally stable particles (microgels [4], and nanogels [5]). Of these, microgels are generally defined as cross-linked networks of water soluble polymers. The special network structure leads microgels to swell with water. Since stimuli responsive microgels were discovered, they have emerged as important building blocks for sensing, [6,7] chemical separation, [8,9] and drug delivery [10,11]. Compared with hydrogels, the size of microgels is in the range of tens of nanometers to several micrometers that gives rise to a rapid response to external stimuli [12]. Additionally, pNIPAm microgels can be functionalized with other comonomers, including acrylic acid (AAc), [13-15] 2-hydroxyethyl methacrylate, [16] N-hydroxymethyl acrylamide, [17] and so on. Of these, AAc is commonly used to functionalize pNIPAm microgels to render them pH responsive. Since AAc has a pKa of ~ 4.25, pNIPAm-co-AAc microgels are negatively charged at pH> 4.25, and “neutral” (initiator affords the microgel with a slight negative charge) at pH< 4.25. As a result, pNIPAm- co-AAc microgels are not completely thermoresponsive at pH> 4.25 due to Coulombic repulsions between the deprotonated AAc in the microgel structure. At pH< 4.25 the microgels are fully thermoresponsive. AAc also allows for facile functionalization of the microgels via reaction of amine containing molecules with the AAc on the microgel via carbodiimide coupling [18,19]. | |
| Various optical sensors have been designed with stimuli-responsive hydrogels/ microgels. For example, Asher et al. [20,21] developed three dimensional (3D) polymerized colloidal crystal array hydrogel sensing materials. Composed of stimuli-responsive hydrogels, these photonic materials (PMs) change their volume in response to external stimuli, resulting in the shift of the Bragg diffraction of the crystalline colloidal array, and therefore yielding evident color changes. Additionally, Asher et al. [22] prepared close-packed two dimensional (2D) polystyrene particle arrays by self-assembly of spreading particle monolayers on mercury surfaces. Color alterations of high-diffraction efficiency 2D photonic crystals indicate environmental changes, and hence allowing them to be developed for sensing. PMs fabricated by a number of groups [23-27] have refractive index order periodicity in 3D and 2D, however PMs can also have structural periodicity in one dimension (1D), yielding their own interesting optical properties and colors as well. In this submission, we review our recent work on pNIPAm microgels based the 1D PM structure [28-33]. | |
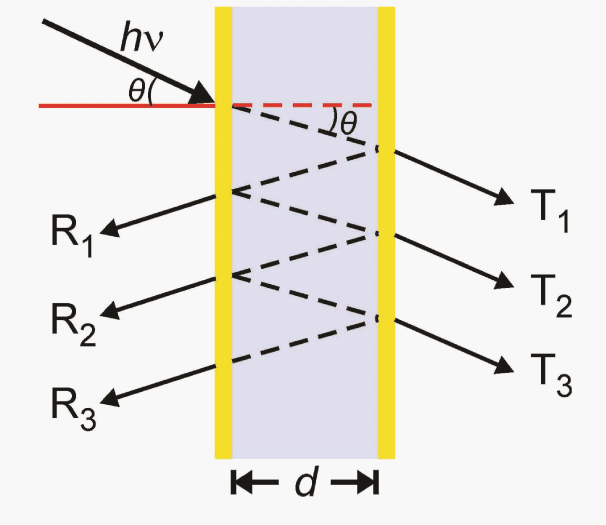
| 1D PMs are generally composed of periodically arranged layers of alternating refractive index in only one dimension (e.g. x, y, or z axis only) such as Bragg mirrors, interferometers, waveguides, and Fabry- Pérot etalons; the reflected/refracted light at the interface of each layer leads to constructive/destructive interference, resulting in color. Recently, Fabry-Pérot etalons (simply etalons) were investigated in our group [28-34] and others. [35-37] Etalons are composed of a dielectric cavity confined between two reflective surfaces (Scheme 1). After entering the etalon, light resonates in the dielectric cavity, and therefore produces light interference. This interference gives rise to specific wavelengths of light that are reflected. Interestingly, the material can exhibit visible color tunability if the dielectric layer is of the appropriate thickness, and can be made to change thickness. Furthermore, the refractive index of the dielectric layer can also affect the visible color. This is accounted for by equation (1): | |
| λm = 2nd cosθ (1) | |
| where the specific wavelength maximum of the peak (λ) depends on the peak order (m), refractive index of the dielectric(n) and the spacing between the mirrors (d), as well as the angle of incidence(θ). [38] In our etalons, Au and pNIPAm-based microgels serve as the mirrors and the dielectric layer, respectively. | |
| Fabrication of pNIPAm Microgel Based Etalons | |
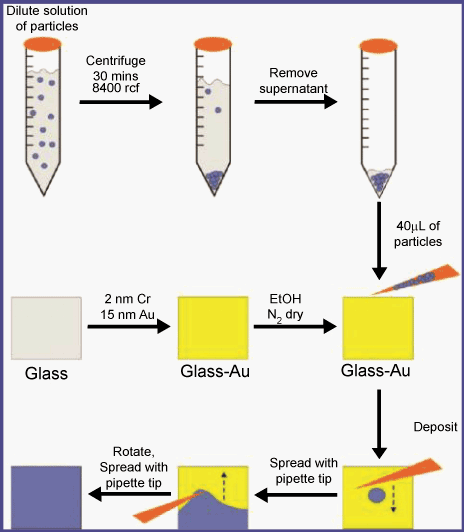
| To fabricate an etalon, a Au coated glass cover slip (2 nm Cr was used as an adhesion layer followed by 15 nm Au) was made using a thermal evaporation system (New Windsor, NY) at a rate of ~1 Å s-1 (Cr), and ~0.2 Å s-1 (Au), respectively. Then, the Cr/Au coated substrates (simply Au substrates) were annealed at 250°C for 3 h, followed by rinsing with ethanol, dried with N2 prior to use. After pNIPAm based microgels were deposited on Au substrate, followed by deposition of an additional 2 nm Cr/ 15 nm Au layer using the aforementioned instrument. Herein, we desire “perfect” microgel monolayers on the Au substrate. To accomplish this, a so called “paint-on” protocol was developed by our group. As seen in Scheme 2, the paint-on protocol requires centrifuging a solution of microgels (microgels synthesized according to [30]) until they were concentrated at the bottom of the centrifuge tube. This takes 30-60 min, depending on the diameter of the microgels. Following centrifugation, the supernatant solution was removed, and a 40 μL aliquot of the concentrated microgels was deposited onto the Au substrate at 30°C. This aliquot was then spread toward each edge using the side of a micropipette tip until the microgels covered the entire Au substrate. The spreading continued until the microgel solution was too viscous to spread over the surface. At that point, the microgels were allowed to dry completely on the substrate for 2 h at 35°C. After 2 h, the dry film was rinsed with copious amounts of DI water and soaked in DI water overnight to remove microgels not bound directly to the Au. This method yields an extremely uniform etalon both spectrally and visually. [29] Moreover, homogeneity of the response of the etalons was also significantly enhanced from spot to spot using our painting protocol. In addition, this painting method can be applied to coat a variety of different microgels on a variety of surfaces [31]. | |
| Application | |
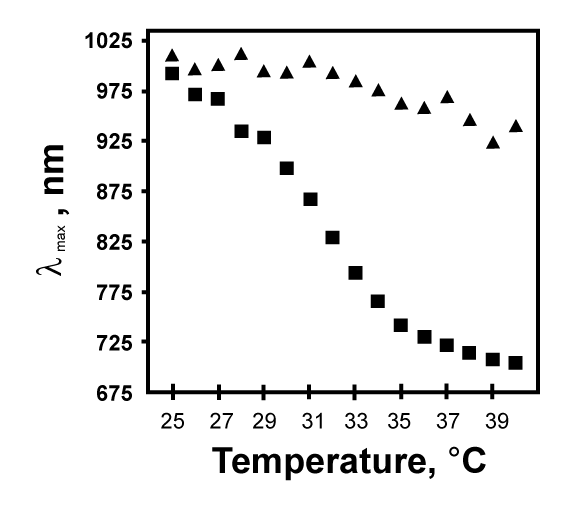
| To investigate the thermo- and pH-responsivity of the etalon, we secure it in a special chamber, Figure 1. The chamber allows us to directly measure the reflectance spectrum from the etalon, and allows us to very precisely control the temperature of the solution the etalon is in. Using this setup, we can easily monitor the wavelength maximum (λmax) in the reflectance spectrum for a given peak order as a function of temperature and pH. From Figure 2, we see that when the etalon is immersed in pH 3.0 solution, the λmax shows a blue-shift of approximately 300 nm over the given temperature range. This shift is attributed to the collapse of the microgels at high temperature bringing the Au mirrors closer to one another shifting λmax accordingly based on equation 1. Moreover, the most dramatic shift occurs between 29 and 35°C, which correlates with the behavior of the microgels in solution. At pH 6.5, this behavior is suppressed due to Coulombic repulsion deprotonated AAc in the microgel structure. This behavior shows the sensitivity of etalons toward temperature and pH changes. | |
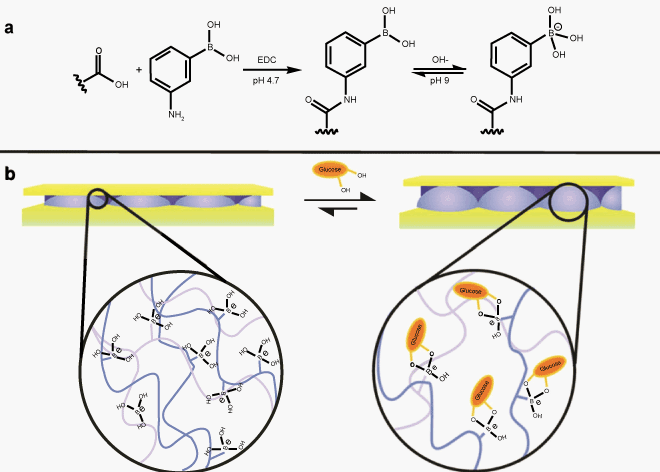
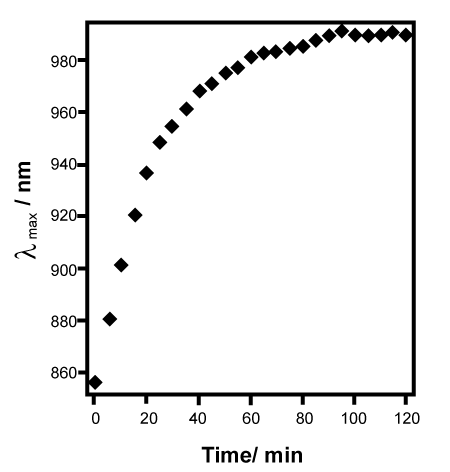
| To assess whether etalons can potentially be developed for point-ofcare (POC) diagnostic applications, we used glucose sensitive etalons for the proof-of-concept [32]. Glucose sensitive microgels were synthesized by modification of the microgels with aminophenylboronic acid (APBA) APBA-functionalized pNIPAm-based microgels have been shown to change solvation state in a manner that depended on glucose concentration. Specifically, they swell in the presence of glucose. Therefore, fabrication of etalons from these microgels should yield a material that changes color in response to glucose addition, as depicted in Figure 3.Therefore, the APBA modified microgels based etalons were fabricated, and we studied their spectral responsive properties with glucose. As seen in Figure 4, there is a significant spectral red-shift (~134 nm) in the presence of 3 mg/mL solution of glucose (pH 9 carbonate buffer) and the majority of the spectral shift occurs within 30 minutes of glucose introduction, therefore yielding a visible color changes (Figure 5). Given that APBA can bind to other diols as well, APBA modified microgel etalons can exhibit a colorimetric and spectral response to other biologically relevant molecules as well, thus, further specificity will need to be built in for future biosensing efforts. | |
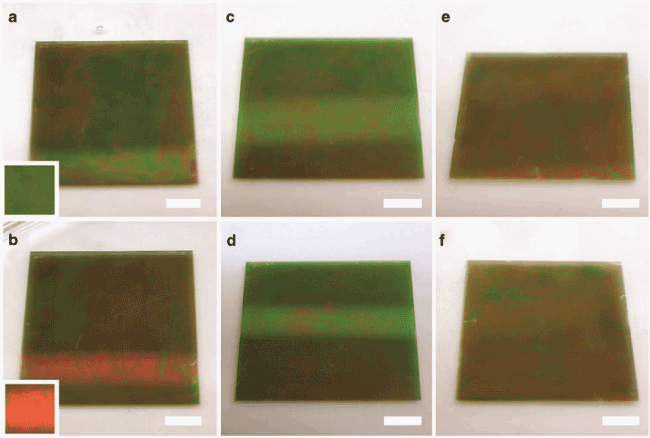
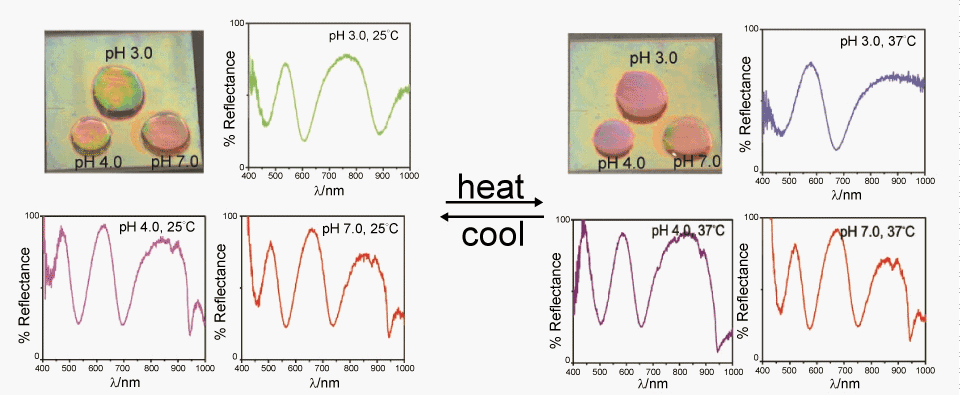
| Thus far, our group and others have focused on investigating the color tunability of a complete PM [29-33]. To move the field forward, one challenge is modulating the color of the material in specific regions, while not affecting the optical properties of another, spatially isolated, region. Recently our group investigated the ability of the etalon to be solvated, and the color modulated, in spatially isolated regions. Specifically, pH 3.0, 4.0, 7.0 solutions were deposited between three separate reflectance probes and an etalon, and the reflectance spectra for the different regions were collected as a function of temperature. As seen in Figure 6, the peak for the spot at pH 3.0 significantly blue-shifts with increasing temperature, while the spots at pH 4.0 and 7.0 shift minimally over the same temperature range. Additionally, the colors of the individual spots are visibly distinct, and change independently as a function of temperature. For example, the pH 3.0 spot visibly changes color from green to red as the temperature increases, while the others do not significantly change. Although three spots are close to each other, independent responsive behavior is observed as expected. This independent responsive behavior on a single etalon enhances the utility of the materials for sensing applications. | |
| So far, our pNIPAm microgel based etalons also show potential applications in humidity sensing, pressure sensing, and organic solvent titration (data not shown). | |
| Conclusions | |
| With the fascinating properties of pNIPAm microgels and the invention of the “paint-on” protocol, it is quite simple to fabricate a variety of pNIPAm microgels based etalons. Given that tunable “lattice” spacing dominates colors in ordered materials, pNIPAm microgels based etalons can be made to respond to pH and temperature, changing color visually and spectrally. APBA functionalized pNIPAm microgels rendered etalons responsive to biologically molecules (i.e., glucose), thus the color of the etalon changes in the presence of biological analytes. Also, the independent tunable optical properties at different regions on a single etalon are realized. Even though pNIPAM micorgels have been widely studied, pNIPAm microgel based etalons require further research. Combining promising physical and chemical properties of pNIPAm microgels with optical properties of etalons, pNIPAm microgel based etalons are likely to become quite appealing biosensors in the near future. | |
| Acknowledgements | |
| M.J.S. acknowledges funding from the University of Alberta (the Department of Chemistry and the Faculty of Science) the Natural Science and Engineering Research Council (NSERC), the Canada Foundation for Innovation (CFI), and the Alberta Advanced Education & Technology Small Equipment Grants Program (AET/ SEGP). LH would thank the China Scholarship Council (CSC) for financial support. | |
References
- Schilli CM, Zhang MF, Rizzardo E, Thang SH, Chong YK, et al. (2004) A new double-responsive block copolymer synthesized via RAFT Polymerization:Poly(N-isopropylacrylamide)-block-poly(acrylic acid). Macromolecules 37: 7861-1866.
- Yin X, Hoffman AS, Stayton PS (2006) Poly(N-isopropylacrylamide-co-propylacrylic acid) copolymers that respond sharply to temperature and pH. Biomacromolecules 7: 1381-1385.
- Lin HH, Cheng YL (2001) In-situ thermoreversible gelation of block and star copolymers of Poly(ethylene glycol) and Poly(N-isopropylacrylamide) of varying architectures. Macromolecules 34: 3710-3715.
- Pelton R (2000) Temperature-sensitive aqueous microgels. Adv Colloid Interface Sci 85: 1-33.
- Reese CE, Mikhonin AV, Kamenjicki M, Tikhonov A, Asher SA (2004) Nanogel nanosecond photonic crystal optical switching. J Am Chem Soc 126: 1493-1496.
- Liu T, Hu JM, Yin J, Zhang YF, Li CH, et al. (2009) Enhancing detection sensitivity of responsive microgel-based Cu(II) chemosensors via thermo-induced volume phase transitions. Chem Mater 21: 3439-3446.
- Wang D, Liu T, Yin J, Liu SY (2011) Stimuli-responsive fluorescent Poly(N-isopropylacrylamide) microgels labeled with phenylboronic acid moieties as multifunctional ratiometric probes for glucose and temperatures. Macromolecules 44: 2282-2290.
- Parasuraman D, Serpe MJ (2011) Poly (N-isopropylacrylamide) microgel-based assemblies for organic dye removal from water. ACS Applied Materials & Interfaces 3: 4714-4721.
- Parasuraman D, Serpe MJ (2011) Poly (N-isopropylacrylamide) microgels for organic dye removal from water. ACS Applied Materials & Interfaces 3: 2732-2737.
- Zhang JG, Xu SQ, Kumacheva E (2004) Polymer microgels: reactors for semiconductor, metal, and magnetic nanoparticles. J Am Chem Soc 126: 7908-7914.
- Zhou JH, Liu J, Wang G, Lu X, Wen Z, et al. (2007) Poly(N-isopropylacrylamide) Interfaces with dissimilar thermo-responsive behavior for controlling ion permeation and immobilization. Advanced Functional Materials 17: 3377-3382.
- Karg M, Hellweg T, Mater (2009) Smart inorganic/organic hybrid microgels: Synthesis and characterisation. J Chem 19: 8714-8727.
- Jones CD, Lyon LA (2000) Synthesis and characterization of multiresponsive core-shell microgels Macromolecules 33: 8301-8306.
- Kratz K, Hellweg T, Eimer W (2000) Influence of charge density on the swelling of colloidal poly(N-isopropylacrylamide-co-acrylic acid) microgels. Colloids and Surfaces A: Physicochemical and Engineering Aspects 170: 137-149.
- Snowden MJ, Chowdhry BZ, Vincent B, Morris GE (1996) Colloidal copolymer microgels of N-isopropylacrylamide and acrylic acid: pH, ionic strength and temperature effects. Journal of the Chemical Society-Faraday Transactions 92: 5013-5016.
- Gan T, Zhang Y, Guan Y (2009) In situ gelation of P(NIPAM-HEMA) microgel dispersion and its applications as injectable 3D cell scaffold. Biomacromolecules 10: 1410-1415.
- Yildiz B, Isik B, Kis M (2001) Synthesis of thermoresponsive N-isopropylacrylamide–N-hydroxymethyl acrylamide hydrogels by redox polymerization. Polymer 42: 2521-2529.
- Suzuki D, Tsuji S, Kawaguchi H (2007) Janus microgels prepared by surfactant-free pickering emulsion-based modification and their self-assembly. J Am Chem Soc 129: 8088-8089.
- Nayak S, Lyon LA (2004) Ligand-functionalized core/shell microgels with permselective shells. Angewandte Chemie-International Edition 43: 6706-6709.
- Liu L, Li P, Asher SA (1999) Entropic trapping of macromolecules by mesoscopic periodic voids in a polymer hydrogel. Nature 397: 141-144.
- Muscatello MM, Stunja LE, Asher SA (2009) Polymerized crystalline colloidal array sensing of high glucose concentrations. Anal Chem 81: 4978-4986.
- Asher SA. Zhang JT, Wang L, Luo J, Tikhonov A, et al. (2011) 2-D array photonic crystal sensing motif. J Am Chem Soc 133: 9152-9155.
- Arsenault AC, Puzzo DP, Manners I, Ozin GA (2007) Photonic-crystal full-colour displays. Nat Photonics 1: 468-472.
- Holtz JH, Asher SA (1997) Polymerized colloidal crystal hydrogel films as intelligent chemical sensing materials. Nature 389: 829-832.
- Debord JD, Lyon LA (2000) Thermoresponsive photonic crystals. J Phys Chem B 104: 6327-6331.
- Lee YJ, Braun PV (2003) Tunable inverse opal hydrogel pH sensors. Advanced Materials, 15: 563-566.
- Kang Y, Walish JJ, Gorishnyy T , Thomas EL (2007) Broad-wavelength-range chemically tunable block-copolymer photonic gels. Nat Mater 6: 957-960.
- Hu L, Serpe MJ (2012) Color modulation of spatially isolated regions on a single poly(N-isopropylacrylamide) microgel based etalon. J Mater Chem 22: 8199-8202.
- Sorrell CD, Carter MCD, Serpe MJ (2011) A "paint-on" protocol for the facile assembly of uniform microgel coatings for color tunable etalon fabrication. ACS Appl Mater Interfaces 3: 1140-1147.
- Sorrell CD, Carter MC, Serpe MJ (2011) Color tunable Poly (N-isopropylacrylamide)-co-acrylic acid microgel–Au hybrid assemblies. Advanced Functional Materials 21: 425-433.
- Sorrell CD, Serpe (2011) Reflection order selectivity of color-tunable poly(N-isopropylacrylamide) microgel based etalons. AdvMater 23: 4088-4092.
- Sorrell CD, Serpe MJ (2012) Glucose sensitive poly (N-isopropylacrylamide) microgel based etalons. Anal Bioanal Chem 402: 2385-2393.
- Carter MC, Sorrell CD, Serpe MJ (2011) Deswelling kinetics of color tunable poly(N-isopropylacrylamide) microgel-based etalons. J Phys Chem B 115: 14359-14368.
- Hu L, Serpe MJ (2012) Color-tunable etalons assembled from Poly (N-isopropylacrylamide) based microgels. Polymers 4: 134-149.
- Hou Y, Huang SW, Ashkenazi S, Witte R, O'Donnell M (2008) Thin polymer etalon arrays for high-resolution photoacoustic imaging. J Biomed Opt 13: 064033.
- Huang SW, Hou Y, Ashkenazi S, O'Donnell M (2008) High-resolution ultrasonic imaging using an etalon detector array. Appl Phys Lett 93: 113501.
- Zhang X, Guan Y, Zhang Y (2012) Ultrathin hydrogel films for rapid optical biosensing. Biomacromolecules 13: 92-97.
- Brooker G (2003) Modern classical optics. Oxford University Press, Oxford, UK.
Tables and Figures at a glance
 |
 |
 |
 |
 |
 |
|||||
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 | Figure 6 |
 |
 |
|
| Scheme 1 | Scheme 2 |
Post your comment
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 15428
- [From(publication date):
May-2012 - Dec 21, 2025] - Breakdown by view type
- HTML page views : 10590
- PDF downloads : 4838
