Research Article Open Access
Simultaneous Estimation of Pregabalin and Methylcobalamine in Pharmaceutical Formulation by RP-HPLC Method
Bhatt KK, Emanual Michael Patelia* and Aswin Mori
Department of Pharmaceutical Chemistry and Analysis, Indukaka Ipcowala College of Pharmacy, Gujarat, India
- *Corresponding Author:
- Emanual Michael Patelia
Department of Pharmaceutical Chemistry and Analysis
Indukaka Ipcowala College of Pharmacy
New Vallabh Vidyanagar–388121, Gujarat, India
E-mail: ricky.emanual@gmail.com
Received date: January 15, 2013; Accepted date: January 28, 2013; Published date: January 31, 2013
Citation: Bhatt KK, Patelia EM, Mori A (2013) Simultaneous Estimation of Pregabalin and Methylcobalamine in Pharmaceutical Formulation by RP-HPLC Method. J Anal Bioanal Tech 4:159. doi: 10.4172/2155-9872.1000159
Copyright: © 2013 Bhatt KK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
A simple, precise, rapid, selective, and economic reversed phase high-performance liquid chromatography (RPHPLC) method has been established for simultaneous analysis of PRG and MC. A Phenomenex C18 (250×4.6 mm i.d) chromatographic column equilibrated with mobile phase water: methanol (60:40 v/v) adjusted to pH 6.5 with Triethylamine (1% v/v) was used. Mobile phase flow rate was maintained at 1 ml/min and effluents were monitored at 218 nm. The sample was injected using a 20 μl fixed loop, and the total run time was 10 min. Experimental conditions such as pH of mobile phase, column saturation time, selection of wavelength, etc. were critically studied and the optimum conditions were selected. The calibration curve 50-300 μg/ml for PRG and 0.5-2.0 μg/ml for MC. The limit of quantification was 24.10 μg/ml for PRG and 0.40 μg/ml for MC respectively. This HPLC procedure is economic, sensitive, and less time consuming than other chromatographic procedures. It is an importance tool for analysis of combined dosage form.
Keywords
HPLC; Phenomenex C18; Pregabaline (PRG); Methylcobolamine (MC)
Introduction
Pregabaline (PRG; (3S)-3-(aminomethyl)-5-methylhexanoic acid; figure 1a) is a widely used Anticonvulsant and Analgesic for Epilepsy [1]. Methylcobalamine (MC; carbanide; cobalt; [5-(5,6-dimethylbenzimidazol- 1-yl)-4-hydroxy-2-(hydroxymethyl)oxolan-3-yl]1-[3- [2,13,18-tris(2-amino-2-oxoethyl)-7,12,17-tris(3-amino-3-oxopropyl)- 3,5,8,8,13,15,18,19- octamethyl-2,7,12,17-tetrahydrocorrin-3- yl] propanoylamino] propan-2-yl hydrogen phosphate figure 1b) is derivative form of vitamin B12. It is used for the treatment of peripheral neuropathy, diabetic neuropathy.
Literature survey revealed that various analytical methods like HPLC [2-5], UV [6-8] and HPTLC [9] have been reported for the determination of PRG and MC either individually or combination with some other drugs. The review of literature prompted us to develop an accurate, selective and precise simultaneous method for the estimation of PRG and MC in combined dosage forms (Figure 2).
Experimental
Chemicals and materials
PRG was procured from Torrent Pharmaceuticals Ltd, Ahmedabad and Mehylcobalamine was obtained from sajal enterprises. Methanol (HPLC Grade), Triethylamine (AR Grade), Ninhydrin (AR Grade), Isopropylalcohol (AR Grade), Ammonia and n-Butanol were used as solvents to prepare the mobile phase. All the reagents used were of Analytical reagent grade and used without further purification. Capsule formulation PREGABALIN M 75 (Torrent Pharmaceuticals Ltd) and NOVA PLUS CAP (Cipla Pharmaceuticals Ltd.) containing labelled amount of 75 mg Pregabalin and 0.75 mg of Methylcobalamine was procured from local market.
Chromatographic conditions
A Phenomenex C18 (250×4.6 mm i.d) chromatographic column equilibrated with mobile phase water: methanol (60:40, v/v, pH 6.5) was used. Mobile phase flow rate was maintained at 1 ml/min and effluents were monitored at 218 nm. The sample was injected using a 20 μL fixed loop, and the total run time was 10 min.
Sample preparation
Twenty capsules were taken and drug content was collect by empty gelatin shell. Capsule powder equivalent to 50 mg PRG (0.5 mg of MC) was taken in 100 ml volumetric flask. Water (50 ml) was added to the above flask and the flask was sonicated for 15 minutes. The solution was filtered using 0.45 μm whatman filter paper and volume was made up to the mark with the water. Appropriate volume of the aliquot was transferred to a 10 ml volumetric flask and the volume was made up to the mark with the mobile phase to obtain a solution containing 200 μg/ml of PRG (2 μg/ml of MC). The solution was injected using HPLC loop system and analyzed for PRG and MC content using the proposed method as described earlier (Figure 3).
Preparation of standard solution: PRG (25 mg) and MC (25 mg) were accurately weighed and transferred to two separated 25 ml volumetric flask and dissolved in few ml of water. Volumes were made up to the mark with water to yield a solution containing 1000 μg/ml of PRG and 1000 μg/ml of MC. Appropriate aliquot from stock solution of MC was taken and diluted with mobile phase to obtain final concentration of 10 μg/ml of MC.
Method validation
The developed method was validated for linearity and range, specificity, accuracy, precision, Limit of detection, Limit of quantitation, robustness and solution stability as per ICH guidelines.
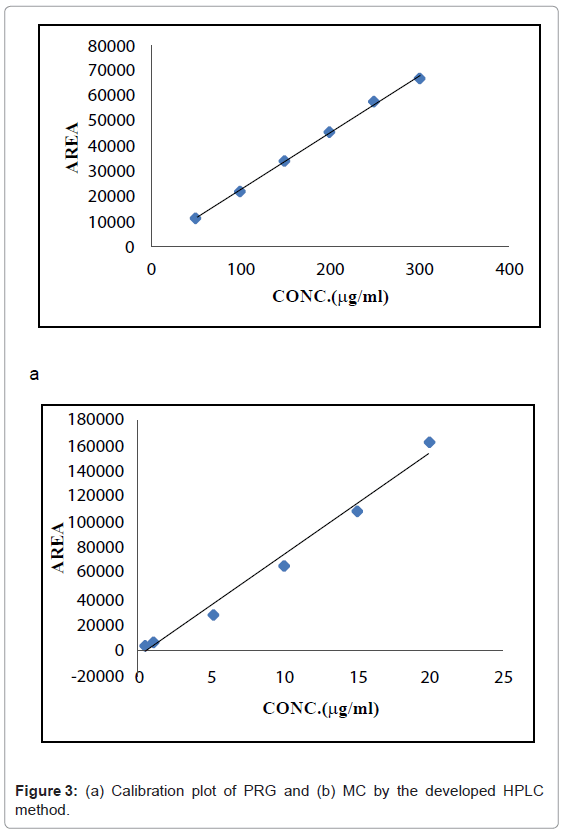
Linearity and range: Linearity of the method was evaluated by constructing calibration curves at six concentration levels over a range of 50-300 μg/ml for PRG and 0.5-20 μg/ml for MC. The calibration curves were developed by plotting peak area versus concentration (n=5).
Specificity: The specificity of the method was ascertained by analyzing PRG and MC in presence of excipients like talc, magnesium stearate and micro-crystalline cellulose were used for capsule formulations. The peak of PRG and MC were confirmed by comparing Rt values and respective spectra of sample with those of standards.
Accuracy (% Recovery): The accuracy of the method was determined by calculating recoveries of PRG and MC by method of standard additions. Known amount of PRG (50%, 100%, 150%) and MC (50%, 100%, 150%) were added to a pre quantified sample solution, and the amount of PRG and MC were estimated by measuring the peak areas and by fitting these values to the straight-line equation of calibration curve.
Method precision (Repeatability): The intra-day and inter-day precision studies were carried out by estimating the corresponding responses 3 times on the same day and on 3 different days for three different concentrations of PRG (50,200,300 μg/ml) and MC (0.5,10,20 μg/ml), and the results are reported in terms of relative standard deviation. The instrumental precision studies were carried out by estimating response of concentrations 200 μg/ml of PRG and 10 μg/ ml of MC for six times and results are reported in terms of relative standard deviation.
Intermediate precision (Reproducibility): The intra-day and inter-day precision studies were carried out by estimating the corresponding responses 3 times on the same day and on 3 different days for three different concentrations of PRG (50,200,300 μg/ml) and MC (0.5,10,20 μg/ml), and the results are reported in terms of relative standard deviation.
Limits of detection (LOD) and Limits of quantitation (LOQ): The limit of detection (LOD) is defined as the lowest concentration of an analyte that can reliably be differentiated from background levels. Limit of quantification (LOQ) of an individual analytical procedure is the lowest amount of analyte that can be quantitatively determined with suitable precision and accuracy. LOD and LOQ were calculated using following equation as per ICH guidelines. LOD=3.3×σ/S; LOQ=10×σ/S; Where σ is the standard deviation of y-intercepts of regression lines and S is the slope of the calibration curve.
Robustness: Small changes in the flow rate and the ratio of mobile phase were carried out and effects on the results were examined. Robustness of the method was determined in triplicate at a concentration level of 200 μg/ml and 10 μg/ml of PRG and MC respectively. The mean and %RSD of peak areas were calculated.
Solution stability: The solutions at analytical concentration PRG (50,200,300 μg/ml) and MC (0.5,10,20 μg/ml) were prepared and stored at room temperature for 24 h and analyzed at interval of 0, 6, 12 and 24 h for the presence of any band other than that of PRG and MC and the results were simultaneously compared with the freshly prepared PRG and MC standard solution of the same concentration in the form of change in %RSD of the response obtained.
Application of validated method to pharmaceutical formulation
Twenty capsules were taken and drug content was collect by empty gelatin shell. Capsule powder equivalent to 50 mg PRG (0.5 mg of MC) was taken in 100 ml volumetric flask. Water (50 ml) was added to the above flask and the flask was sonicated for 15 minutes. The solution was filtered using 0.45 μm whatman filter paper and volume was made up to the mark with the water. Appropriate volume of the aliquot was transferred to a 10 ml volumetric flask and the volume was made up to the mark with the mobile phase to obtain a solution containing 200 μg/ml of PRG (2 μg/ml of MC). The solution was injected using HPLC loop system and analyzed for PRG and MC content using the proposed method as described earlier.
Results and Discussion
Method development and optimization of chromatographic conditions
The mobile phase water: methanol (60:40% v/v, pH 6.5 adjusted using 0.1% triethylamine) was found to be satisfactory and gave two symmetric and well-resolved peaks for PRG and MC. The retention time for PRG and MC were 6.4 min and 7.9 min, respectively. The resolution between PRG and MC was found to be 2.02, which indicates good separation of both the compounds. The asymmetric factors for PRG and MC were 1.43 and 1.14, respectively. The mobile phase flow rate was maintained at 1 ml.min-1. Overlay UV spectra of both the drugs showed that PRG and MC absorbed appreciably at 218 nm, so detection was carried out at 218 nm.
Validation of the method
Linearity: The calibration curve for PRG was found to be linear in the range of 50-300 μg/ml with a correlation coefficient of 0.9991. The calibration curve for MC was found to be linear in the range of 0.5-20 μg/ml with a correlation coefficient of 0.9900.
Specificity: The specificity study was carried out to check the interference from the excipients used like microcrystalline cellulose, magnesium stearate and talc in the formulations by preparing synthetic mixture containing both the drugs and excipients. The chromatogram showed peaks for both the drugs without any interfering peak and the recoveries of both the drugs were above 98%.
Accuracy: The accuracy of the method was determined by calculating recoveries of PRG and MC by method of standard addition. The recoveries found to be 99.44-100.26% and 97.88-99.18% for PRG and MC respectively. The high values indicate that the method is accurate (Table 1).
| Amount of sample taken (μg/ml) | Amount of standard drug added (μg/ml) | Amount of drug recovered (μg/ml) | % Recovery ± % RSD (n=3) | ||||
|---|---|---|---|---|---|---|---|
| PRG | MC | PRG | MC | PRG | MC | PRG | MC |
| 100 | 1 | 0 | 0 | 99.19 | 0.99 | 99.90 ± 1.56 | 99.18 ± 0.91 |
| 100 | 1 | 50 | 0.5 | 150.39 | 1.51 | 100.26 ± 0.98 | 97.88 ± 1.17 |
| 100 | 1 | 100 | 1 | 200.23 | 1.98 | 100.11 ± 1.29 | 98.53 ± 0.97 |
| 100 | 1 | 150 | 1.5 | 248.62 | 2.48 | 99.44 ± 1.37 | 99.14 ± 0.67 |
Table 1: Results from accuracy study.
Precision: Instrument precision was determined by performing injection repeatability test and the %RSD values for PRG and MC were found to be 0.56 and 0.86 respectively. The intra-day and inter-day precision studies were carried out and the results are reported in table 2.
| Parameters | PRG | MC |
|---|---|---|
| Range | 50-300 μg/ml | 0.5-20 μg/ml |
| Retention time (min) | 6.4 | 7.9 |
| Tailing factor | 1.43 | 1.14 |
| Resolution | 2.02 | |
| Theoretical Plates | 10509 | 6757 |
| Detection limit (ng/ml) | 8.10 | 0.12 |
| Quantitation limit (μg/ml) | 24.50 | 0.40 |
| Accuracy (%) | 99.11-100.26 | 97.88-99.14 |
| Precision (%RSD) | ||
| Intra-day (n=3) | 0.73-1.27 | 0.37-1.05 |
| Inter-day (n=3) | 0.63-0.99 | 0.54-1.40 |
| Instrument precision (%RSD) | 0.56 | 0.86 |
| Specificity | Specific | Specific |
Table 2: Summary of validation parameters of developed HPLC method.
Limits of detection (LOD) and Limits of quantification (LOQ): The detection limits for PRG and MC were 8.10 μg/ml and 0.12 μg/ml respectively, while quantitation limits were 24.50 μg/ml and 0.40 μg/ml respectively. The above data shows that a microgram quantity of both the drugs can be accurately and precisely determined (Table 2).
Robustness: Acceptable %RSD values obtained after making small deliberate changes in the developed HPLC method indicates that the method is robust for the intended purpose (Table 3).
| Parameter | Method condition | Rt | % RSD of peak area | ||
|---|---|---|---|---|---|
| PRG | MC | PRG | MC | ||
| Flow rate | 0.9 ml/min | 6.98 | 8.56 | 0.69 | 0.87 |
| 1.1 ml/min | 5.21 | 6.80 | 0.97 | 0.49 | |
| Mobile phase ratio Water:methanol | 55 : 45 | 5.10 | 6.75 | 0.54 | 0.54 |
| 65 : 35 | 7.10 | 8.70 | 0.71 | 0.59 | |
Table 3: Results from the robustness study of method.
Solution stability: The solutions at analytical concentration PRG (50,200,300 μg/ml) and MC (0.5,10,20 μg/ml) were prepared and stored at room temperature for 24 h and analyzed at interval of 0, 6, 12 and 24 h for the presence of any band other than that of PRG and MC and the results were simultaneously compared with the freshly prepared PRG and MC standard solution of the same concentration in the form of change in %RSD of the response obtained.
Method application
Marketed formulation was analyzed using proposed method which gave percentage recovery for PRG and MC more than 97% (Table 4).
| Formulation | Label claim (mg) | Amount found | % of label claim (n=5) ± % RSD (n=5) | |||
|---|---|---|---|---|---|---|
| PRG | MC | PRG | MC | PRG | MC | |
| Pregalin M 75 | 75 | 0.75 | 74.50 | 0.738 | 99.33 ± 1.02 | 98.4 ± 0.63 |
| Nova Plus CAP. | 75 | 0.75 | 73.68 | 0.733 | 98.25 ± 0.74 | 97.7 ± 1.37 |
Capsule formulation PREGABALIN M 75 (Torrent Pharmaceuticals Ltd.) and NOVA
PLUS CAP (Cipla Pharmaceuticals Ltd.) containing labelled amount of 75 mg
Pregabalin and 0.75 mg of Methylcobalamine n=number of determinations
Table 4: Results from analysis of Pregabalin and Methylcobalamine in the combined capsule dosage form.
Conclusions
This developed and validated method for simultaneous analysis of PRG and MC in pharmaceutical preparations is very rapid, accurate, and precise. The method was successfully applied for determination of PRG and MC in its pharmaceutical capsule formulations. Moreover it has advantages of short run time and the possibility of analysis of a large number of samples, both of which significantly reduce the analysis time per sample. Hence this method can be conveniently used for routine quality control analysis of PRG and MC in its pharmaceutical formulations.
Acknowledgements
The authors are very thankful to Sophisticated Instrumentation Center for Applied Research & Testing (SICART), Vallabh Vidyanagar, India), for providing necessary facilities to carryout research work. The authors are also thank full to indukaka Ipcowala College of pharmacy (IICP) for providing laboratories facilities.
References
- Baldwin DS, Ajel K (2007) Role of pregabalin in the treatment of generalized anxiety disorder. Neuropsychiatr Dis Treat 3: 185-191.
- Saravanan.J, Shajan A, Joshi NH, Varatharajan R, Valliappan K (2010) RP-HPLC method for the estimation of methylcobalamin in bulk and capsule dosage form. International journal of chemical and pharmaceutical sciences 1: 13-16.
- Hari Babu B, Nageswara Rao M, Rambabu A, Srinivasan P (2011) Development and validation of HPLC method for the estimation of methylcobalamine in bulk drugs and Pharmaceutical formulations. Journal of Pharmacy Research 4: 1685-1687.
- Katsushi Y, Cheie K, Yoshihiko S (2008) Stability of methylcobalamin injection. Journal of Applied Therapeutic Research 6: 15-18.
- Kannapan N, Nayak SP, Venkatachalam T, Prabhakaran V (2009) Analytical RP-HPLC Method for Development and Validation of Pregabalin and Methylcobalamine in Combined Capsule Formulation. Journal of Applied Chemical Research 13: 85-89.
- Galande VR, Baheti KG, Dehghan MH (2010) UV-VIS Spectrophotometric Method for Estimation of Gabapentin and Methylcobalamin in Bulk and Tablet. International Journal of ChemTech Research 2: 695-699.
- Sharma MC, Sharma S, Sharma AD (2011) Simultaneous Estimation and Validation of Gabapentin and Methylcobalamin in Tablet Dosage form: hydrotropic approach. Drug Invention Today 3: 95-97.
- Sengamalam R, Ravindran M, Gunjan M, Meena S (2011) Analytical Method Development and Dissolution Profile of Duloxetine and Methylcobalamin By Vierodt's Method. J Pharm Res 4: 449-451.
- Baheti KG, Galande VR (2011) Validated Simultaneous Estimation of Gabapentin in the Presence of Methylcobalamin in Tablet by HPTLC Method. International Journal of Research in Pharmacuetical & Biomedical science 2: 1199-1202.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 17890
- [From(publication date):
February-2013 - Nov 11, 2025] - Breakdown by view type
- HTML page views : 12778
- PDF downloads : 5112