Research Article Open Access
Simultaneous Estimation of Tenofovir and Emtricitabine in Human Plasma Using HPLC after Protein Precipitation Extraction
Arti Soni* and Seema Thakral
GVM College of Pharmacy, Murthal Road, Sonipat, India
- *Corresponding Author:
- Arti Soni
GVM College of Pharmacy
Murthal Road
Sonipat, India
E-mail: artisoni26@gmail.com
Received date: May 22, 2013; Accepted date: August 05, 2013; Published date: August 07, 2013
Citation: Soni A, Thakral S (2013) Simultaneous Estimation of Tenofovir and Emtricitabine in Human Plasma Using HPLC after Protein Precipitation Extraction. J Anal Bioanal Tech 4:170. doi: 10.4172/2155-9872.1000170
Copyright: © 2013 Soni A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Tenofovir (TNF) and emtricitabine (FTC) are both reverse transcriptase inhibitors, often used in combination for anti-retroviral therapy. In the present study, a reverse phase high performance liquid chromatographic method was developed and validated for the simultaneous estimation of TNF and FTC in human plasma using stavudine as the internal standard. Protein precipitation extraction procedure utilizing perchloric acid was employed to extract the drugs from human plasma. Estimation of the drug contents was done by using a mixture of sodium dihydrogen orthophosphate buffer (pH 6.9) and methanol as the mobile phase and absorbance was read at 259 nm for TNF and 280 nm for FTC. Retention time was found to be 6.6 (± 0.1) min for TNF and 17.1 (± 0.2) min for FTC. The recovery, selectivity, linearity, precision as well as the accuracy of the method was evaluated from spiked human plasma samples during the course of validation. The result revealed that the analytical technique presented here demonstrates acceptable accuracy and precision, shorter and simpler sample preparation, and a reduced need for complicated equipment along with a tolerable analysis time.
Keywords
Tenofovir; Emtricitabine; HPLC; Plasma
Introduction
Tenofovir (TNF), (phosphorylmethoxy) propyl-adenine (Figure 1a), a commonly used anti-retroviral agent, is a nucleotide analogue reverse transcriptase inhibitor [1]. Pharmacokinetic profile of the drug is characterized by the elimination half-life of ˜15 hours and a poor bioavailability [2]. Tenofovir disoproxilfumarate (TDF) is a prodrug of TNF, which is known to demonstrate improved bioavailability. When administered in vivo, TDF is hydrolyzed to TNF and subsequently phosphorylated to the active metabolite, tenofovir diphosphate, within the cell where it functions as a competitive inhibitor of reverse trancriptase and as a HIV-DNA chain terminator [3,4].
Emtricitabine (5-fluoro-1-[2-hydroxy methyl]1,3-oxathiolan-5 yl)-cytosine, (FTC) (Figure 1b) is adeoxycytidine nucleoside analogue that was recently approved for the treatment of HIV infection [5]. FTC is activated by intracellular phosphorylation to its 5’-triphosphate (FTC5’-TP), which is reported to be a competitive inhibitor of the HIV reverse transcriptase [6]. It has been established as a potent and selective inhibitor against HIV type I and II and hepatitis B virus [5,7]. FTC is reported to exhibit mean plasma elimination half-life of 8-10 hours [8]. TDF and FTC are prescribed as fixed dose combination tablets containing 300 mg of TDF and 200 mg of FTC (Truvada®1) for once daily dosing to be used in combination with other antiretroviral drugs [9]. TDF and FTC have synergistic antiviral effects on both HIV-1 and HIV-2 [10]. The combined formulation is considered to be an efficacious therapy that can be used in multi-drug HIV treatment regimens [11,12].
In view of the frequent use of the combination therapy of TDF and FTC, it is relevant to establish a suitable analytical method for the simultaneous estimation of TDF and FTC in human plasma. A previously reported method for the purpose is based solid phase extraction followed by estimation using high performance liquid chromatography (HPLC) equipped with photodiode array detector using 2,3-dideoxyuridine as internal standard [13]. Another HPLC
Experimental
Materials
Blank human plasma and drugs TNF, FTC and Stavudine (Figure 1c) were provided by Ranbaxy Research Labs (India). Following HPLC grade chemicals were used in the study: methanol (SD Fine Chemicals Ltd., Mumbai), perchloric acid, sodium dihydrogen orthophosphate and sodium hydroxide pellets (Qualigens Fine Chemicals, Mumbai). HPLC grade water was obtained from Millipore water purifying unit.
Equipment
High performance liquid chromatographic equipment consisted of model Shimadzu LC-10AD (integrated system), eppendorf refrigerated centrifuge 5810R, sonicator power sonic405 (Hwashin Technology SEOUL, Korea), pH meter of cyberscan 2500 pH, micropipettes of finnpipette (Thermo Electron Corporation V89771-4500), repeater of handy step 12C 7515 and UV detector was used.
Preparation of standards
Preparation of dilutions of standard master stock solutions: As TNF has limited solubility in most of the common organic solvents like acetonitrile, ethyl acetate,acetone, isopropanol and it exhibits good solubility in water (Houghton et al. [15]), the drug stock solution was prepared in water. Thus accurately weighed quantity (5.11 mg) of TNF (molecular weight=287.213) was suitably diluted with water to obtain a solution of conc. 1005.026 μg/ml. Similarly aqueous FTC (molecular weight=247.25) solution was also prepared by dissolving accurately weighed quantity (9.56 mg) of drug in water and suitably diluting to contain a conc. of 953.63 μg/ml. The stock solutions were subsequently diluted with mobile phase for spiking in plasma to obtain calibration curve standards. Spiking of blank human plasma was done by diluting 200 μL of each of the dilutions to 10 ml with blank plasma to achieve the standards for CC and QC, as per Table 1. One ml aliquot of each of these standards transferred to polypropylene tubes, which were stored at -25°C to -30°C till time of analysis.
| Drug | A | B | C | D | E | F | G | H | LQC | MQC | HQC |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TNF | 31.7 | 51.3 | 82.0 | 136.6 | 227.7 | 350.4 | 539.0 | 663.3 | 82.6 | 229.6 | 542.7 |
| FTC | 200.6 | 324.6 | 518.6 | 864.3 | 1440.6 | 2216.3 | 3409.6 | 4196.0 | 519.0 | 1441.7 | 3408.3 |
Table 1: Preparation of drugs stock dilutions for CC and QC spiking.
Internal standard (IS) preparation: An IS stock solution of concentration 1.0 mg/ml was prepared using methanol as solvent while, working solution was prepared by diluting aliquot from stock solution in mobile phase to a final concentration of 10 μg/ml.
Sample pre-treatment: All the spiked plasma samples, stored at the ˜ -25°C, were brought to room temperature on the day of analysis.
Protein precipitation method: To 500 μl of plasma sample, 50 μL of IS dilution was added. The samples were vortex mixed for about 1 min to facilitate mixing of IS with the analytes. Subsequently, protein precipitation was achieved by adding 200 μl of perchloric acid and the samples were again vortex mixed. These were subjected to refrigerated centrifuge for 15 min at 14000 rpm. After centrifugation the supernatant was transferred to HPLC autosampler vials and 50 μl of the solution was injected into the HPLC column for analysis.
High performance liquid chromatographic conditions: A Hypersil C18 (250 mm×4.0 mm, 5 μm particle size) was used for separation of analytes and for their quantification. During elution, the absorbance was monitored at three different wavelengths; 259 nm for TNF, 280 nm for FTC and 265 nm for stavudine. Mobile phase used was a mixture (96:4) of sodium dihydrogen orthophosphate buffer (pH 6.9 ± 0.05) and methanol. Rinsing solution was a mixture of methanol and water (1:1). All the estimations were performed at 40°C, with mobile phase pumped at a flow rate of 1.0 ml/min. The injection volume was 50 μl. The retention time was found to be 6.6 (± 0.1) min for TNF and 17.1 (± 0.2) min for FTC.
Selectivity: The selectivity of the method was verified by checking the interference of endogenous compounds in human plasma at retention time of the drugs and IS by evaluating six lots of blank plasma.
Method validation
Precision and accuracy: The precision of the method was determined by the percent coefficient of variation (%CV) over the six replicates of all the quality control samples of the drugs during the course of validation. The accuracy of the assay was defined as the peak area ratio response of the drugs and internal standard versus concentration of the quality control samples to their respective nominal values, expressed as percentage.
Linearity: The linearity of the method was determined by the weighted least square regression analysis of standard plot associated with an eight-point standard curve.
Recovery: The percentage recoveries for the drug and the internal standard were determined by comparing the peak areas of the response of drug extracted from plasma quality control samples with that of the peak areas of the non processed standard solutions containing the same concentration of the drug and the internal standard.
Stability
Long term stability: Six replicates of the stored LQC and HQC samples were analyzed after storage at a temperature ˜-25°C for 25 days. The peak area ratios of drugs to internal standard of stored frozen samples were compared to that of freshly spiked samples containing equivalent concentrations.
Freeze-thaw stability: The freeze-thaw stability in matrix was assessed by evaluating six replicates of both LQC and HQC samples previously frozen and thawed over three cycles against freshly spiked standards. The first freezing was done at -25°C for at least 40 hrs, while the subsequent two freezings were done for 24 hrs at the same temperature, using unassisted thawings after each freezing. The samples were then processed for extraction and analyzed.
Bench top stability: The bench top stability was also determined for six replicate of LQC and HQC samples. The samples were processed after keeping them at bench top (room temperature) for about 6 hours and then analyzed against freshly spiked calibration curve standards.
In-injector stability: In-injector stability was assessed by extracting six replicates of LQC and HQC and putting the processed samples in the auto sampler. The samples were injected after 96 hours along with freshly spiked calibration standards.
Results and Discussion
The present study is an attempt to estimate simultaneously two anti-retroviral combination drugs TNF and FTC, in human plasma. The method is based protein precipitation to extract the drugs from human plasma followed by their estimation using high performance liquid chromatographic method.
Selectivity
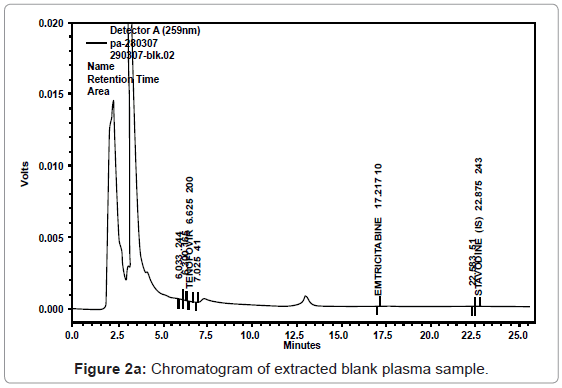
As evident from the chromatogram of blank plasma (Figure 2a), the response of the interfering peak was found to be insignificant at the retention time of both the analytes i.e. at around 6.6 and 17.1 min. The chromatogram of blank plasma with IS also depicted no interfering peaks at the retention time of the internal standard in selected blanks. (Figure 2b).
Precision and accuracy
Results from the validation of this method from human plasma were within acceptable range. Within batch precision of the method was found to range from 2.2 to 11.5% for TNF and 1.5 to 2.7% for FTC. Within batch accuracy of the method for the drug was found in the range of 96.2 to 114.60% for TNF and 95.8 to 108.0% for FTC (Table 2).
| Within batch | LOQQC | LQC | MQC | HQC | ||||
|---|---|---|---|---|---|---|---|---|
| TNF | FTC | TNF | FTC | TNF | FTC | TNF | FTC | |
| Mean | 32.69 | 204.58 | 82.38 | 560.316 | 211.40 | 1421.826 | 509.57 | 3286.311 |
| S.D (+/-) | 4.327 | 17.877 | 3.173 | 33.650 | 13.352 | 74.349 | 28.841 | 163.249 |
| C.V (%) | 13.2 | 8.7 | 3.9 | 6.0 | 6.3 | 5.2 | 5.7 | 5.0 |
Table 2: Precision and accuracy.
Linearity
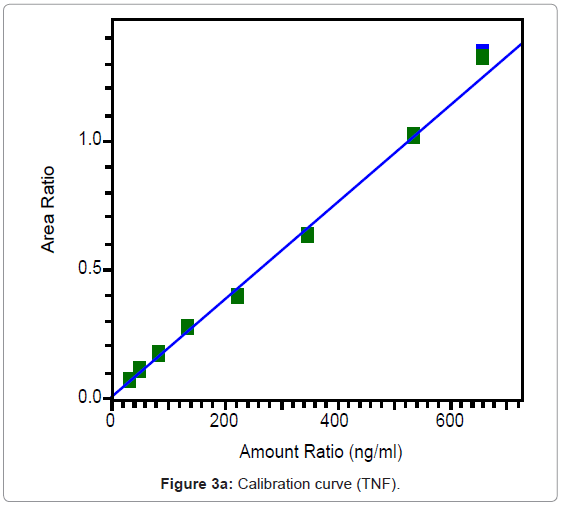
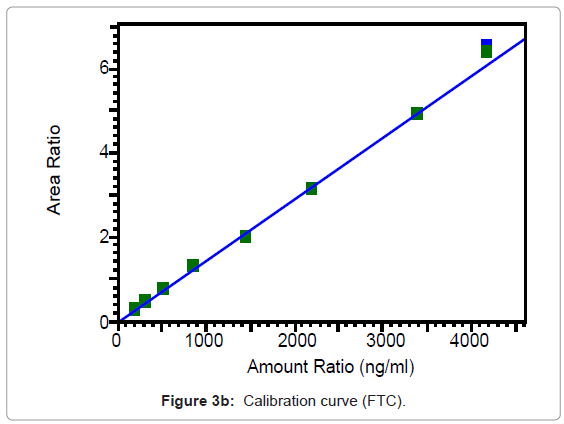
The calibration curves of TNF and FTC are represented in Figures 3a and 3b and were found to be linear from 31.6 to 663.3 ng/ml for TNF and from 200.1 to 4196 ng/ml for FTC. Best fit calibration lines of peak area ratio response of drug and internal standard versus concentration of calibration standards were determined by weighted least square regression analysis with a weighting factor of 1/X for TNF and 1/X2 for FTC. The regression coefficients were consistently greater than 0.99 for both the drugs during the course of validation.
Recovery
The extraction efficiency for TNF and FTC using protein precipitation method were calculated using ratio of analyte concentration in blood plasma to the identical concentration of analyte prepared in mobile phase without extraction. The analyte recovery for the TNF and FTC was found to be 62.6% and 66.8% respectively and that of the internal standard was 78% (Table 3).
| TNF | FTC | dt4 | |
|---|---|---|---|
| Mean | 62.9 | 66.8 | 78.195 |
| S.D. (±) | 2.61 | 0.89 | 6.7860 |
| C.V. (%) | 4.2 | 1.3 | 1.8 |
| N | 18 | 18 | 6 |
Table 3: Recovery of TNF, FTC and dt4.
Stability
Long-term stability: The concentration of the stability samples ranged between 99.2 to 110.8% for TNF and 101.7 to 114.3% for FTC of the nominal value (Table 4).
| S.No | LQC (ng/ml) | HQC (ng/ml) | ||
|---|---|---|---|---|
| TNF | EMF | TNF | EMF | |
| Long term stability: After 25 days | ||||
| Mean | 91.56 | 593.130 | 538.27 | 3467.564 |
| S.D (±) | 8.983 | 59.7163 | 9.580 | 48.9096 |
| C.V. (%) | 9.8 | 10.1 | 1.78 | 1.41 |
| Freeze thaw stability: after three cycles | ||||
| Mean | 85.554 | 569.886 | 582.298 | 3870.032 |
| S.D (±) | 9.2058 | 77.8234 | 0.362 | 158.456 |
| C.V. (%) | 10.8 | 13.7 | 9.1 | 4.1 |
| Bench top stability: after 6 hrs | ||||
| Mean | 96.792 | 542.262 | 525.769 | 3465.435 |
| S.D (±) | 12.474 | 50.037 | 47.813 | 295.701 |
| C.V. (%) | 12.9 | 9.2 | 9.1 | 8.5 |
| Injector stability: after 48 hrs | ||||
| Mean | 85.58 | 581.09 | 549.18 | 3297.31 |
| S.D (±) | 8.409 | 48.379 | 8.580 | 62.771 |
| C.V. (%) | 9.8 | 8.3255 | 1.56 | 1.9037 |
Table 4: Stability of Tenofovir and Emtricitabine.
Freeze-thaw stability: The percentage degradation was determined by back calculating their concentration against freshly spiked calibration curve samples. The concentration of the freeze-thaw samples was calculated to be 103.6 to 107.3% (TNF) and 109.8 to 113.5% (FTC) of the nominal concentration indicating the stability of the drug over three freeze-thaw cycles.
Bench top stability: The drug was found to be stable for at least 6 hours at bench top (at room temperature). The back calculated concentration against freshly spiked calibration standards was within a range of 96.9 to 110.2% (TNF) and 101.7 to 104.5% (FTC) of the nominal concentration.
In-injector stability: The results reveal that the samples were stable for at least 48 hours in the auto injector. The back calculated concentration ranged from 101.2 to 103.6% for TNF and 96.7 to 112.0% for FTC of the nominal concentration.
Conclusion
The bioanalytical method described above is valid for the analysis of TNF and FTC in human plasma over a range of 31.6 to 663.3 ng/ml and 200.1 to 4196 ng/ml using a hypersil C18 column (4.0×250 mm) with UV detection at 259 nm and 280 nm respectively.
Acknowledgements
We wish to thank Ranbaxy research laboratories Gurgaon, India for providing us samples, chemicals, materials and all other facilities used to complete this project.
References
- Kearney BP, Flaherty JF, Shah J (2004) Tenofovir disoproxil fumarate: clinical pharmacology and pharmacokinetics. Clin Pharmacokinet 43: 595-612.
- Barditch-Crovo P, Deeks SG, Collier A, Safrin S, Coakley DF, et al. (2001) Phase i/ii trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob Agents Chemother 45: 2733-2739.
- Pfeiffer S, Mayer B (1998) Lack of Tyrosine Nitration by Peroxynitrite Generated at Physiological pH. J Biol Chem 273: 27280-27285.
- Lyseng-Williamson KA, Reynolds NA, Plosker GL (2005) Tenofovir disoproxil fumarate: a review of its use in the management of HIV infection. Drugs 65: 413-432.
- Schinazi RF, McMillan A, Cannon D, Mathis R, Lloyd RM, et al. (1992) Selective inhibition of human immunodeficiency viruses by racemates and enantiomers of cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine. Antimicrob Agents Chemother 36: 2423-2431.
- Wang LH, Begley J, St Claire RL 3rd, Harris J, Wakeford C, et al. (2004) Pharmacokinetic and pharmacodynamic characteristics of emtricitabine support its once daily dosing for the treatment of HIV infection. AIDS Res Hum Retroviruses 20: 1173-1182.
- Abobo CV, Ni L, Schinazi RF, Liotta DC, Boudinot FD (1994) Pharmacokinetics of 2',3'-dideoxy-5-fluoro-3'-thiacytidine in rats. J Pharm Sci 83: 96-99.
- Molina JM, Cox SL (2005) Emtricitabine: a novel nucleoside reverse transcriptase inhibitor. Drugs Today (Barc) 41: 241-252.
- Huang F, Scholl P, Huang DB, MacGregor TR, Taub ME, et al. (2011) Concomitant administration of BILR 355/r with emtricitabine/tenofovir disoproxil fumarate increases exposure to emtricitabine and tenofovir: a randomized, open-label, prospective study. Basic Clin Pharmacol Toxicol 108: 163-170.
- Louie M, Hogan C, Hurley A, Simon V, Chung C, et al. (2003) Determining the antiviral activity of tenofovir disoproxil fumarate in treatment-naive chronically HIV-1-infected individuals. AIDS 17: 1151-1156.
- Gallant JE, DeJesus E, Arribas JR, Pozniak AL, Gazzard B, et al. (2006) Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med 354: 251-260.
- Pozniak AL, Gallant JE, DeJesus E, Arribas JR, Gazzard B, et al. (2006) Tenofovir disoproxil fumarate, emtricitabine, and efavirenz versus fixed-dose zidovudine/lamivudine and efavirenz in antiretroviral-naive patients: virologic, immunologic, and morphologic changes-a 96-week analysis. J Acquir Immune Defic Syndr 43: 535-540.
- Rezk NL, Crutchley RD, Kashuba AD (2005) Simultaneous quantification of emtricitabine and tenofovir in human plasma using high-performance liquid chromatography after solid phase extraction. J Chromatogr B Analyt Technol Biomed Life Sci 822: 201-208.
- Sentenac S, Fernandez C, Thuillier A, Lechat P, Aymard G (2003) Sensitive determination of tenofovir in human plasma samples using reversed-phase liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci 793: 317-324.
- Houghton SR, Melton J, Fortunak J, Ripin DHB, Boddy CN (2010) Rapid, mild method for phosphonate diester hydrolysis: development of a one-pot synthesis of tenofovir disoproxil fumarate from tenofovir diethyl ester. Tetrahedron 66: 8137–8144.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 16500
- [From(publication date):
August-2013 - Dec 20, 2025] - Breakdown by view type
- HTML page views : 11535
- PDF downloads : 4965