Research Article Open Access
Biodegradation of Tertiary Butyl Mercaptan in Water
| R. Karthikeyan1*, S.L.L. Hutchinson2 and L. E. Erickson3 | |
| 1Biological and Agricultural Engineering, Texas A&M University, College Station, TX 77845-2117, USA | |
| 2Biological and Agricultural Engineering, Kansas State University, Manhattan, KS 66502, USA | |
| 3Chemical Engineering, Kansas State University, Manhattan, KS 66502, USA | |
| Corresponding Author : | R. Karthikeyan Associate professor, Biological and Agricultural Engineering Texas A&M University, College Station, TX 77845-2117, USA Tel: 979-845-7951 Fax: 979-862-3442 E-mail: karthi@tamu.edu |
| Received March 26, 2012; Accepted June 04, 2012; Published June 06, 2012 | |
| Citation: Karthikeyan R, Hutchinson SLL, Erickson LE (2012) Biodegradation of Tertiary Butyl Mercaptan in Water. J Bioremed Biodeg 3:156. doi: 10.4172/2155-6199.1000156 | |
| Copyright: © 2012 Karthikeyan R, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Tertiary butyl mercaptan (TBM) belongs to the alkyl mercaptan family and possesses a characteristic odor. Tertiary butyl mercaptan (TBM) can enter aquatic environments through anthropogenic activities as well as the natural processes. Undefined microbial cultures from different soils along with a pure culture were used to study the biodegradation of TBM in water under aerobic conditions. There were about 17% losses in gas phase TBM concentrations attributed to abiotic losses over the period of 14 days. Environmental microbial consortium from sandy soils with low organic matter content and significantly lower heterotrophs resulted in the lowest biodegradation, only slightly higher than abiotic losses. Microbial cultures isolated from soils with previous contamination history resulted in higher degradation rates. In general, biodegradation of TBM in water followed first-order kinetics. The first-order kinetic constant ranged from 0.002 to 0.005 h -1 . TBM was partly degraded to two significant intermediate products and partly mineralized to CO 2 in water with mixed culture isolated from a petroleum contaminated soil. The half- life of TBM in water with this mixed culture was only six days. A Gram-ve bacterium isolated from a grey-water bioprocessor, Alcaligines faecalis subsp. phenolicus subsp. nov, was able to mineralize 50% of TBM within four days under laboratory conditions. The degradation rate was slightly increased with the addition of tertiary butyl alcohol while slightly inhibited with the addition of phenol.
| Keywords |
| Malodorants; Remediation; Sulfur compounds; Water quality |
| Introduction |
| Potential human health problems related to sulfur compounds such as hydrogen sulfide and mercaptans may occur due to deterioration of sewer and wastewater networks [1-4]. Mercaptans are primarily produced from anaerobic decomposition of proteins and are grouped under Volatile Sulfur Compounds (VSCs). Mercaptans possess a characteristic odor. Tertiary butyl mercaptan (TBM) belongs to the alkyl mercaptan family and has been listed as hazardous under Occupational Safety & Health Administration (OSHA) regulations [5,6]. Exposure to TBM may cause dizziness, eye and skin irritation, nausea, and other allergic reactions [7]. Commercial uses of TBM include natural gas odorizing agent for leak detection and starting material in the manufacture of several agricultural chemicals. Physical and chemical properties of TBM are listed in table 1 [7]. |
| Based on a thorough literature review, Kalainesan et al. [5] reported that there were no studies on the degradation characteristics of TBM. They studied the rate and extent of disappearance of TBM in six different soils under aerobic conditions and reported that 99% of TBM disappeared in 33 days. This was attributed to biodegradation and/or chemical oxidation. The estimated first-order degradation rate constant ranged from 0.044 to 0.14 day-1. Tertiary butyl mercaptan (TBM) can also enter aquatic environments through anthropogenic activities as well as the natural processes [8]. Abiotic and biotic losses determine the persistence of TBM in aquatic systems. Microcosm studies were conducted to study the biodegradation potential of TBM in water. Undefined microbial cultures from different soils along with a pure culture were explored to study the biodegradation of TBM under aerobic conditions. Experimental details and results from this laboratory study are presented in this manuscript. |
| Materials and Methods |
| Microbial cultures |
| Undefined mixed microbial cultures from six different soils (Table 2) were extracted as follows: One gram soil sample was extracted with 9 mL 0.7% NaCl buffer in a 10 mL test tube. The test tube was vortexed for 2 min to disperse microorganisms from soil particles. The solution was serially diluted to result in 10-2 dilution. One milliliter solution was taken from this dilution using a sterile disposable pipette and added to corresponding treatment bottle. The rest of the solution was stored at -10°C. Pure culture bacterium identified as Alcaligines faecalis subsp. Phenolicus subsp. Nov [9] was obtained from the researchers and maintained in M9 mineral medium at pH 7.0. M9 medium is a defined medium containing per liter of water, 7 g Na2HPO4, 3 g KH2PO4, 0.5 g NaCl, 1 g NH4Cl, 0.02 g CaCl2, and 0.2 g MgSO4. One milliliter of pure culture was taken using a sterile disposable pipette and added to corresponding treatment bottle. The rest of the solution was stored at -10°C. |
| Water microcosms |
| Serum bottles (160 mL) containing 20 mL (approximately 20 g) autoclaved DI water (see next section for autoclaving protocol) and 10 μL of TBM were used as treatments and controls. One milliliter of microbial culture from six different soil solutions (10-2 dilution) and from the pure culture flask was added to each serum bottle. Serum bottles that did not receive any microbial culture were considered as controls. All treatments and controls were carried out in triplicates. All serum bottles were crimped tightly with a Teflon-lined septum that could withstand multiple punctures with negligible leakage, covered with aluminum foil to avoid any photodegradation, agitated continuously using an orbital-reciprocal shaker (Cole-Palmer, Vernon Hills, IL), and incubated at 22 ± 2°C. |
| Autoclaving protocol |
| Autoclaving of water was carried out as follows: the required amount of water was taken in Pyrex bottles (Fisher Scientific, Pittsburgh, PA) and placed in an autoclave at 125°C for one hour [10,11]. The bottles were allowed to cool for 2 hr, incubated at 30°C for 2 hr, and autoclaved again as before. This process was repeated three times to ensure maximum sterilization. All serum bottles, glassware, syringes, and other utensils were also sterilized. If not sterilized, pre-packed sterilized supplies were used throughout the experiment. |
| Analytical methods |
| All chemicals and standards were purchased through Fisher Scientific (Pittsburgh, PA). Headspace of each microcosm was sampled for TBM analysis at regular intervals. TBM was analyzed by gas chromatography using a method developed the first author of this manuscript and applied by [5]. Hewlett-Packard (HP, Avondale, PA; now Agilent Technologies) 5890 Series II Gas Chromatograph (GC) with ChemStation integration software was used in the analysis. TBM injection was 10 μL splitless. The column used was 30 m HP-1 (J&W Scientific, Folsom, CA) mega-bore column with internal diameter 0.53 mm and 4 μm film thickness. The carrier gas was H2 (99.999%), the make-up gas was N2 (99.999%), and the support gas was dry compressed air (zero-grade). The column oven temperature program began at 60°C for 2 min, increased at 10°C/min to 160°C, and then held at 160°C for 2 min. The injection port and flame ionization detector were kept at 160°C and 300°C, respectively. |
| The chromatogram obtained showed a distinct TBM peak appearing at about 2 min. This peak was verified using GC/MS by running standards and samples using the same temperature program with a minor modification (MS detector was kept at 280°C) and a similar column. The peak areas were obtained through automatic integration. Standard curves (with r2 = 0.999) were developed using a set of standards prepared. Standards were run before, after, and in between every analysis to check for any deterioration. Blanks (acetone or methanol) were run in between samples to avoid any carryover due to column bleeding and cross contamination of samples and standards. |
| Bacterial enumeration |
| Heterotrophic bacteria were enumerated from each sample at the beginning of the experiments before being added to the microcosms. Ten-fold serial dilutions were performed as required to obtain appropriate colony numbers, and samples were plated in triplicate by the spread plate method on Difco nutrient agar. Plates were incubated at 34 ± 2°C for 24 hours and then counted. Plating was not done during the experiment because of the odor nuisance. |
| Results and Discussion |
| Degradation of tert-butyl mercaptan (TBM) in water with environmental microbial consortia |
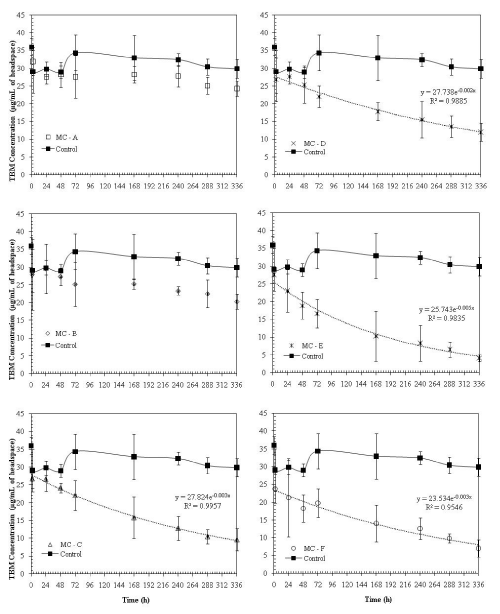
| Initial incubation studies were conducted for a 14 day period (336 hrs) to observe the degradation of TBM in water. The dissipation of TBM in water microcosms over time is presented in Figure 1. There were about 17% losses in gas phase TBM concentrations in control microcosms over the period of 14 days (Figure 1). These losses may be attributed due to leakage in the septum, any biotic losses due to incomplete sterilization, and other abiotic losses such as photodegradation or dissolution of TBM in water. However, these losses are lower compared to microcosms inoculated with microbial cultures from different soils (Figure 1). |
| In general, treatments with microbial cultures from soils with no previous contamination history had significantly lower (p = 0.05) TBM dissipation rates compared to treatments with microorganisms from contaminated soils (Figure 1). This may be due to the fact that microorganisms extracted from contaminated soils were acclimated to organic pollutants (Table 2). About 85% of TBM was lost in 14 days due to abiotic and biotic degradation in water with environmental microbial consortium E, isolated from a petroleum contaminated site; whereas only 23 to 27% losses were observed in environmental microbial consortium resulting from sandy soils (A and B) with no known contamination history. It should be noted that these two sandy soils had the lowest organic matter and significantly lower total heterotrophic counts compared to other soils from which initial microbial cultures were obtained (Table 2,3). |
| TBM dissipation rates in microcosms with microbial cultures from methyl-tert-butyl-ether (MTBE) contaminated soil (mixed culture F) were slightly less than the dissipation rates with environmental microbial consortium E (Figure 1). Microcosms with mixed culture C and D had the dissipation rates of 64 and 55%, respectively. It is evident from the results that increase in the organic matter content and initial heterotrophic bacterial counts resulted in increase in TBM dissipation rates in water. If the soils had previous contamination history, the TBM dissipation rates were even higher. This phenomenon is very commonly observed in several field-scale and lab-scale biodegradation studies [12-18]. |
| Degradation rates followed first-order decay in all microcosms except the ones with environmental microbial consortia A and B. The first-order rate constant for TBM degradation in water ranged from 0.002 to 0.005 hr-1 (Figure 1). Kalainesan et al. [5] reported similar degradation rates in soil, ranging from 0.0018 to 0.0058 hr-1. However, it should be noted that even with dilute concentrations of heterotrophs inoculated in water, the organisms were able to degrade TBM comparable to the rates found in soils. Environmental microbial consortium E with the highest TBM degradation rate was utilized in further TBM degradation studies. We obtained reproducible TBM degradation rates in water (first-order decay constant = 0.005 hr-1) during our second batch-incubation studies conducted over the period of 21 days using this consortium (Supporting Information, Figure S1). However, isolating an organism from this consortium that would degrade TBM in water was not successful. Addition of external carbon sources such as glucose and beef extract did not significantly increase the TBM degradation rates (data not shown). |
| Degradation of tert-butyl mercaptan (TBM) in water with Alcaligines faecalis subsp. phenolicus subsp. nov |
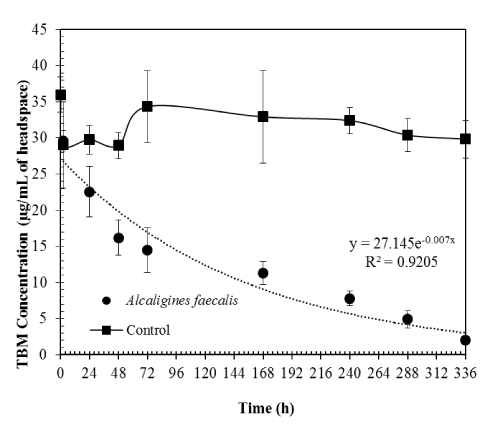
| The pure culture of Alcaligines faecalis subsp. phenolicus subsp. nov resulted in about 93% TBM degradation in water over 14 days. TBM degradation rates followed first-order kinetics with the rate constant of 0.007 hr-1 (Figure 2). This is a Gram-ve, coccobacillary bacterium isolated from a grey-water bioprocessor in a previous study [9]. This organism was found to grow on either 0.1% phenol or 0.5% TBM as the sole carbon and energy source in solid media plates [5,9]. The doubling time of this bacterium in water with 0.5% TBM was reported to be 98 h [5]. However, that study was conducted with no replication and tertbutyl alcohol (TBA) was supplemented initially along with TBM. We were able to reproduce the results (doubling time approximately 99 h) with replications and no added TBA in our present study. |
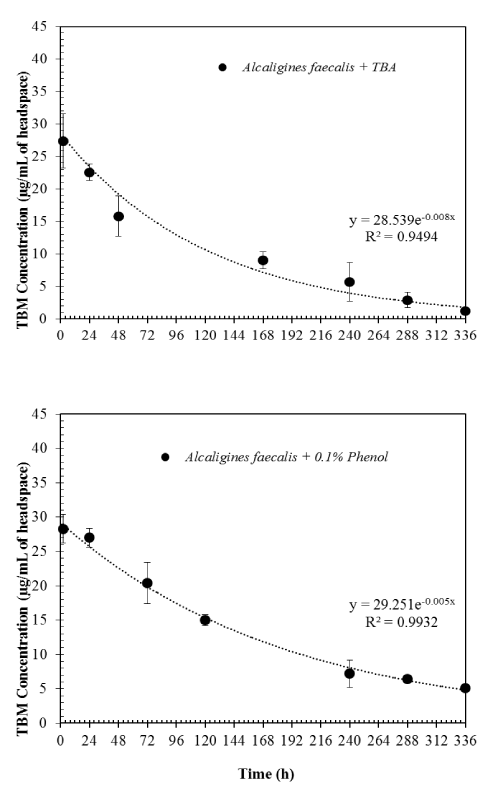
| When TBA was added to the microcosms along with TBM at the beginning of the experiments, TBM degradation rates were slightly increased. The first-order rate constant was 0.008 hr-1 (Figure 3). This clearly shows that TBM can be cometabolically degraded when TBA is present. However, when 0.1% phenol was added to the microcosms with TBM at the beginning of the experiments, TBM degradation was inhibited. The first order rate constant of 0.005 hr-1 was obtained for TBM degradation. This may be due to Alcaligines faecalis subsp. phenolicus subsp. nov preferentially degrading phenol. Since we did not monitor the degradation of phenol, this statement is rather speculative than conclusive. However, it should be noted that this organism was originally isolated as a phenol-degrading organism [9]. |
| Significance of the biodegradation study results |
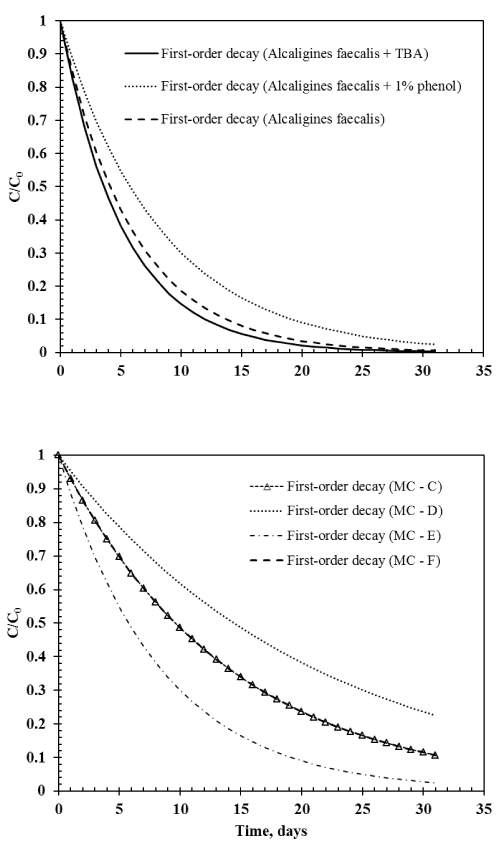
| Figure 4 shows the relative gas phase TBM concentration with first-order decay over a period of one month for different treatments: Environmental microbial consortia C, D, E, and F; Alcaligines faecalis subsp. phenolicus subsp. nov, Alcaligines faecalis subsp. phenolicus subsp. nov with TBA, and Alcaligines faecalis subsp. phenolicus subsp. nov with 1% phenol. In general, the half-life of TBM in water ranged between less than four days and 15 days (Figure 4). Half-life of TBM in water with microbial consortium obtained from soil with no contamination history (MC-D) was about 15 days where as the half-life was only four days in water with Alcaligines faecalis (Figure 4). Addition of external carbon sources such as TBA slightly decreased the half-life of TBM in water with Alcaligines faecalis while addition of phenol slightly increased the half-life at the given conditions. This demonstrates that while addressing the fate of TBM in water, the presence or absence of other organic substrates should be considered. When groundwater is contaminated with TBM either due to micro anaerobic conditions in the aquifer material or due to leakage of underground sewer lines and natural gas lines, in situ microorganisms can potentially degrade TBM. In our study, the environmental microbial consortia obtained from a previously contaminated site degraded about 97% of TBM in less than a month. However, in sandy aquifers where the microbial population is relatively low, TBM might persist for a longer time. These sandy aquifers can be enriched with pure cultures such as Alcaligines faecalis subsp. phenolicus subsp. nov used in this study along with trace amount of external organic carbon sources. Even though this culture is not yet tested at field-scale, bioaugmentation with pure cultures has resulted in promising end points in treating contaminated groundwater in the past [18-25]. |
| Proposed biodegradation pathway |
| Headspace samples were analyzed using GC/MS during the second batch study with microbial consortia E. In all headspace sampling, two other significant peaks appeared in addition to TBM peak throughout the experiment (note: these two peaks appeared during the first experiment as well; however, these peaks were not confirmed using GC/MS). The first peak (retention time = 12.4 min) was identified as di-tertiary-butyl-disulfide (DTBD; molecular formula: C8H18S2) with 95% similarity. The magnitude of this peak increased with time up to 10 days and then significantly decreased. Another peak appeared at 4.5 min, may be another intermediate product during the degradation of TBM, was identified as bis (1,1-dimethylethyl) disulfoxide (molecular formula: C8H18O2S2) with 88% similarity. The magnitude of this peak decreased with time as well. Since we did not have standards for DTBD and sulfoxide, we could not report actual concentration of these two compounds in the gas phase. |
| The proposed TBM degradation pathway in water involves initial oxidation of TBM (specifically S-H bonds) to DTBD (S-S bond). This oxidation can be catalyzed by chemical catalysts such as MnO2 or vanadium [26-29] as well as oxygenase enzymes present in microorganisms [30-37]. Kalainesan et al. [5] have reported that MnO2 as well as soil microorganisms oxidize TBM to DTBD before complete mineralization. DTBD can be further oxidized to sulfoxides or sulfones. Our assumption is part of TBM is completely transformed to CO2 and part is oxidized to disulfide and then further oxidized to sulfoxides before being oxidized to CO2. It should be noted that even though 50% of TBM disappeared in six days during this treatment, only after 10 days was a significant CO2 peak noticed in headspace. This clearly suggests that TBM undergoes intermediate oxidation steps; and complete mineralization in water could take longer than half life for mere disappearance of TBM [38]. |
| In pure culture experiments with no external carbon sources, we did not notice any other significant peaks other than CO2. This may be due to complete mineralization of TBM to CO2 by Alcaligines faecalis subsp. phenolicus subsp. nov in water. Since there are no toxic byproducts, bioaugmentation of TBM contaminated aquifers with this organism is advantageous to native organisms. |
| Conclusions |
| Laboratory studies were conducted to study the biodegradation potential of tertiary butyl mercaptan (TBM) in water. There were about 17% losses in gas phase TBM concentrations attributed to abiotic losses over the period of 14 days. Undefined microbial consortia from different soils with varying organic matter content and initial heterotrophic microorganisms were used to study the biodegradation of TBM under aerobic conditions. Environmental microbial consortium from sandy soils with low organic matter content and significantly lower heterotrophs resulted in the lowest biodegradation, only slightly higher than abiotic losses. Microbial consortia from soils with previous contamination history resulted in higher degradation rates. |
| In general, biodegradation of TBM in water followed first-order kinetics. The first-order kinetic constant ranged from 0.002 to 0.005h-1. TBM was partly degraded to two significant intermediate products and partly mineralized to CO2 in water with mixed culture isolated from a petroleum contaminated soil. The half-life of TBM in water with this mixed culture was only 6 days. |
| A Gram-ve bacterium isolated from a grey-water bioprocessor, Alcaligines faecalis subsp. phenolicus subsp. nov, was able to mineralize 50% of TBM within four days under laboratory conditions. The firstorder rate constant was 0.007 h-1. The degradation rate was slightly increased with the addition of tertiary butyl alcohol while slightly inhibited with the addition of phenol. |
| Acknowledgements |
| The authors thank Dr. James Urban for providing the pure culture of Alcaligines faecalis subsp. phenolicus subsp. nov. The authors acknowledge the help rendered by Dr. Scott Smith and Prini Gadgil in analyzing samples using GC/ MS. The authors also thank Dr. Charles Rice for allowing us to use the autoclaving facility. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 14452
- [From(publication date):
June-2012 - Sep 21, 2024] - Breakdown by view type
- HTML page views : 9973
- PDF downloads : 4479
