Review Article Open Access
Current Applications of Tissue Engineering in Biomedicine
| Cristina Castells-Sala, Mireia Alemany-Ribes, Teresa Fernández-Muiños, Lourdes Recha-Sancho, Patricia López-Chicón, Caterina Aloy- Reverté, Javier Caballero-Camino, Alejandro Márquez-Gil and Carlos E Semino* | |
| IQS School of Engineering, Ramon Llull University, Via Augusta 390, 08017 Barcelona, Spain | |
| Corresponding Author : | Carlos E Semino IQS School of Engineering, Ramon Llull University Via Augusta 390, 08017 Barcelona, Spain E-mail: carlos.semino@iqs.url.edu |
| Received May 06, 2013; Accepted August 20, 2013; Published August 22, 2013 | |
| Citation: Castells-Sala C, Alemany-Ribes M, Fernandez-Muiños T, Recha-Sancho L, Lopez-Chicon P et al. (2013) Current Applications of Tissue Engineering in Biomedicine. J Biochip Tissue chip S2:004. doi:10.4172/2153-0777.S2-004 | |
| Copyright: © 2013 Castells-Sala C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Bioengineering and Bioelectronics
Abstract
Tissue Engineering (TE) is a scientific field mainly focused on the development of tissue and organ substitutes by controlling biological, biophysical and/or biomechanical parameters in the laboratory. The result corresponds, in most cases, to the elaboration of three-dimensional cellular constructs with properties more similar to natural tissues than classical monolayer cultures. These systems enable the in vitro study of human physiology and physiopathology more accurately, while providing a set of biomedical tools with potential applicability in toxicology, medical devices, tissue replacement, repair and regeneration. To succeed in these purposes, TE uses nature as an inspiration source for the generation of extracellular matrix analogues (scaffolds), either from natural or synthetic origin as well as bioreactors and bio-devices to mimic natural physiological conditions of particular tissues. These scaffolds embed cells in a threedimensional milieu that display signals critical for the determination of cellular fate, in terms of proliferation, differentiation and migration, among others. The aim of this review is to analyze the state of the art of TE and some of its application fields: bone, cartilage, heart, pancreas, vascular and cancer.
| Keywords |
| Tissue engineering; Biomedicine; Scaffold; Bone tissue engineering; Cardiac tissue engineering; Cartilage tissue engineering; Vascular tissue engineering; Pancreas tissue engineering; Cancer; Drug discovery |
| Abbreviations |
| 2D: Two-dimensional; 3D: Three-dimensional; ASCs: Adult Stem Cells; BMPs: Bone Morphogenic Proteins; CTE: Cardiac Tissue Engineering; ECM: Extra-Cellular Matrix; EMT: Epithelial Mesenchymal Transition; ESCs: Embryonic Stem Cells; FGF: Fibroblast Growth Factor; GFs: Growth Factors; HA: Hydroxyapatite; HF: Heart Failure; iPSCs: Induced Pluripotent Stem Cells; LV: Left Ventricular; MSC: Mesenchymal Stem Cells; PCL: Polycaprolactone; PDGF: Platelet-Derived Growth Factor; PEG: Polyethylene glycol; PGA: Polyglycolic acid; PLA: Polylactic acid; PLG: Poly(lactide-coglycolide); PLGA: Poly(lactic-co-glycolic acid); PPF: Poly(propylene fumarate); RGD: Tripeptide arginine-glycine-aspartic acid; SHH: Sonic Hedgehog Homolog; TE: Tissue Engineering; TGFβ: Transforming Growth Factor; VEGF: Vascular Endothelial Growth Factor |
| Introduction |
| Tissue Engineering is an interdisciplinary discipline addressed to create functional three-dimensional (3D) tissues combining scaffolds, cells and/or bioactive molecules [1]. This field involves scientific areas such as cell biology, material science, chemistry, molecular biology, engineering and medicine. The term Tissue Engineering (TE) was first presented to the broad scientific community in 1993 by Langer and Vacanti [2]. Their definition is still applied nowadays and states that the ultimate goal in TE is the development of biological substitutes that maintain, improve or restore tissue function [2]. Therefore, TE could sidestep the problems associated with tissue damage, in the present treated with transplants, mechanical devices or surgical reconstruction. These three medical therapies have saved and improved countless patients’ lives, but they present associated problems. For example, organ transplants show important limitations such as transplant rejection and lack of donor to cover all the worldwide demand. Mechanical devices are not capable of accomplishing all the functions associated with the tissue and cannot prevent progressive patient deterioration. Finally, surgical reconstruction can result in long-term problems [3]. Therefore, TE arises from the need to provide more definitive solutions to tissue repair in clinics and aims to achieve this goal by the development of in vitro devices that would repair in vivo the damaged tissue. |
| TE also leads to engineered tissues, which could allow us to study human physiology in vitro [1,4]. Cells in the body grow within an organized 3D Extra-Cellular Matrix (ECM), surrounded by other cells. Indeed, the interactions between cell-cell and cell-ECM can determine whether a given cell undergoes proliferation, differentiation, apoptosis or invasion. However, studies on cellular biology have commonly been performed on 2D cultures, where cells grown under non-physiological conditions. Specifically, they are unnaturally polarized having one side attached to a rigid and flat substrate and the other one exposed to culture media, which reduces cell-cell and cell-ECM interactions. |
| Consequently, 2D cultures do not recreate properly in vivo systems in terms of cellular communication, gene and protein expression pattern and diffusion of soluble molecules (oxygen, nutrients, growth factors, etc.) [4,5]. At the opposite end of experimental platforms, animal models can display the integrated responses that result from complex interactions between tissues and organs. Nonetheless, they fail to capture important facets of human responses, are very costly, time-consuming and ethically controversial [4,6]. As a result, 3D cultures are currently being developed to provide the third dimension, which is essential to integrate mechanical and chemical signals, better mimicking the in vivo microenvironment. These 3D cultures fulfill the need for in vitro approaches that enable an accurate study of the molecular mechanisms underlying human diseases and a better prediction of drugs and therapies effect [1,7-9] (Figure 1). |
| TE involves basically three basic elements: scaffolds, cells and biomolecules. Therefore, it is necessary to carefully choose which combination better fits for the desired application. For this reason, we will give a general overview for each component. |
| Scaffolds |
| A major goal in TE is the design of scaffolds capable of recreating the in vivo microenvironment, which is mainly provided by the ECM. Thereby, these structures should incorporate the appropriate biophysical, biomechanical and biochemical cues that guide cell proliferation, differentiation, maintenance and function [10]. |
| Regarding biophysical signaling, an essential function of the ECM is to give anchorage to cells. Indeed, the ECM highly porous nanostructure provides them a proper 3D microenvironment and imparts biochemical signaling through two mechanisms: (i) the binding of a wide variety of soluble Growth Factors (GF), enzymes and other effector molecules, controlling their diffusion and local concentrations and (ii) the exposure of specific motifs that are recognized by cellular adhesion receptors. As a result, ECM is dynamically integrated with the intracellular signaling pathways that regulate gene expression and participate in cell phenotype determination [11-14]. |
| Additionally, the cells are able to sense the matrix stiffness, which results in mechanical signaling. Cells routinely contract to pull on the milieu to which they are attached, generating internal tension. This mechanical stimulus is converted into a chemical response through a process known as mechanotransduction, which has been reported to influence directly on cell differentiation [15-17]. Due to the complexity and interaction among all these cues, TE focuses on mimicking the most relevant ECM properties to develop scaffolds custom tailored depending on the tissue to be recreated [11,18,19]. |
| Specifically, parameters as scaffold dimensions pore size, chemical signaling motives and stiffness should be carefully examined. |
| An ideal biomaterial designed for clinical applications should fulfill a serial of requirements. First of all, biocompatibility and biodegradability are required; allowing scaffold replacement by proteins synthesized and secreted by native or implanted cells [19-21]. Besides, the material must be clinically compliant (Good Manufacturing Practice) to minimize inflammatory and immunological response avoiding further tissue damage [19]. Moreover, as cell degradation products are toxic to other cells, it would be important that the material allow host macrophages to infiltrate and remove cellular debris [22]. Finally, material production, purification and processing should be easy and scalable [10,23]. |
| Scaffolds for TE can be divided in natural and synthetic, depending on its origin. Natural scaffolds are readily accessible and provide a broad range of cues that in vivo participate in the process of morphogenesis and function acquisition of different cell types. However, its composition strongly depends on the specific animal origin and the isolation and purification procedures, compromising assay reproducibility [1,19]. On the other hand, synthetic scaffolds can be custom tailored to mimic specific ECM properties, providing controllable cellular environments. |
| Paradoxically, this advantage makes this class of biomaterials far more challenging because wide range of factors has to be identified and precisely incorporated. Indeed, unless surface-modifications are applied (adhesion of peptides or biological molecules), scaffolds only serve to hold and guide cells in 3D space until they produce their own physiological matrix environment [1,22,24]. |
| Cells |
| An important decision to make when designing strategies for TE is the cell source selection. This step becomes a critical issue especially when these strategies are designed to be clinically applied. Importantly, cells should fulfill a basic requirement: integrate themselves in the specific tissue and secret various GF and cytokines that activate the endogenous tissue regeneration program. The first approach in cellbased techniques is the use of native progenitor cells. |
| The main problem is the inherent difficulty of growing some specific cell types to obtain large quantities. As a consequence, stem cells either Embryonic (ESCs) or Adult (ASCs) have emerged as promising alternative cell sources [25]. ESCs are pluripotent cells that are able to differentiate into any lineage but their use is highly restricted due to ethical controversies and their potentiality to produce teratomas. On the other hand, ASCs are multipotent cells, so they have more limited capacity to differentiate than ESCs. Nonetheless, they overcome some problems associated with ESCs, being more appropriate for TE [26]. For instance, ASCs and tissues derived from them are currently believed less likely to initiate rejection after transplantation. Research on ASCs is progressing rapidly and up today, they have been isolated from different tissues including bone marrow [27], muscle and adipose tissue [28] and umbilical cord [29,30]. |
| An alternative cell type under study for their application in TE are induced Pluripotent Stem Cells (iPSCs), which were first generated by Yamanaka and collaborators [31] from mouse fibroblasts and subsequently obtained from adult human cells [31,32]. Basically, iPSCs are somatic cells that have been reprogrammed to a pluripotent state through the introduction of a defined set of transcription factors. The main advantages of these cells are their autologous character, their differentiation capacity and the robustness and simplicity of reprogramming procedure. |
| However, there are several barriers to overcome before extensively using iPSCs. Specifically, the molecular mechanisms underlying reprogramming should be precisely characterized before its use in clinical applications [33,34]. |
| Biomolecules |
| Besides an appropriate scaffold and cell source, signaling molecules represent an interesting tool in TE to modulate several aspects of cell biology, from proliferation capacity to specific phenotypic features of fully differentiated cells [18,35,36]. In the cellular milieu, the presence and gradient of soluble factors such as GF, chemokines, and cytokines play an important role in biological phenomena such as chemotaxis, morphogenesis and wound healing. In particular, these signals are tightly controlled and unique to each organ. |
| Signaling molecules used in TE can be added to the culture media as soluble factors or attached to the scaffold by covalent and non-covalent interactions. First of all, the direct delivery of these molecules in the media is frequently used to in vitro evaluate the effect of these cues. However, these biomolecules are rapidly degraded and deactivated by some cell-secreted enzymes, responsible for their short biological half-live. For this reason, for clinical applications, bounding factors to the matrix helps to protect them from degradation [37]. Consequently, the controlled release of different factors from scaffolds allows their constant renewal, having a great potential to direct tissue regeneration and formation. Several matrix systems, micro particles and encapsulated cells have been reported to locally deliver bioactive factors and to maintain effective concentrations for their use in the application areas, such as musculoskeletal, neural and hepatic tissue [38-40]. |
| This review summarizes progress in the two TE application fields: the regeneration of damaged tissue and the development of in vitro human models. Within the regenerative purpose, we carefully examine the concerted efforts realized in bone, cartilage, cardiac, pancreas and vascular tissue engineering. On the other hand, 3D in vitro models can transform the way we understand cancer disease, helping to optimize future therapeutic approaches (Table 1). |
| TE For Regeneration of Damaged Tissues |
| Bone tissue engineering |
| Bone is the calcified connective tissue responsible for supporting body structure and protecting internal organs. It consists mainly of a natural organic mineral composed by collagen type I and diverse forms of calcium phosphate such as hydroxyapatite. It is formed by an outer layer with low porosity (10-30%) and high mechanical strength, and an internal layer with higher porosity (50-90%) and reduced mechanical properties. Both layers contain a highly vascularized network, which plays a crucial role supplying oxygen and nutrients and removing waste products. These features are challenging to reproduce in vitro, making difficult to obtain an ideal scaffold for bone tissue regeneration [41-44]. |
| Unlike other tissues, bones possess the capacity for regeneration, remodeling and repair in response to injury. However, the supply of bone grafts is needed when the required bone regeneration exceeds the natural potential for self-healing, as in large bone defects occurring after trauma, infection, tumor resection or skeletal abnormalities [45]. In the clinical setting, the gold standard to treat bone defects consists in the transplantation of autologous bone grafts, usually harvested from the iliac crest or the fibula. However, the disadvantages of these grafts are the limited availability of autologous material and donor-site morbidity [45]. Possible alternatives are allografts and xenografts, but their use is often associated with infection, disease transmission and immunological rejection [46]. |
| One of the most applied strategies in bone tissue engineering consists on the development and application of 3D porous scaffolds with similar composition to the bone. Bioceramic scaffolds, mainly Hydroxyapatite (HA), have been widely used since their chemical composition is comparable to the mineral component of natural bone and its biocompatibility and bioactivity successfully promotes new bone formation in vivo [47,48]. Instead, low mechanical properties of ceramics materials may result in their fracture upon applying load, making them unsuitable for the regeneration of large bone defects. For this reason, composites made of HA and natural hydrogels, such as collagen type I, take the advantage of both components and better mimics the bone structure, showing an improvement in the in vivo results [49]. Some other combinations of HA with chitosan, alginate, gelatin, PLA and other polymers have been developed and studied in order to find the best bone substitute [47,50-52]. |
| Next generations of scaffolds are osteo-inductive, promoting new bone formation through biomolecular signaling and progenitor cells recruitment [41]. Signaling molecules can be incorporated into scaffolds through simple dispersion or stronger immobilization by electrostatic and covalent bonding [44]. Several types of nanoparticles have been designed for the release of osteogenic factors, like some Bone Morphogenic Proteins (BMPs), and showed an enhanced in vitro and in vivo osteogenic differentiation in bone defect models [42,44]. However, the delivery of a single growth factor can enhance but not emulate the complex process of bone regeneration in vivo. Indeed, since bone is a highly vascularized tissue, the ideal scenario would be the delivery of a cascade of different GFs to simultaneously induce angiogenesis and osteogenesis in order to produce a vascularized and functional bone tissue substitute [42,53]. Another approach is to supply GFs together with cells, which is expected to stimulate a more effective repair since the GFs can act directly on the seeded cells, promoting their proliferation and differentiation [44]. In general, cellularized grafts provide better integration with the host tissues and remodeling than acellular counterparts. Several studies show that Mesenchymal Stem Cells (MSCs) stabilize vascularization and form functional bone in vivo, being as good as autologous bone grafts [54-57]. In addition, other studies use co-cultures of MSCs and endothelial cells in order to enhance the establishment of vascular networks [42]. |
| For clinical applications, it is necessary to develop manufacturing processes that guarantee an automated and controlled bone production [45,58]. Up to date, the use of bioreactors together with computerassisted imaging and modeling has been reported. As an example, it was published the development of a temporomandibular joint bone graft by using hMSCs and a biomimetic scaffold-bioreactor system [58]. The main drawback is that as the similarity to native bone tissue increases in cell-seeded scaffolds, the readiness for clinical application decreases compared to cell-free scaffolds [46]. |
| Even with the development of aforementioned strategies, challenges still remain in the inability to reproduce an engineered well vascularized bone that truly mimics natural bone blood vessels. New frontiers of research should be directed in a more fundamental understanding of the biological processes during bone tissue regeneration. |
| Cartilage tissue engineering |
| Cartilage is a stiff and flexible connective tissue, composed of chondrocytes embedded in a highly hydrated ECM, being their main components collagens, proteoglycans and elastin [59]. Cartilage can be classified in elastic cartilage, hyaline cartilage and fibro cartilage, which differ in the relative amounts of ECM components. Within hyaline class, repair of articular cartilage remains an unmet medical need due to its little capacity for self-regeneration. Particularly, as it is an avascular tissue, either chondrocytes or progenitor cells cannot migrate towards the lesion to produce more matrixes [60]. As a consequence, even minor lesions may lead to progressive damage and osteoarthritic joint degeneration, resulting in significant pain and disability [61]. Aiming to promote cartilage repair, tissue engineering strategies that combine appropriate cell sources with scaffolds, mechanical stimulation and GFs are being developed. |
| However, there are a number of challenges that remain to be accomplished due to the complex biological environment that is necessary to mimic. |
| Theoretically, autologous chondrocytes would be the ideal donor cell type for cartilage repair due to their intrinsic properties in terms of function and immune compatibility. However, they are obtained by invasive techniques and they de-differentiate to fibroblasts phenotype when cultured in monolayer. Therefore, alternative cell sources are being considered. Since chondrogenesis is initiated by a condensation phase of mesenchymal precursor cells, MSCs collected from different sources, such as adipose tissue or bone marrow have generated great interest [62]. MSCs have a vast proliferative capacity, can be easily cultured in vitro and have the ability to differentiate towards osteogenic, adipogenic, chondrogenic and myogenic lineages [63]. Consequently, an appropriate biochemical and biomechanical microenvironment is required to in vitro guide chondrogenic commitment and stable cartilaginous tissue formation [64]. |
| In articular cartilage, numerous GFs have been long recognized as important candidates to work in concert to regulate development and homeostasis [65]. Specifically, biomolecules as transforming growth factor-β superfamily (TGFβ), Bone Morphogenic Proteins (BMPs) and Fibroblast Growth Factor (FGF) have been proposed to play a crucial role on these processes [66]. They act via specific membrane-bound receptors and their effects are mediated by signal transduction processes, leading to the induction of cell proliferation and differentiation. Moreover, correct, duration and intensity of this stimulation are key factors in cell behavior to induce a regenerating environment. For this reason, important efforts are focused on the delivery of these factors in the affected zone. |
| For the last two decades, several natural scaffolds have been studied for their application in cartilage repair: collagen [67,68], fibrin [69,70], hyaluronan [71], agarose [72,73], alginate [74-76] or gelatin [77]. In addition to natural scaffolds, synthetic scaffolds are regarded with a serial of advantages, being the most relevant their defined properties and, thus, their reproducibility. For instance, polyurethane [78], Poly (Ethylene Glycol) (PEG) [79,80], elastin-based polymers [81] and selfassembling peptides, such as KLD-12 [73] and RAD16-I [82-84] are being widely used. Interestingly, these biomaterials can be decorated to incorporate signaling motives that better recreate cartilage ECM. Up to date, peptide sequences such as RGD moieties to stimulate cell adhesion [85,86] and decorin moieties to bind and release GFs [87] have been reported to enhance chondrogenesis and reach the same levels of differentiation obtained with natural scaffolds. Additionally, composites consisting of two or more materials incorporated in a single scaffold are also under development. This group can include a mixture of fibers from different natural materials and a synthetic or naturally derived hydrogel infused into a synthetic mesh. For instance, coatings of collagen and fibronectin are used for cartilage applications in order to improve cell adhesion, based on the cell-integrin receptors. This combination is an interesting approach to replicate the complex structure to provide the functional in vivo properties [88]. On the other hand, a more simplified culture system for chondrogenesis is pellet culture or micromass culture that consists in pellets compromising between 200.000 and 500.00 cells cultured in vitro with the appropriate chondrogenic media inductors [62]. This scaffoldless approach avoids the complexities of creating tailored matrices, since cells create and remodel their extracellular matrix. |
| As cartilage is a tissue that acts as one entity to distribute applied load and therefore accomplish its mechanical function, future directions for cartilage TE are related to the integration of the engineered construct with the native host tissue. If implanted or injected immediately, the scaffold should maintain its shape and possess robust mechanical characteristics similar to native cartilage to match the loading environment. However, in vitro culturing systems do not require scaffolds with these strict properties, since during the culture period new tissue forms and acquires slowly the chondrogenic commitment. |
| Cardiac tissue engineering |
| Heart is the muscular organ responsible of pumping blood throughout arteries and veins to supply nutrients and oxygen all along the body. The muscular tissue is divided in three layers: epicardium, myocardium and endocardium. Interestingly, myocardium is composed by cardiomyocytes that have the unique ability to selfcontract without central nervous system intervention. Their inherent contractile activity is critical for blood pumping and, hence, loss or dysfunction of cardiomyocytes results in Heart Failure (HF), the major cause of death and disabilities in the world. Nowadays, the current treatments for HF are focused on drug therapies. Unfortunately, at the end stage, heart transplantation is the only available solution [89]. Since transplants are limited due to lack of donors and the immuneresponse of the host recipient, cell-therapies are emerging as promising strategies to induce heart regeneration [22]. These therapies have been approached from different angles: direct injection of isolated cells suspension [90-93]; injection of biomaterials with or without cells and/ or GFs [94-100] and implantation of biomaterials previously prepared with or without cells and/or GFs [26,101-104]. |
| Several cell types are being tested as potential sources for Cardiac Tissue Engineering (CTE). Since the primary goal is to increase the number of contractile cells in the necrotic zone, cardiac myocytes and skeletal myoblasts were the first evident cell source due to their natural electro-physiological, structural and contractile properties. However, these cells are difficult to obtain and expand. On the other hand, embryonic stem cells can differentiate into cardiac lineage, but implanting them in vivo might have the risk of producing teratomas [26,103,105,106] and/or arrhythmias [107,108]. Nowadays, extensive research focuses on the use of adult stem cells, since they are not ethical controversial and can be easily isolated from the own patient. Interesting detailed reviews have been published the last year regarding to advantages, drawbacks and different advances developed in this issue: [19,103,107,109-113]. |
| Different studies have faced the problem of HF combining biomaterials, biomolecules and cells. A lot of natural biomaterials are being tested due to their intrinsic characteristics. Chitosan has been widely used as soft and injectable material and it has been proved that its application in ischemic myocardium could improve myocardial infarction microenvironment [96]. Additionally, chitosan has been modified in different ways to improve its mechanical characteristics [98,114] and/or its effect on cell differentiation [115], maintaining the biological properties of the material. Diverse proteins like gelatins, collagens [116,117], laminin, silk [118], vitronectin [119], fibrin [120] between others, with or without modifications has also been examined for their effect on cell behaviour both in 2D and 3D microenvironment in vitro. |
| On the other side, diverse synthetic biomaterials are under development. A blend of POC/PLCL (poly(1,8-octanediol-co-citrate)/ poly(L-lactic acid)-co-poly-(3-caprolactone)) has been designed to obtain a material with appropriate mechanical properties, with a special focus on its tensile strength to enable heart beating [121]. In addition, poly (D, L-lactic-co-glycolic acid) (PLGA) porous beads have been reported to improve cell retention, maintaining them in the infracted area after implantation [95]. Besides elasticity and cell retention, biomaterials for CTE need to accomplish other properties. For instance, the poor conductivity of the materials, which limits the patch from contracting strongly as a unit, has to be faced. This issue has been approached from different points of view like the incorporation of gold nanowires within alginate scaffolds [122] or the combination of PLGA with carbon nanofibers in different blend ratios [123]. Moreover, materials for CTE need to be especially resistant to the formation of a fibrous capsule, which may result in electrical isolation of the transplanted tissue. Because of its low protein adsorption properties, PEG has shown to be an excellent candidate [124]. Extensive research is still needed in order to design a material that integrates the most relevant properties to fulfill heart demands. |
| Moreover, some efforts have been directed to GFs release into the infarcted area [107]. The use of nanoparticles or micro encapsulations for GFs and/or cells delivery can provide sustained slow-release and a safe refuge to escape recognition by the host immune system [125,126]. Parallel, hydrogels have been investigated as alternative vehicles for release or immobilization of GFs [94,127,128]. In general, the results showed that substrate immobilization for its slow administration is a powerful tool for the development of useful strategies for regenerative medicine in CTE. |
| Apart from the correct selection of cells, materials and GFs, other factors should be taken into account: patient selection, concomitant procedures, cell transplantation, timing, cell survival, cell tracking, dose, age, regulatory issues and funding [129]. Interestingly, it has been showed that the injection of the biomaterial immediately after infarct inductions in a rat model resulted on no observable improvement, but a delay of one week significantly increased scar thickness and fractional shortening, as well as decreased end-systolic diameter against saline controls [97]. On the other hand, it is possible that pre-seeding of the material with the cells can enhance therapeutic efficacy [130,131]. |
| Although the experimental basis of myocardial cell therapy is still incomplete, several clinical applications have already been initiated. During the last 10 years, major findings in clinical trials have shown an improved contractile and systolic function, improvement in Left Ventricular (LV) remodeling parameters and a decrease of LV end systolic volume [107,110,111]. Therefore, CTE is emerging as a promising approach to treat cardiomyopathies. Anyway there is still a long way to go. |
| Pancreas tissue engineering |
| Pancreas is an organ comprised of two separated functional units, the exocrine and the endocrine. The exocrine compartment consists of acinar and ductal cells, which synthesize and secrete digestive enzymes. On the other hand, the endocrine compartment is composed of α, β, δ, ε and pancreatic polypeptide cells assembled into islets (islets of Langerhans) that secrete hormones into the bloodstream. Approximately, 80% of these cells are β-cells, responsible for producing insulin and, thus, regulating glucose levels in blood [132]. The destruction of β-cells by the immune system causes a disease known as Diabetes Type 1 [133]. The classic treatment consists of daily injections of exogenous insulin. Therefore, a concerted effort is being made to find alternative treatments that improve life quality of patients. Based on this statement, TE proposes the replacement of the damaged cells for regulated insulin-producing cells [133]. |
| Islets of Langerhans are 3D structures surrounded by a double basement membrane, mainly composed of laminins and type IV collagen. Signals originating from this specialized ECM are crucial for β-cell function [134]. As a consequence, the culture of these cells in monolayer leads to the loss of insulin expression, compromising their potential use for clinical applications [135]. On the other hand, 3D cultures are capable of re-establishing critical ECM-β-cell signaling, improving the outcome of islet culture techniques used between islet isolation and transplantation [136]. |
| Within 3D cultures, the most commonly used scaffold for pancreatic TE has been Matrigel™, a solubilized basement membrane preparation extracted from the Engelbreth-Holm-Swarm mouse sarcoma. Several authors reported a considerable increase in the insulin accumulation when growing islet cell clusters in Matrigel™, compared to classical 2D cultures [137,138]. Alternatively, Matrigel™ has also been used to obtain new sources of functional β-cells, since a major drawback of islet transplantation is the limited amount of donor tissue [139]. Interestingly, human ductal cells grown in 2D as an epithelial sheet and covered with a thin layer of Matrigel™, could be differentiated into insulin positive cells [140]. In addition to Matrigel™, other materials have been purposed as suitable 3D scaffolds for pancreatic TE. For instance, promising results have been obtained by using collagen [141] and PEG hydrogel with matrix proteins entrapped [136]. Additionally, 3D cultures have been supplemented with different biomolecules, aiming to promote specific biological events that improve the functionality of transplanted islets. An example is the vascularization of the graft through the delivery of angiogenic factors, like VEGF [142]. |
| Although islet transplantation permits to correctly control glucose levels, this procedure presents several obstacles. The major barrier is the need to use long-term immunosuppressive drugs to overcome rejection of cross-patient transplanted islet cells [143,144]. Furthermore, as the islets remain in the hepatic portal vein, they are exposed to high levels of toxic drugs and circulating toxins [145]. A strategy proposed to avoid some of these associated-problems is islet encapsulation [146]. In this technique, transplanted cells are separated from the host immune system by a semi permeable and biocompatible membrane, which allows the islets to regulate blood glucose levels through insulin release while excluding the larger proteins and cells of the immune system [144,146,147]. In particular, the most studied experimental procedure is microencapsulation and consists in encapsulating single or groups of islets within spherical droplets [146]. Hydrogels based on alginates are the most frequently used biomaterials for microencapsulation due to their abundance and easy gelling properties [148,149]. However, nowadays it is still being argued whether immune-suppression is required in microencapsulation techniques [150]. |
| In conclusion, pancreatic islet regeneration and transplantation have achieved a significant progress during the last decades thanks to the better understanding of the complex microenvironment that surrounds β-cells and the advances in fields such as biomaterials and immunology. Nevertheless, some issues, as the long-term survival of transplanted islets and shortage of islets, still need to be overcome. |
| Vascular tissue engineering |
| Vascularization is the process through which blood vessels and capillaries are formed in living tissues. The anatomy of blood vessels can be divided in three layers: the Tunica Intima is the inner layer facing the blood and it is composed of endothelial cells and variable amounts of connective tissues; the Tunica Media is composed of smooth muscle cells with elastic fibers and the Tunica Adventitia is composed basically of connective tissue. The role of blood vessels is crucial, since they have to maintain a specific balance in blood distribution to avoid an insufficient or excessive delivery of oxygen and nutrients that could lead to the development of many diseases. Particularly, diseases like tumors are associated with new blood vessel growth to fulfill the metabolic demand of the altered cells [151]. Up to date, increasing number of studies are under development trying to inhibit de novo vascularization in the area of cancer disease [152-154], while some efforts are performed with the aim reproduce well vascularized devices for tissue repair [155-157]. |
| Tissues that grow beyond 100-200 μm (oxygen diffusion limit) require the formation of new blood vessels to maintain cell viability [158]. According to that, one of the major challenges in TE is the vascularization of the engineered tissue. To succeed in this objective, it is first needed the complete understanding of this process. Basically, it occurs by two different mechanisms that are interdependent: vasculogenesis and angiogenesis. First of all, vasculogenesis is de novo formation of the primary blood system by endothelial cells in response to local cues (such as GFs and ECM) [159,160]. On the other hand, angiogenesis is defined as the creation of new capillaries from pre-existing blood vessels. Upon angiogenic stimulation, endothelial cells are activated and begin to degrade their surrounding basement membrane. Then, they migrate into the interstitium to form capillary buds and sprouts and proliferate, elongating the newly blood vessel [158,161]. The strategies currently used to vascularized engineered tissues are: new scaffolds design, angiogenic factor delivery and in vitro or in vivo pre-vascularization. |
| The use of biomaterials is a versatile strategy, easy to develop and translate into multiple tissues. However, after implantation, the limitation of oxygen and nutrient supply remains an issue. The success of the bio implant relies on vessel ingrowth from host tissue and, therefore, scaffold design has a deep impact in vascularization rate after surgery. Nowadays, there are several studies confronting this issue using scaffolds from different sources [158,162,163], converging that pore size and interconnectivity is the most critical parameters. Besides the development of vascularized grafts, it is also necessary to develop structures that contribute to repair the damaged vasculature [164,165]. |
| Another strategy to improve blood perfusion is angiogenic factors delivery. It is well established that the addition of angiogenic factors to tissue-engineered constructs can enhance their vascularization after implantation. The most studied are Vascular Endothelial Growth Factor (VEGF) [157,166-168] and basic Fibroblast Growth Factor (bFGF) [169,170], because they directly promote the formation of new vessels. Parallel, there is a set of factors that stimulate cells close to the vascularization site to produce angiogenic factors. Some examples are Sonic Hedgehog Homolog (SHH) [171] and the Bone Morphogenetic Protein (BMP)-2, -4 or -6 [172–174] between others. Additionally, there are other GFs that are involved in stabilizing new vessels, including Platelet-Derived Growth Factor (PDGF) [166,175,176], Transforming Growth Factor β (TGF-β) [177] and angiopoietin 1 [178-181]. |
| Finally, both in vivo and in vitro pre-vascularization are interesting strategies to obtain vascularized grafts for TE. In vitro prevascularization is based on the use of endothelial cells cultured under specific conditions to form pre-vascular structures. On the other hand, in vivo pre-vascularization consists in implanting a tissue-engineered construct into a region with an artery suitable for microsurgical transfer. Despite in vitro approach does not rely on vessel ingrowth from host, avoiding consequently the need for extra surgery, it lacks the complex biological machinery that in vivo regulates vessel maturation and anastomosis. Interestingly, in vitro pre-vascularization has been used for the regeneration of many tissues, like skin, skeletal muscle, cardiac muscle or bone [182,183]. In vivo methodology is a promising strategy since it can generate a mature and organized vasculature. However, two surgeries are required: the first one to connect the graft with the irrigation system for its proper vascularization and the second to implant the vascularized grafts into the damaged area. Moreover it is highly probable to obtain an implant filled with fibrous tissue [158]. |
| Vascularization should be integrated in most TE fields, since the proper delivery of oxygen, nutrient and soluble effector molecules as well as removal of metabolites is essential to improve regeneration of the damaged tissue. Although there are several existing techniques to induce vascularization, they need to be optimized in order to overcome their associated problems. |
| TE for Modeling Human Physiology |
| Another growing application of TE is the creation of in vitro human models, which help us to identify and comprehend the factors that drive cellular processes. In particular, the idea is to deconstruct the complex cellular microenvironment into simpler systems in order to analyze the role of different chemical, mechanical and/or physical factors. In fact, these models fulfill the need for reductionist approaches to understand the in vivo molecular mechanisms that rule human physiological as well as pathological processes and, in turn, better predict the effect of drugs and medical therapies. |
| Important efforts are being directed towards the study of different disorders such as arrhythmia [184], skin fibrosis [185] and wound healing [186,187] and the comprehension of the biological function of healthy organs such as skin [188-191], blood-brain barrier [192] and mammary gland [193] development. In this review we will focus our attention in 3D modeling for drug discovery and cancer disease. |
| Cancer |
| Cancer is a leading cause of disease worldwide, accounting for approximately 12% of all deaths [194,195], only preceded by cardiovascular and infectious afflictions. Significantly, 90% of these deaths are produced by the metastatic spread of primary tumors [196]. This critical process is initiated when tumor cells gain the capacity to degrade their basement membrane and invade the surrounding tissue. Subsequently, they enter to lymphatic and blood vessels in order to disseminate into the circulation and undergo growth in distant organs or tissues [197-199]. This cascade of events occurs through Epithelial- Mesenchymal Transition (EMT) that involves complex changes in cell architecture and function. During EMT, tumor cells lose their epithelial phenotype, resulting in basal-apical polarity disruption, intercellular adhesions down-regulation and a dramatic remodeling of the actin cytoskeleton. As a consequence, cells turn into a mesenchymal phenotype that switches on cell motility programs. This transition is driven by tumor microenvironment, especially by the mechanical and chemical cues that arise from ECM and neighboring cells, arranged in a 3D pattern [198,200-202]. |
| A major challenge in cancer research is the development of in vitro models that recreate the process of tumor progression, with particular focus on migration and invasion key steps. To reach this objective, it is necessary an accurate modeling of tumor microenvironment. In classical 2D cultures, tumor cells are unnaturally polarized and have their surface mainly exposed to cultured media and rigid substrates [10]. On the other hand, 3D cultures can better capture tumor architecture, characterized by having quiescent or necrotic cells located at the internal core of the tumor and highly proliferative cells at the surface. This situation occurs due to naturally arising mass transfer phenomena, which are caused by an elevated deposition of ECM components and a poorly organized vascular network. Therefore, 3D cultures can provide the micro-environmental conditions that control tumorogenesis [203-205]. |
| During the last two decades, a wide range of biomaterials have been carefully designed in order to guide and promote tumor progression. Natural biomaterials have been extensively used, being collagen and Matrigel™ the most prominent ones. A further step in cancer research has consisted of developing synthetic biomaterials that provide both a reproducible cellular microenvironment and the flexibility to individually tune a physical or chemical characteristic with the aim of analyzing its specific role on the disease. Thus far, polymers as PEG [206,207], PLG [203], PLA and PLGA [208] and hydrogels such as RAD16-I (PuraMatrix™) have been used to model cancer. |
| Importantly, there are two ECM parameters that act as key regulators of cellular response in cancer and, therefore, it is essential to characterize and control them: matrix stiffness and molecular composition [6]. First of all, tumors progressively stiffen their microenvironment; breast cancer tissue can be 10 times stiffer than healthy tissue [12]. This phenomenon is produced by an elevated deposition and remodeling of ECM components, mainly fibrilar collagen and hyaluronic acid, secreted by cancer cells and resident fibroblasts of the stroma [11,209]. It has been largely demonstrated that cells sense the mechanical properties of the ECM through their tension machinery (Rho/ROCK signaling pathway). In particular, Rho proteins induce contraction of the actomyosin cytoskeleton, increasing intracellular tension, down-regulating ECM specific ligands and activating key genes in tumor progression, such as metalloproteinases [16,209,210]. Traditionally, in vivo stiffness values have been achieved by increasing concentration, composition or cross-linking density of the biomaterials. Natural scaffolds suffer modifications of fiber architecture, adhesiveness and pore size when increasing their stiffness. To overcome this drawback, it has been described the use of self-assembling peptides (laminin-absorbed RAD16-I) to individually tune tumor compliance microenvironment, without affecting other physical parameters [211]. Therefore, they enable the study of the molecular mechanism whereby ECM stiffness regulates tumorogenesis, without introducing an array of confusing biophysical cues. In particular, it has been observed that ECM stiffness per se could initiate tumor progression through modulation of integrin dynamics. Secondly, ECM binding motifs and GFs constantly interact with cells, activating signaling transduction cascades that determine cell fate. This effect has been largely demonstrated by culturing cancer cells within natural ECM components, mainly collagen and Matrigel™. The results showed that expression of key tumoral genes (EMT markers, matrix metalloproteinases, pro-angiogenic factors, etc.) resembled that of the in vivo situation [212-215]. A further step in cancer research is the development of synthetic scaffolds that incorporate this ECM molecular composition, enabling the profound study of their specific role on the disease. An important example is the design of PEG scaffolds functionalized with integrin-binding domains (RGD), which cause a major cell cluster formation and, therefore, point that integrins act as direct regulators of cell survival and proliferation [206,207]. |
| A part of biomaterial perspective, tumor microenvironment is also comprised of stroma cells, which include fibroblasts, pro-angiogenic cells (endothelial cells, pericytes and smooth muscle cells) and immune system cells (lymphocytes, macrophages and mast cells). They are responsible for the synthesis, deposition and modeling of a large portion of the ECM proteins. Furthermore, they secrete several paracrine GFs that participate in cancer cell growth. Hence, these cells act as active participants in tumorogenesis rather than passive bystanders [216,217]. For this reason, co-cultures have been introduced in cancer research. Up to date, fibroblasts [218], endothelial cells [219] and macrophages [220] have been cultured together with tumor cells in collagen gels. Results showed that stroma cells contributed to tumor cells migration and vasculature sprout formation trough the up-regulation of proteases and the delivery of angiogenic factors respectively. |
| Nowadays many questions about cancer biology remain to be answered, but TE modeling enriches the toolbox to understand disease progression and, thus, improve therapeutic approaches. To achieve this goal, it would be interesting to use more extendedly 3D models within the scientific community. |
| The above-mentioned 3D cultures are based on combining cells, scaffolds and biomolecules. However, we can reach a higher degree of complexity by integrating microchip and microfluidic approaches to TE. First of all, microfabrication include techniques such as photolithography, replica molding and microcontact printing, which enable the creation of structures with well-defined shapes on the micrometer scale, so that we can control cell position, morphology and function. Secondly, microfluidics consists of manipulating small amounts (10-9 to 10-18 L) of fluids in hollow chambers and, therefore, allows us to generate and precisely tune spatiotemporal gradients of soluble effector molecules (nutrients and oxygen). The combination of both techniques can lead to organ-on-chips microdevices, which notably improve the level of cell differentiation and organization achieved with 3D models and constitute potential substitutes for animals in drug screening processes [221]. |
| Drug discovery |
| Nowadays, it is estimated that a drug candidate typically requires 15 years of discovery and preclinical evaluation, having only 8% of chances to reach the bedside [4,222]. A leading cause for this high failure rate is the use of models that miss or alter many tissue-related functions and, as a consequence, impair their predictive power. For this reason, 3D cultures are being introduced into drug screening as promising platforms to analyze the effect of drug action, improving the effectiveness and reducing the investment of this process. They are able to recreate in a more realistic way the complexity of human tissues, while retaining the ability for high-throughput screening and cellular level imaging. |
| The relevance of 3D cultures becomes evident in the assessment of a drug safety profile, in terms of its interaction with the liver [223]. This organ has the function of controlling the biotransformation and elimination of toxic waste substances from the body. Hepatocytes cultured in monolayers dedifferentiate after few passages and lose liver-specific functions, such as the expression of drug metabolizing enzymes [222,223]. On the other hand, liver toxicity is species specific; therefore the results obtained with animal models cannot be always directly translated to humans [222]. To avoid these inconsistencies, significant efforts have been made in “humanizing” mice by transplanting human cells. However, these animals are still challenging and expensive to adopt in an assay format [224]. As a consequence, 3D cultures have been proposed as alternative cellular systems predictors in drug screening processes, since it has been shown that hepatocytes regained their morphology and expression of key-liver proteins (urea, fibrinogen, albumin and drug-metabolizing enzymes) when cultured in alginate [222] and synthetic self-assembling peptides by the sandwich method [225]. |
| An unresolved matter for TE is the translation of 3D cultures from the academia to the pharmaceutical industry. To achieve this goal, 3D cultures should meet a set of requirements, apart from biological relevance: standardization, high throughput applicability and economic feasibility. Hence, major efforts are being made in this direction [226]. |
| Conclusions |
| TE is a promising approach to promote, guide and enhance the innate capacity of tissues to engage regeneration, assisting to recover function and shape where naturally would not. In fact, encouraging advances have been accomplished during the last two decades in several areas such as bone, cartilage, heart, pancreas and vasculature. Furthermore, TE is transforming the way we study human physiology and pathophysiology, having a deep impact on the development of new therapies. As 3D cultures can bridge the gap between 2D cultures and animal models, they emerge as a valuable tool for next generation biology and biomedical research. Further research is still needed in TE. Particularly, the selection of the material appears to be a major challenge. Concerted efforts should be directed to the optimization of synthetic scaffolds, since they can be custom-tailored depending on their specific applications. In addition to the classical issues to be faced in every engineering discipline, TE requires special efforts to be pointed on the needs of living organisms. Either when designing models for in vitro biological studies or grafts for in vivo regeneration, it should be taken into accounts the complexity of tissue architecture. For instance, the implementation into 3D cultures of vascularization networks that facilitate a constant turnover of oxygen and nutrients is under extensive studies. |
| References |
|
Tables and Figures at a glance
| Table 1 |
Figures at a glance
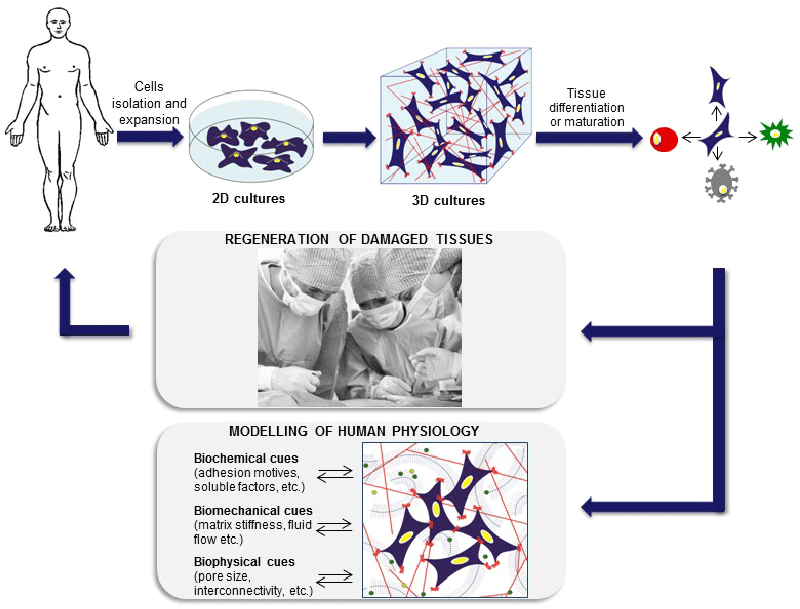 |
| Figure 1 |
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 58946
- [From(publication date):
specialissue-2013 - Nov 05, 2025] - Breakdown by view type
- HTML page views : 52968
- PDF downloads : 5978
