Research Article Open Access
Effect of Pesticide (Chlorpyrifos) on Soil Microbial Flora and Pesticide Degradation by Strains Isolated from Contaminated Soil
| Hindumathy CK* and Gayathri V | |
| Department of Biotechynology, Vinayaka Mission University, Salem, Tamil Nadu, India | |
| Corresponding Author : | Hindumathy CK Department of Biotechynology Vinayaka Mission University Salem-636308, Tamil Nadu, India Tel: +91 9790690164 E-mail: hindumathyck@rediffmail.com |
| Received: November 27, 2012; Accepted: January 23, 2013; Published: January 25, 2013 | |
| Citation: Hindumathy CK, Gayathri V (2013) Effect of Pesticide (Chlorpyrifos) on Soil Microbial Flora and Pesticide Degradation by Strains Isolated from Contaminated Soil. J Bioremed Biodeg 4:178. doi:10.4172/2155-6199.1000178 | |
| Copyright: © 2013 Hindumathy CK, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
In the present study, the effect of pesticide (Chlorpyrifos) on rhizospheric soil and non–rhizospheric soil of two plants marigold and Canna has been investigated. Further, microorganisms have been isolated from Rhizospheric and Non–Rhizospheric soil, characterized and their pesticide degradation ability was investigated. Most of bio process materials have been taken and analyzed for microbial composition. The efficiency of microbial consortium obtained from each of this bio process material for chlorpyrifos degradation has been studied. The result indicates that presence of glucose supports more biomass, which in turn brings about higher degradation and dissipation of pesticide. Maximum 84.5% dissipation was observed through bacterial isolate in presence of glucose as compared to 73.3% dissipation in absence of glucose. In case of fungal isolate 76% dissipation occurred in presence of glucose and only 58% was dissipated in absence of glucose. Both the isolates showed resistance to chlorpyrifos at 10 ppm concentration and also brought about significant dissipation of this pesticide. Therefore, these isolated could be potential candidates for microbe mediated bioremediation of chlorpyrifos contaminated soils.
| Keywords |
| Pesticide; Chlorpyrifos; Rhizospheric; Non– Rhizospheric; Degradation |
| Introduction |
| Bioremediation is an environmental clean–up technique that is currently being investigated for use on a wide variety of chemicals. It is the use of naturally occurring microorganisms to enhance biodegradation, or normal biological breakdown. It involves establishing the condition in contaminated environment so that appropriate microorganisms flourish and carry out the metabolic activities to detoxify the contaminants [1] and is also safe, viable remedy for the detoxification of environmentally hazardous chemicals [2]. There are three primary approaches to bioremediation; Biostimulation, Bioaugmentation {which may include genetically engineered microorganisms (Gem’s)} and Phytoremediation. Biodegradation of organic pollutants is a natural process whereby, bacteria and other organisms alter and breakdown organic molecules into substances, eventually producing carbon dioxide and water or methane. Although the ultimate aim of the biodegradation is to degrade the organic contaminants completely into harmless constituents such as the carbon dioxide and water, many intermediate metabolites can also be formed. What makes bioremediation so desirable is that it is a permanent solution; it destroys the contaminant, focuses on detoxification rather than waste translocation [3]. |
| In view of the above and the literature survey findings, present study is taken up with the following objective of biological dissipation of pesticide in the Chlorpyrifos contaminated soil and effect of pesticide on rhizosphere and non-rhizosphere soil micro flora in case of Marigold and Canna plants and pesticide degradation by isolate/ consortium obtained from contaminated soil. |
| Chlorpyrifos (0,0-diethyl-3,5,6-trichloro-2- pyridylphosphorothioate) is a insecticide/acaricide for treatments of crops, lawns, ornamental plants. It is a widely used insecticide and effective against a broad spectrum of insect pests of economically important crops. It is also used for the control of mosquitoes (larvae and adults), flies, various soil pests, many foliar crop pests and household pests. Additionally it is used for ectoparasite control of cattle and sheep. It persists in the soil for 60-120 days, and has very low solubility in water (2 mgl-1), but is readily soluble in most organic solvents. Chlorpyrifos undergo transformation in the soil by the abiotic hydrolysis and microbial degradation. A few studies have attempted to separate abiotic chemical hydrolysis from microbial processes and to determine their relative importance [4,5]. The half lives of in muck (48% Organic Matter [OM] and loam (2.7% OM) were determined in sterilized and natural soils at 3 temperatures (3, 15, and 28°C). The half lives of in muck (48% Organic Matter [OM] and loam (2.7% OM) were determined in sterilized and natural soils at 3 temperatures (3, 15, and 28°C). Degradation of the pesticide depends upon the type of the soil, soil property, moisture content of the soil and pH [6,7]. The bacterial systems were able to rapidly degrade fenamiphos and between 15 and 35°C. Singh et al. and Singh et al. [8,9] investigated the degradation behavior of three insecticides (bifenthrin, and imidacloprid) at termiticidal application rates under standard laboratory conditions [10,11]. |
| Materials and Methods |
| In the present study, the effect of pesticide on rhizospheric and non–rhizospheric Marigold and Canna has been investigated. Further, microorganisms have been isolated from rhizospheric and non–rhizospheric soil, characterized and their pesticide degradation ability was investigated. Most of bio process materials have been taken and analyzed for microbial composition. The efficiency of microbial consortium obtained from each of this bio process material for degradation has been studied. |
| Samples used |
| • Rhizospheric soil without pesticide-“Marigold” control, and “Canna” control. |
| • Rhizospheric soil with pesticide-“Marigold” with pesticide and “Canna” with pesticide. |
| • Non-Rhizospheric soil with pesticide–“Soil” with pesticide. |
| • Non-Rhizospheric soil without pesticide-“Soil only”. |
| • Bioprocess materials such as Heap manure, Biogas slurry, Vermicompost, and Mushroom spent were used as soil amendments. |
| Conventional method for the isolation of microflora in pesticide contaminated soil |
| Microorganism isolation and cultivation conditions: Microorganism which was employed here was isolated from the organophosphorus pesticides contaminated soils using an enrichment culture technique [12]. The enrichment medium (Czapek-Dox) containing (in gram per litre) 30 g of sucrose, 2 g of NaNO3, 0.5 g of KCl, 0.5 g of MgSO4, 1 g of K2HPO4, 0.01 g of Fe2(SO4)3, 0.5 g peptone and the Mineral Salt Medium (MSM) containing (in gram per litre) 2.0 g of (NH4)2SO4, 0.2 g of MgSO4·7H2O, 0.01 g of CaCl.2H2O, 0.001 g of FeSO4·7H2O, 1.5 g of Na2HPO4·12H2O, 1.5 g of KH2PO4 were used for the isolation of fungal strains. Enrichment and isolation of fungi were performed as described by Chen et al. [13]. In brief, two gram of soil sample was transferred into a 250 ml Erlenmeyer flask containing 50 ml MSM with the addition of 50 mg·L−1 chlorpyrifos as the sole carbon source and incubated at 28°C for 7 days in a rotary shaker at 150 rpm. 5 ml of the enrichment culture was transferred into 50 ml fresh enrichment medium and incubated for another 7 days. After five rounds of transfer, the final culture was serially diluted and spread on Czapek-Dox agar plates. The organism that could make use of chlorpyrifos as the sole carbon source was used for further study. |
| Characterization of bacteria and fungi: Study of motility by hanging drop method and gram staining were performed to characterize bacteria. |
| Pesticide degradation by isolate: The isolate was identified from the plates in which the soil sample treated with pesticide was plated. 100 ppm of the pesticide was added to the pre-sterilized 100 ml Erlenmeyer flasks in 1ml acetone. After evaporation of acetone in 24 hours, 45 ml of the mineral salt medium which contains MgSO4.7H2O (0.2 g); K2HPO4 (0.1g); CaSO4 (0.4g); FeSO4.7H2O (0.001g); distilled water, 1L; with pH 6.5 were placed in 100 ml Erlenmeyer flasks and the flasks were shaken at 26 ± 1 in 200 rpm on a orbital shaker (Syngenics Model). Medium not inoculated served as control. One flask with additional glucose (1000 mg/l) was added. Both the inoculated and non- inoculated samples were incubated under intermittent shaking to aerobic conditions. At periodic intervals, the samples were withdrawn aseptically from flasks and analyzed for by Gas Liquid Chromatography (GLC) after the extraction in hexane [13,14]. The growth of isolates was monitored by protein estimation. |
| Pesticide Degradation by Different Bioprocess Materials: Degradation was studied in the mineral salt medium inoculated with suspension of various bioprocess materials such as heap manure, vermicompost, mushroom spent, biogas slurry, soil with pesticide, and the concentration of 100 ppm was added to 50 ml sterilized Erlenmeyer flasks in 1 ml acetone. After evaporation of acetone, 45 ml of sterile mineral salt medium was added [15] dispensed into each flask. However to one set of flasks glucose (1000 mg/l) was supplemented as additional carbon source. The mineral medium supplemented was equilibrated on a rotary shaker for 24 hours to allow the solubilization of pesticides. Both sets of mineral salt medium, with or without glucose were inoculated with bioprocess materials suspension (prepared by suspending 10 gm in 90 ml distilled water). Uninoculated mineral salt medium served as control. Flasks were incubated at 27 ± 1°C on an orbital shaker. At regular intervals of 0, 5 and 10 days, about 5 ml aliquots were aseptically removed from all the experimental flasks. |
| Microbial biomass and pesticide residue analysis |
| Analysis of protein content of Biomass: Microbial biomasses in the various bioprocess materials flasks and from the isolate flasks with and without glucose were quantified at regular intervals (0, 5, and 10 days) by Bradford’s method for the estimation of total protein. 10 ml of the culture was taken and centrifuged at 12,000 rpm for 12 minutes and the biomass pellet was taken and 2-3ml of 0.1 N NaOH was added in it. Kept in boiling water bath for 15 minutes, cooled it, and centrifuged to remove cell debris and ml supernatant was taken and to this 4.5 ml Bradford’s reagent was added and Incubated at room temperature for 2-4 minute. Absorbance was noted at 595 nm and protein content (μg/ml) was calculated with the help of standard curve. |
| Extraction Procedure for Pesticide Residue Analysis: The Mineral Salt Medium (inoculated and non-inoculated) were extracted by shaking portions (1-2 ml) of the culture in flasks with 1-5 ml of hexane and 3 g of sodium sulphate for 5 minutes and it was evaporated using rotary vacuum evaporator (model-Heidolph) for extraction of residues from samples, the extracted pesticide residue was analyzed by Gas Chromatography (GC). |
| Methodology: 1 g of the soil sample was dissolved in 10 ml of distilled water. The bottle was shaken vigorously to suspend all the microbes in water. It is allowed to for a while to enable settling of coarse particles at the bottom, while microbes appear in Brownian motion in the suspension. 1 ml of the sample was taken from the suspension with the help of 5 ml capacity syringe. The sample was added in a sealed vial, mixed gently. The vials were kept for observation, and recorded the colour faded time and matched it with the microbial population chart. |
| Results and Discussion |
| Effect of on non-rhizospheric soil |
| In the non-contaminated soil bacterial population was higher than fungal population. In contrast, in pesticide contaminated soil the bacterial populations were greatly suppressed and fungal population raised and became dominant. These results show that fungal strains were more resistant and possibly had the capacity to degrade into harmless metabolites [16,17]. On the other hand bacterial population, in general is not able to survive and multiply well in presence of pesticide. It has been reported that one of the primary metabolites of (3,5,6-trichloro-2-pyridinol) possesses antibacterial properties [18]. Significant decline in bacterial populations observed in the present study could be attributed to generation of such antibacterial metabolites [19,20]. |
| Effect on bacterial population in rhizospheric soils of marigold and canna |
| Rhizospheric soil of Marigold and Canna were compared. The roots of both the plants were well grown and proliferated. The rhizospheric soils of both plants were aseptically withdrawn and the bacterial colonies were quantified. In the absence of pesticide, Canna rhizospheric soil (3rd week sample) had significantly higher bacterial population as compared to the marigold rhizosphere. However, in presence of, the bacterial population in Canna rhizosphere crashed while there was no substantial impact or lowering of bacterial population in marigold rhizospheric soil (Tables 1 and 2). |
| Change in bacterial load of -contaminated rhizospheric soils of marigold and canna |
| The observations were recorded after 3rd week of plantation in contaminated soil. Repeated observations were made after 14th week of the experiment. The bacterial population in Canna rhizosphere was greater than Marigold rhizosphere both in the 3rd week as well as 14th week. In general an increase in bacterial colonies was observed in 14th week sample as compared to the 3rd week sample. This was more significant in pesticide contaminated rhizospheric soil. In case of Canna rhizospheric soil, the viable bacterial population almost doubled in the 14thweek sample as compared to the 3rd week sample. Similarly enhancement in rhizospheric bacterial population was observed in case of Marigold. These results (Table 3) indicate that in the 3rd week of plantation in pesticide contaminated soil, the proliferation of the bacterial population is inhibited either due to the presence of pesticide itself or its toxic metabolites [21]. However, with time the pesticide residues might get dissipated due to combined impact of abiotic process as well phytoremediation effect [22]. Therefore, significantly higher bacterial population has been observed in the 14th week sample [23,24]. |
| Effect of fungal population in rhizospheric soils of marigold and canna |
| The fungal population in rhizospheric soil of both Marigold and Canna was higher than the non-rhizospheric soil. The degradation was declined (by almost 50%) in fungal population was observed in the rhizospheric soils of both Canna and Marigold (Table 3). |
| Characterisation of microflora |
| The colonies from Canna rhizospheric soil with pesticide were taken and characterized. The bacteria by hanging drop method, flagella staining, gram staining and fungi by staining with lactophenol cotton blue. |
| Bacteria |
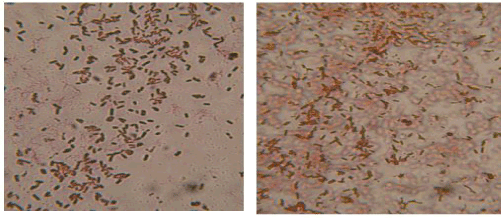
| Most of the bacterial isolates appeared to be non-motile (few are motile), rod shaped, gram-negative bacteria (Figure 1). |
| Fungi |
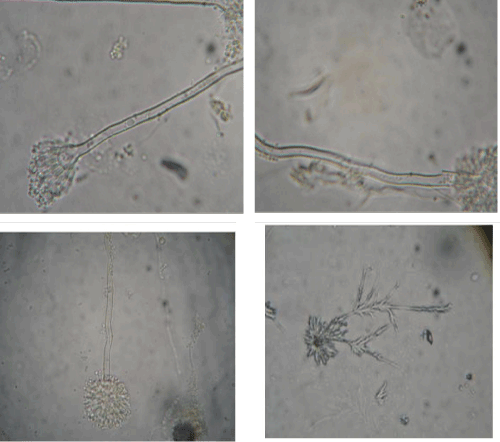
| Most of the fungal groups displayed aseptate hyphae and resembled Aspergillus sps. (Figure 2). |
| Pesticide degradation by isolate/coinsortium |
| The inoculated samples from the mineral salt medium were withdrawn periodically (0, 5 and 10th day) in aseptic condition and analyzed for total protein and pesticide residue analysis. |
| Protein estimation |
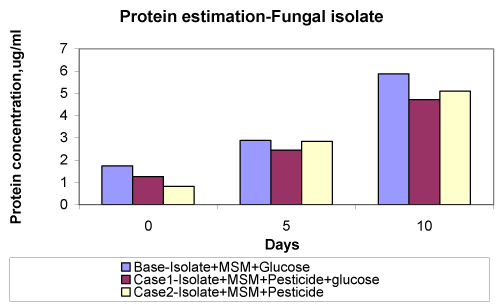
| The fungal isolate used in this study was isolated from Canna plate and the phase contrast micrograph of the same is shown in figure 1. In general the result implies that in all the case fungi growth increases slowly from 0 day and maximum growth is observed in 10th day, but the magnitude of growth is different in each cases. No significant inhibition of growth is observed in the presence of pesticide. In case when no glucose was added, the fungal isolate were able to survive even in the absence of any carbon source. These results indicate that the isolate was able to utilize the energy source and hence there is a continuous growth period during 0 to 10th days (Figure 3). |
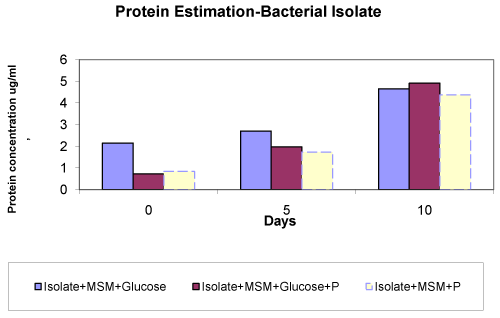
| The bacterial isolate used in this study was isolated from Canna rhizospheric soil with pesticide and found to be rod shaped gram negative bacteria. It was reported that the bacterial isolate showed maximum growth in the presence of glucose. However, in the absence of carbon source also the bacterial isolate was able to survive and showed the continuous growth during 0 day-10th day. As compared to the fungal isolate, the bacterial isolate showed quite less growth in terms of the protein concentration (Figure 4). Nevertheless, results showed that the bacterial isolate was also able to utilize the energy source and carbon source [25]. Several investigations [26,27] carried out earlier also reported the requirement of electron donating co-substrates such as glucose and yeast extract for the reduction of azo bonds by bacteria [28], Moosvi et al. [25] have also reported the importance of carbohydrate and nitrogen source in the form of glucose and yeast extract respectively for the maximum degradation of Reactive Violet 5 by bacterial consortium RVM11.1. |
| The results of pesticide dissipation by the isolates are shown in table 1. After 5th day maximum pesticide dissipation occurred in when both glucose and pesticide were present in the case of bacterial as well as fungal isolate [21,22]. This result indicates that presence of glucose supports more biomass, which in turn brings about higher degradation and dissipation of pesticide. Maximum 84.5% dissipation was observed through bacterial isolate in presence of glucose as compared to 73.3% dissipation in absence of glucose. In case of fungal isolate 76% dissipation occurred in presence of glucose and only 58% was dissipated in absence of glucose. Both the isolates showed resistance to at 10 ppm concentration and also brought about significant dissipation of this pesticide. Therefore, these isolated could be potential candidates for microbe mediated bioremediation of contaminated soils. |
| Pesticide degradation by bioprocess materials |
| Microbial Enumeration of Bioprocess Materials: Bioprocess materials such as vermicomposting, heap manure, mushroom span, and biogas slurry act as a biostimulant as well as source of microflora [20]. For this enumeration biomanures diagnostic kit was used and this microbial kit helps to determine the quality of biomanures in terms of microbial count. Hence, high microbial population is desirable for quality manure. |
| Pesticide degradtion by consortium from bioprocess materials |
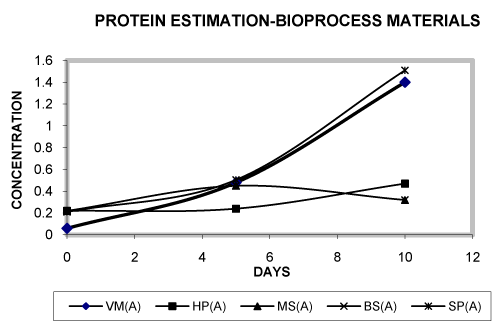
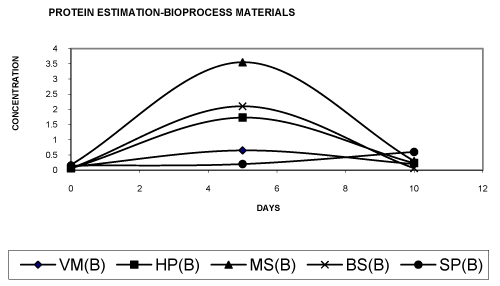
| • The total protein has been estimated in case of bioprocess materials by Bradford’s method. Our results shows that in the different bioprocess materials vermicomposting biogas slurry and soil pesticide has shown an increased growth curve [29]. From the figure 5 it has been concluded that growth of biogas slurry is more than the other bioprocess materials from the period of 0 day to 10th day, in case of vermicomposting and soil pesticide the growth is slightly decreased while compared with the biogas slurry. So it has been concluded that: BS>VM>SP>HP>MS. The consortiums present in the materials are able to survive in the presence of pesticide as a energy source [20,21]. Further, many researchers have mentioned that a higher degree of biodegradation and mineralization can be expected when co-metabolic activities within a microbial community complement each other [26-29]. |
| • From the figure 6 it has been concluded that in the presence of glucose the consortium present in the materials attains maximum growth on the 5th day which gets subsequently declined in 10th day. As compared to the case in absence of glucose, much higher protein concentrations were recorded in presence of glucose. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
 |
 |
 |
| Figure 4 | Figure 5 | Figure 6 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 21422
- [From(publication date):
February-2013 - Sep 25, 2024] - Breakdown by view type
- HTML page views : 16262
- PDF downloads : 5160
