Make the best use of Scientific Research and information from our 700+ peer reviewed, Open Access Journals that operates with the help of 50,000+ Editorial Board Members and esteemed reviewers and 1000+ Scientific associations in Medical, Clinical, Pharmaceutical, Engineering, Technology and Management Fields.
Meet Inspiring Speakers and Experts at our 3000+ Global Conferenceseries Events with over 600+ Conferences, 1200+ Symposiums and 1200+ Workshops on Medical, Pharma, Engineering, Science, Technology and Business
Editorial Open Access
Bioavailability in Biodegradation and Function of Herbicides
| Gerald K Sims* | |
| Department of Entomology Plant Pathology and Weed Science, New Mexico State University, Las Cruces, New Mexico 88011, USA | |
| Corresponding Author : | Gerald K Sims Department of Entomology Plant Pathology and Weed Science New Mexico State University, New Mexico, USA Tel: 575 646-3154 Fax: 575 646-8087 E-mail: gksims@nmsu.edu |
| Received February 25, 2014; Accepted February 26, 2014; Published March 03, 2014 | |
| Citation: Sims GK (2014) Bioavailability in Biodegradation and Function of Herbicides. J Bioremed Biodeg 5:e144. doi:10.4172/2155-6199.1000e144 | |
| Copyright: © 2014 Sims GK. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
| Soil retentive processes and biodegradation kinetics are key parameters in predicting offsite transport of herbicides [1,2]. Sorptive and physical entrapment processes interact with highly compartmentalized tortuous pore space to impose limitations on the availability of herbicides to the plants they are designed to kill, as well as the microorganisms required for biodegradation [3,4]. Since degree of water saturation determines the cross sectional area for diffusion of solutes, water content profoundly affects herbicide bioavailability. Product labels for soil-active herbicides often prescribe application rates based on soil texture or organic matter content to deliver sufficient product to overcome plant bioavailability limitations caused by sorption [5]. In fact, the concentration of herbicide in solution can be a good predictor for both herbicide activity and degradation kinetics [6]. For these reasons, variation in soil properties over a field scale has been used for precision herbicide applications [7]. Since limited bioavailability may protect herbicides from biodegradation, some products can be detected long after use [8] or may be re-released within the same growing season when conditions change, typically due to rainfall after a drought [9]. Most herbicides exhibit sorption-desorption hysteresis and in unsaturated soils, the non-equilibrium “apparent” sorption coefficient tends to increase with time as slow desorption kinetics fail to replenish material lost from solution by degradation or leaching [10]. This slows down biodegradation as residues “age” in the soil matrix [11]. The ubiquity of bioavailability-driven persistence has led to the development of various treatments, such as the addition of surfactants to release sorbed materials or conversely promoting persistence by enhancing binding of herbicides to soil organic matter [12]. However, while chemical and physical interactions with soil may be intrinsic properties of a herbicide, the resulting effects on biodegradation rates are a function of microbial population sizes, and thus may be subject to change. |
| A particularly revealing case study in the dynamic nature of physicochemical constraints on adaptive biological systems is the subtle role of bioavailability in the persistence of the herbicide, atrazine. This product was introduced in 1959 and quickly became the most widely used herbicide in the world, holding that title until displaced in recent years by the use of glyphosate on GMO crops [13]. Atrazine is inherently biodegradable, and numerous bacteria able to grow rapidly on the herbicide as a C or N source have been identified [14]. Though only moderately retained by soil sorption, atrazine is generally protected from degradation by bioavailability limitation, owing to the limited driving force for diffusion produced by small populations of atrazine degraders typical of most soils [15]. In recent years, numerous sites have been reported to exhibit reduced atrazine phytotoxicity and concomitantly more rapid atrazine degradation, typically after many years of atrazine use [16]. Populations in these soils are only slightly amplified, however, though there appears to be a shift in the representation of atrazine degradation genes in favor of those found in gram positive bacteria [17]. The tendency of gram positives to sustain more stable populations in soil may explain the recent increases in degradation rates. Alternatively, it has been shown that the subtle increases in degrader populations are just enough to overcome the limitations on atrazine degradation rate imposed by tortuous diffusion [18,19]. In this case, the bioavailability to weeds is unchanged, whereas bioavailability to degraders has increased (increased population constitutes a greater sink), depleting the residual concentration of herbicide and reducing weed control. |
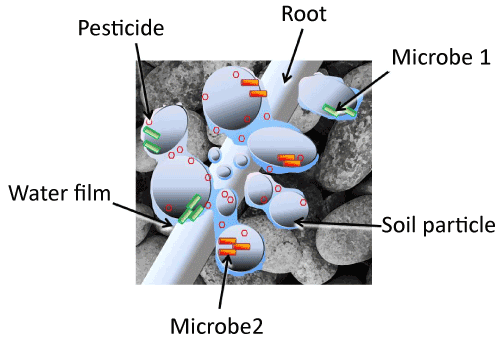
| Figure 1 depicts a soil ecosystem at the scale of the pore environment inhabited by soil microorganisms. Soil solids and water films are shown inhabited by two pesticide degraders (depicted in green and orange). Also present is a pesticide represented by red hexagons, depicted in both the water film and adsorbed to solid surfaces to demonstrate potential to replenish the solution as it is depleted by degradation. A root hair is included for scale. The soil solids are organized into clusters surrounded by continuous water films, showing that in unsaturated conditions, part of the pore space allows free diffusion while diffusion between other compartments is constrained by too-thin water films. Also evident is the possibility that water pools containing pesticide can be isolated from degraders, or degraders may be present in pools devoid of pesticide. It is also clear that some degraders may lack competition for the pesticide within the water film they occupy, whereas other water films may overlap different degrader populations, resulting in direct competition for resources. This simple diagram obviates the complexity of relationships among microorganisms, weeds, the soil matrix, and herbicides introduced to control weeds. Compartmentalization protects pesticides from degradation as well as degraders from competition, and limits exposure of weed roots to pesticides in solution. Changes in water levels will have obvious and profound effects on bioavailability and changes in microbial populations will change the driving force for diffusion through thin water films. |
| As we broaden our understanding of compartmentalization within the soil environment, some of the peculiarities observed in environmental behavior of herbicides become easier to predict or interpret. Lacking, however in the literature are studies in which tools of microbial ecology, such as stable isotope probing [9,14] have been deployed on scales relevant to address bioavailability questions. As these tools become more affordable and increasingly sensitive, it will be possible to gain a clearer understanding of herbicide biodegradation and function, and why these processes may change as use patterns shift and microbial populations adapt to substrates. |
References
|
Figures at a glance
 |
| Figure 1 |
Post your comment
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 13652
- [From(publication date):
February-2014 - Sep 25, 2024] - Breakdown by view type
- HTML page views : 9217
- PDF downloads : 4435
