Hydro-geochemical and Geophysical Study of Groundwater in the Suburb of Osogbo, South Western Nigeria
Received: 13-May-2014 / Accepted Date: 18-Jun-2017 / Published Date: 28-Jun-2014 DOI: 10.4172/2157-7617.1000205
Abstract
In an attempt to carry out the assessment of groundwater quality from hand dug wells and streams in Onibu-Eja and Aduramigba communities, suburb of Oshogbo, South Western Nigeria, with a view to determine the impact of a nearby dumpsite on the groundwater quality, twenty (20) water samples were taken randomly at varying depths within the vicinity. The cation and heavy metal analyses were done in accordance to APHA standard using the Atomic Absorption Spectrophotometry (AAS) while the anion analyses were carried out using the ion chromatography and titrimetric methods. Physical parameters were measured on the field. The results observed showed that the water in the study area contains a relatively high amount of calcium, magnesium, and bicarbonate, an indication of temporary hardness. The hydro-geochemical contour maps of the ions shows that cations, anions and heavy metal content where mostly concentrated in the western and eastern parts of the study area. The analyses of the water samples and streams in the study area fall within the acceptable range of World Health Organization (WHO) and the Nigerian Standard of potable drinking water with the exception of the stream despite their proximity to the dumpsite. Vertical Electrical Sounding (VES) carried out on the dumpsite showed that the study area is overlain by a 0.3 to 1.1 m thick lateritic topsoil with resistivity values between 104 ohm m and 437 ohm m which serves as a hard pan over a 7 to 13 m thick predominantly clayey / weathered layer interval with a resistivity value between 35 to 80 ohms meter. Both of these prevent the leachate from percolating into the groundwater in the study area.
Keywords: Groundwater, Dumpsite, Leachate, Vertical electrical sounding, Physico-chemical, Cation
8042Introduction
Groundwater generally experience quite a number of chemical and impact processes as it moves from one region to another below the subsurface. Most of these processes are as a result of geogenic and/or anthropogenic causes. These processes include chemical composition of the recharge water, interaction with gases within the unsaturated zone, solid material located between land surface and the water table, chemical reaction between water and solid material within the aquifer or saturated zone and interaction with water from human activities. The ultimate results of all these processes lead to alteration in the chemical composition, colour, taste and odour of groundwater; hence posing adverse physiological effect on the entire users. Increasing population and anthropogenic impacts warrants hydro-geochemical quality assessment. Dumpsites and landfills have been identified as some of the major treats to water resources around the vicinity [1]. The practice of landfill system as a method of waste disposal in many developing countries is usually far from standard recommendations in most cases [2,3].
The issue of water quality is of great importance not only for man's immediate consumption but also for industrial use. Several workers have discussed extensively about it. Appelo and Postma [4] observed that chemical interactions between the solution and the sediment do change the dispersivity in the course of the reaction and this may have profound effect on water chemistry. Such interactions may comprise dissolution of minerals present in the sediment or the precipitation of new minerals. Also, Krishan et al. [5] who worked on the groundwater quality in the Bist-Doab catchment, Punjab, India observed that chemical properties of groundwater are usually altered by processes taking place in the recharge zone as well as by the geochemical processes that occur during subsurface flow. Mineral enrichment from underline rocks is believed to have a greater impact on the chemistry of groundwater thus making it unsuitable for human and livestock consumption [6]. Others include the works of Drever [7] and Saleh et al. [8].
Groundwater is generally believed to be relatively protected compared to other sources of water because it is held within the pore spaces, fractures and weathered regolith depending on the geologic setting which makes it generally free from sediments. As a result, groundwater becomes the principal source of water to rural communities in Nigeria, especially areas underlined by basement rocks [9].
This project is centered on hydro-geochemical and geophysical studies at Onibu-Eja and Aduramigba communities; a suburb of Oshogbo and it is of interest due to the fact that there are quite a number of residential and small-scale industries springing up newly in the vicinity. These small scale industries and the individuals living in the vicinity depend more on the resource for their activities. The objective is to understudy the viability of the water in this area to consumption by the inhabitants.
Location and geology of the study area
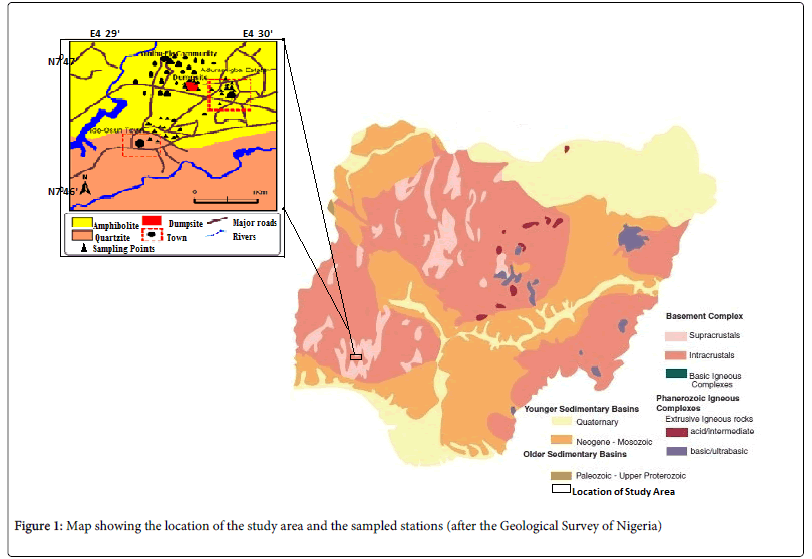
The study area is part of the South Western Nigeria, and lies between longitudes 07o47′03″N and 07o 47′50″N and latitudes 07o47 03 N and 07o47′03″ N. Like other parts of the South Western Nigeria, Onibu-Eja and Aduramigba lies within the tropical rain forest and is marked with two prominent seasons - the wet season and the dry season, experienced between April and October and between November to March respectively (Figure 1).
Geologically, the study area belongs to the migmatite-gneiss-quartzite zone of the basement complex of South Western Nigeria and lies within the zone of Pan African reactivation of 600 ± 150 m.y. [10]. The major rock groups in the study area are amphibolites schist, quartzite and pegmatite that occur within the Iwo schist belt. These rock types have weathered to form the soils that cover Onibu-Eja and Aduramigba community. Surficial materials are characterized by relatively deeply weathered soil profile in the low lying areas, due to the humid climatic conditions. Greater proportions of the top soils are lateritic soils. Usually lateritic soils are formed as a result of the weathering of parent sedimentary rocks (limestone), metamorphic rocks (schists, gneisses, migmatites); igneous rocks (granites, basalts, gabbros, peridotites) [11]. In this case, the lateritic soils are formed as a result of the weathering of metamorphic rocks (schists, gneisses, migmatites) leaving the more insoluble ions, which are predominantly iron and aluminum. Hence, lateritic soils are rich in aluminum and iron and it is commonly found associated with tropical environment especially in the basement complex terrains of Nigeria.
Materials And Methods
Water samples were collected randomly from twenty (20) wells at varying depths between March and April, 2012. This was a period when the raining season was just starting. Some water samples were close to the dumpsite while others were far away from it. The in situ physical parameters such as Temperature, Colour, Taste, Conductivity, and Total Dissolved Solid (TDS) were measured using the Ec/pH Multi parameter instrument. Water samples taken for Cation analyses were preserved using drops of nitric acid to prevent homogenization. They were taken to the laboratory to estimate the heavy metal and cation content using Atomic Absorption Spectrophotometer (AAS). Anion analysis was done using Iron Chromatographic method and titrimetric method was used for SO42- and HCO3-. All tests were done in accordance to the prescription of APHA 2005. Four anion, Nitrate (NO3-), Bicarbonate (HCO3-), Chloride (Cl-) and Sulphate (SO42-) were analyzed for while the thirteen metals include Calcium, Magnesium, Potassium and Sodium, together with heavy metals: Manganese, Iron, Copper, Zinc, Cobalt, Chromium, Cadmium, Lead and Nickel. The results obtained were compared with the World Health Organization (WHO) standard and Nigeria Drinking Water Standard (NDWS) in order to determine its quality in line with these standards. Also, hydro-geochemical contour maps were generated in order to study the regional distribution of the Physico-chemical parameters.
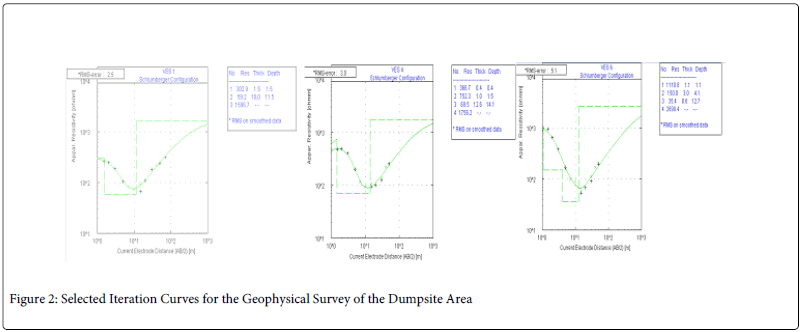
Also, six vertical electrical soundings were taken in all; some at locations within the dumpsite and others far from it to serve as control. The results of the water quality analysis are linked with the interpretation of the vertical electrical soundings carried out in order to study the influence of the dumpsite on the groundwater in the study area. An ABEM SAS 300c tetrameter was used for the resistivity measurements using the popular Schlumberger electrode configuration. The field resistivity data was interpreted using the 2-dimension inversion programs; Win Resist software. Figure 2 shows selected iterative curves obtained out of the six electrical soundings carried out while the geo-electric section is shown in Figure 3.
Figure 2: Selected Iteration Curves for the Geophysical Survey of the Dumpsite Area
Results And Discussion
Physical parameters
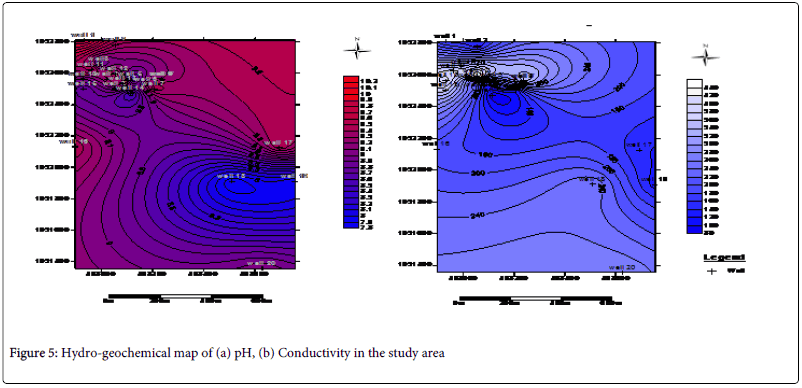
The physical parameters of concern are temperature, pH, TDS and conductivity. The pH value ranges from as low as 7.8 in well 19 to as high as 10.2 in well 1 with average value of 9.09. This falls within acceptable standard of the World Health Organization (WHO) and Nigeria drinking water. The TDS measurements vary from 61.5 to 308.25 with average values of 172.31 while the conductivity measurements vary from 82.00 μs/cm to 459.00 μs/cm with average value of 249.95 μs/cm (Table 1). The results obtained from these analyses are in conformity with the works of Tijani et al. [12,13] that indicated that the TDS values of water samples within Oshogbo and its environs are generally low and fall within the acceptable WHO Standardized values and Nigeria Standardized values for drinking water (Table 2).
| Sample No | Elev. (m) | Depth to Water (m) | Depth to Bottom (m) | pH | Temp (°C) | EC (μs/cm) | TDS | Comment on well/stream |
|---|---|---|---|---|---|---|---|---|
| 1 | 315 | 5.7 | 6.6 | 10.2 | 28.1 | 155 | 116.25 | Colorless and odorless. Well is also covered |
| 2 | 312 | 4.1 | 5.3 | 9.4 | 27.1 | 147 | 110.25 | Covered with steel plate and the water slightly turbid. |
| 3 | 320 | 5.5 | 6.2 | 8.8 | 26.2 | 335 | 251.25 | Opened and unlined well with dirt stored in it. Slightly turbid with choking smell |
| 4 | 317 | 4.5 | 6.1 | 9.3 | 25.6 | 256 | 192 | Plant stem dropped in it colorless and odourless. |
| 5 | 317 | 7 | 8.1 | 8.5 | 26.5 | 249 | 186.75 | Lined, neat with steel plate cover. Odourless and colorless. Treated with chlorine, salt NaCl and aluminum salt. |
| 6 | 305 | 2 | 4.8 | 9.3 | 26 | 221 | 165.75 | Soda and HTH are added to the water every 3 months, close to the stream. |
| 7 | 306 | 0.5 | 1.2 | 9.5 | 22.7 | 126 | 94.5 | Stream flowing through a swampy side |
| 8 | 310 | 3.6 | 6.6 | 9.1 | 24.9 | 226 | 169.5 | Colourless and odourless. |
| 9a | 310 | 1 | 4.4 | 9.2 | 24.2 | 357 | 267.75 | Not frequently Fetched |
| 9b | 310 | 0.6 | 4.0 | 9.5 | 23.1 | 162 | 121.5 | Slightly turbid. |
| 10 | 271 | 6 | 10.5 | 9 | 26.1 | 411 | 308.25 | Colourless and odourless water, lined and covered with steel plate |
| 11 | 319 | 4 | 8.6 | 9.5 | 27.1 | 389 | 291.75 | Colourless and odourless |
| 12 | 314 | 2.6 | 6.1 | 9.5 | 27.1 | 459 | 344.25 | Lined and covered with steel plate in a relatively neat environment |
| 13 | 318 | 3.3 | 3.6 | 9.5 | 25.3 | 117 | 87.75 | Newly dug well that is not lined and not covered |
| 14 | 308 | 2.7 | 3.4 | 8.4 | 26.4 | 82 | 61.5 | Covered with cement but not lined and relatively neat |
| 15 | 319 | 5.2 | 7.3 | 8.7 | 26 | 366 | 274.5 | Newly dug, lined and not covered |
| 16 | 321 | 4.3 | 6.0 | 9.5 | 27.4 | 187 | 140.25 | Constantly in use in a block making industry |
| 17 | 315 | 1.2 | 6.3 | 9.5 | 23.5 | 144 | 108 | Stream flowing NE-SW: highly turbid and disturbed |
| 18 | 324 | 5.4 | 6.3 | 7.8 | 27.1 | 259 | 194.25 | Lined and covered with steel plate. Colourless and odourless water. Newly dugged. |
| 19 | 317 | 7.3 | 8.3 | 7.9 | 27.6 | 127 | 95.25 | Lined and covered with steel cover. Actively in use. Colourless and odourless. |
| 20 | 326 | 7.5 | 14.6 | 9.1 | 27.4 | 279 | 209.25 | Lined and covered with steel plate. Colourless and odourless water |
Elev. = Elevation (m), Temp. = temperature (oc), TDS = Total Dissolved Solute, EC = Electrical Conductivity
Table 1: Showing the Physical Parameters and Location of Each Well
| Cations | Heavy Metals | Anions | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Well | mg/l Ca | mg/l Mg | mg/l K | mg/l Na | mg/l Mn | mg/l Fe | mg/l Cu | mg/l Zn | mg/l Co | mg/l Cr | mg/l Cd | mg/l Pb | mg/l Ni | NO3- | HCO3- | Cl- | SO4- |
| well 1 | 20.11 | 3.59 | 2.67 | 4.12 | 0.04 | 0.23 | 0 | 0.041 | 0 | 0 | 0 | 0 | 0 | 0.463 | 61 | 259.2 | 1.235 |
| well 2 | 15.62 | 6.6 | 1.59 | 5.04 | 0.05 | 0.41 | 0 | 0.01 | 0 | 0 | 0 | 0 | 0 | 0.57 | 61 | 86.4 | 0.493 |
| well3 | 6.18 | 27.7 | 1.73 | 2.89 | 0.13 | 2.95 | 0 | 0.003 | 0 | 0 | 0 | 0 | 0 | 0.397 | 45.75 | 100.8 | 0.866 |
| well 4 | 25.45 | 16.95 | 0.81 | 2.56 | 0.6 | 0.11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.587 | 61 | 136 | 0.493 |
| well 5 | 17.94 | 12.35 | 0.87 | 1.33 | 0.02 | 0.2 | 0 | 0.054 | 0 | 0 | 0 | 0 | 0 | 0.676 | 61 | 115.2 | 1.359 |
| well 6 | 19.51 | 7.83 | 1.17 | 9.97 | 0 | 0.21 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.57 | 30 | 86.4 | 2.224 |
| well 7 | 6.97 | 4.34 | 0.92 | 7.71 | 0.3 | 2.4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.748 | 47.75 | 194.4 | 1855 |
| well 8 | 38.22 | 3.21 | 2.87 | 4.32 | 0 | 0.08 | 0 | 0.027 | 0 | 0 | 0 | 0 | 0 | 0.392 | 30 | 93.6 | 11.119 |
| well 9 | 46.51 | 12.5 | 3.69 | 20.12 | 0 | 0.05 | 0 | 0.01 | 0 | 0 | 0 | 0 | 0 | 1.139 | 45.75 | 93.6 | 2.717 |
| well 9' | 1.032 | 30 | 100.8 | 1.235 | |||||||||||||
| well 10 | 39.33 | 30.81 | 5.75 | 5.54 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.356 | 45.75 | 136 | 0.743 |
| well 11 | 38.74 | 26.74 | 4.07 | 6.35 | 0 | 0.08 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.175 | 30 | 100 | 1.112 |
| well 12 | 46.9 | 32.85 | 3.75 | 4.11 | 0 | 0.12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.961 | 30 | 115.2 | 0.37 |
| well 13 | 2.53 | 10.52 | 1.56 | 6.12 | 0.05 | 0.29 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.748 | 61 | 144 | N.D |
| well 14 | 1.49 | 3.02 | 0.97 | 8.07 | 0 | 0.13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.961 | 61 | 208 | 0.246 |
| well 15 | 33.01 | 19.05 | 5.46 | 14.72 | 0 | 0.08 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.068 | 61 | 122 | N.D |
| well 16 | 22.05 | 3.72 | 4.28 | 8.12 | 0.04 | 0.12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.246 | 91.5 | 100.8 | 0.989 |
| well 17 | 4.58 | 6.23 | 4.26 | 9.56 | 0.44 | 2.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.424 | 91.5 | 144 | 1.482 |
| well 18 | 25.48 | 6.6 | 7.54 | 9.44 | 0.02 | 0.07 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.246 | 30 | 331.2 | N.D |
| well 19 | 17.65 | 2.95 | 8.56 | 3.21 | 0.09 | 0.14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.691 | 30 | 223.2 | 0.37 |
| well 20 | 43.65 | 4.3 | 6.31 | 6.11 | 0.05 | 0.21 | 0 | 0.037 | 0 | 0 | 0 | 0 | 0 | 0.691 | 30 | 158.4 | N.D |
| WHO-2004 | 75 | 50 | NIL | NIL | 0.05 | 0.3-1.0 | 1-1500 | 5-1500 | 0.05 | 0.5 | 0.05 | 0.1 | 0.2 | 50-100 | 30-400 | 200-600 | 200-400 |
| NDWS | NS | 0.2 | NS | 200 | 0.2 | 0.3 | 1 | 3 | NS | 0.5 | 0.003 | 0.01 | 0.02 | 50 | NS | NS | 100 |
Table 2: Showing the Results of the Minor and Major Element Composition
Anions and cations
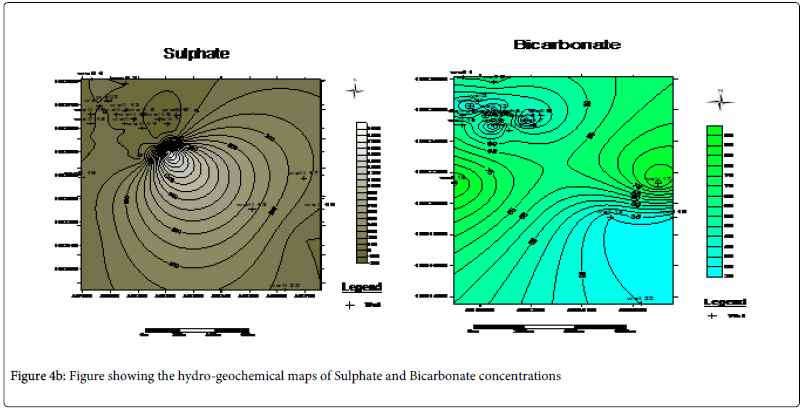
The anions analyzed include; chloride ion (Cl-), sulphate ion (SO42-), nitrate ion (NO3-) and the bicarbonate ion (HCO3-). The chloride ion value range from as low as 86.40 mg/L to as high as 331.20 mg/L with average value of 145.20 mg/L. SO42- ion across the wells reveals values ranging from 0.246 mg/L to 11.119 mg/L with average value of 1.70 mg/L. NO3- ion varies from 0.392 mg/L to 1.424 mg/L with average values of 0.816 mg/L and the HCO3- ion vary from 30.00 mg/L to 91.50 mg/L with average value of 49.28 mg/L. See Table 2.
Ca2+ on concentration range from 46.9 mg/L in well 12 and 1.4 mg/L in well 14 whereas Mg2+ concentration increased to 32.85 mg/L in well 12. Na+ and K+ ions seem not to be too high.
However, analysis of the anions show that Cl->HCO3->SO42-> NO3- decrease in that order while cations analysis shows Ca2+>Mg2+>Na+>K+ decrease in that order.
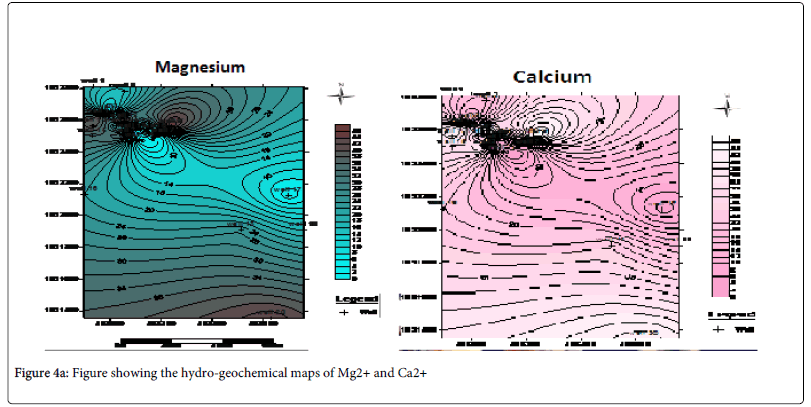
Hydro-geochemical map of the cations which reveals that Ca2+ , Mg2+ and HCO3- possessing the highest regional distribution and concentrated at the North western part of the study area is an indication of hardness due to the presence of the magnesium bicarbonate in these area (Figures 4a and 4b).
Heavy Metals
The heavy metals analysis for in the twenty (20) wells in the field showed that heavy metals values fall within accepted World Health Organization (WHO) Standardized values. Analysis showed that most of the analyzed water samples had little or no heavy metal content except for Fe, Mn and Zn which have slightly increased values (Table 2). These observed values are not due to the presence of pollution within the groundwater but rather due to the presence of the lateritic topsoil that dominate the study area. Lateritic materials according to Tardy (1997)[11] are usually rich in Iron, Aluminum and little Zinc in association with Manganese.
Geophysical investigation
The result of the water analysis showed that most of the Physico-chemical parameters under investigation fall within accepted WHO and NSWD, despite the proximity of the wells to a dumpsite. Six (6) vertical electric soundings measurements were carried out in the vicinity with the purpose of understanding the lithologic and geo-electric composition of the entire study area. The iteration of the data collected is as shown below in Figure 3.
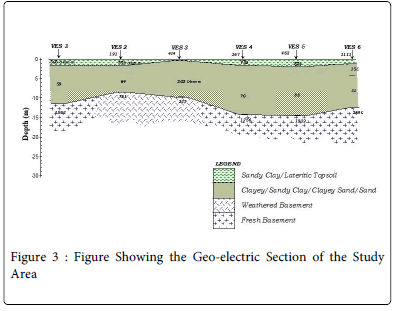
The geo-electric section of the entire study area showed that the area is overlain by resistive lateritic topsoil with values of between 306Ω m in VES 1 to 462 Ω m in VES 5 and a thickness of between 0.3 m in VES 1 to 1.1 m in VES 6. This is quickly followed by a thick sandy clay or clayey sand subsurface with a low resistivity ranging from 35 Ω in VES 6 to 202 Ω in VES 3 and thickness of 6 m in VES 2 to 12 m in VES 5. Below the thick clayey sand layer lies a region of weathered layer/ weathered basement with fresh basement rock below the strata.
From the analysis, it can be explained that the thin but hard lateritic topsoil surface in the study area was responsible for concentrating the leachate within the dumpsite without having a pronounced influence on the majority of the water in the vicinity. This can be seen from the iterative interpretative curves in Figures 4 where the curves exhibit a sharp drop in the resistivity values. Laterite being a highly resistive material is serving as a firm hard pan which tends to prevent intensive percolation of the leachate from penetrating into the neighboring groundwater wells. Rather, the leachate is concentrated on top of the lateritic topsoil only to be washed off by run-off into the streams. This is the basis for the observed high regional distribution of conductivity measurements in the vicinity of the dumpsite alone on the hydro-geochemical conductivity map as shown in Figure 5. Well 16 which happen to be a surface stream is likely to be more affected by the pollutants from the dumpsite. This is evident from the observed values obtained from the anions and cation analysis.
Conclusion
The results obtained from the research clearly indicate that the result from the analysis of groundwater within the study area reflects the geochemistry of the underlying bedrock, as these are residual soils despite their proximity to a nearby dumpsite. It was found that the vertical electrical sounding technique (Table 3) is a good geophysical tool in the study of the geo-electric layering and lithologic differences of the study area using their resistivity measurements. The leachate from the dumpsite was found not be have a pronounced effect of the groundwater potential in the area due to the presence of a lateritic hardpan which tend to prevent percolation of the surface water in the area into the subsurface. Thus, information obtained from studies can be employed to supplement other geophysical methods for both local and regional hydrogeology.
| VES 1 | VES 2 | VES 3 | ||||
|---|---|---|---|---|---|---|
| Layers | Resisitivity (Ωm) | Thickness (m) | Resisitivity (Ωm) | Thickness (m) | Resisitivity (Ωm) | Thickness (m) |
| 1 | 302.9 | 1.5 | 191.1 | 0.5 | 433.7 | 0.3 |
| 2 | 59.2 | 10 | 308.4 | 1.1 | 201.7 | 9.5 |
| 3 | 1596.7 | - | 64.6 | 7 | 236.6 | - |
| 4 | - | - | 80.9 | - | - | - |
| 5 | - | - | - | - | - | - |
| Rms error = 1.06% Rms error = 1.i3% | ||||||
| Layers | VES 4 | VES 5 | VES 6 | |||
| Resisitivity (Ωm) | Thickness (m) | Resisitivity (Ωm) | Thickness (m) | Resisitivity (Ωm) | Thickness (m) | |
| 1 | 366.7 | 0.4 | 462.4 | 0.7 | 1110.8 | 1.1 |
| 2 | 752.3 | 1 | 623.5 | 1.3 | 150 | 3 |
| 3 | 69.5 | 12.6 | 88 | 12.3 | 35.4 | 8.6 |
| 4 | - | - | 1931.7 | - | 2690.4 | - |
| 5 | - | - | - | - | - | - |
Rms error = 1.06% Rms error = 1.i3%
Table 3: Summary table of the Six (6) Vertical Electrical Sounding (VES)
Acknowledgements
The authors wish to acknowledge the assistance of the staff of the Agronomy Departmental water laboratory, University of Ibadan where the analyses was carried out. We also wish to appreciate Mr. Aladejana of the Department of geology, University of Ibadan who assisted during field work. We also wish to thank the reviewers of this paper as well as those who gave good and constructive criticism about the work.
References
- Charles OA, Olabanji OA, Abimbola AJ, Olamide AO (2013) Assessing the effect of a dumpsite on groundwater quality: case study of Aduramigba Estate within Osogbo metropolis. J Env Earth Sci 3: 120-130.
- Mull EJ (2005) Approaches toward Sustainable Urban Solid Waste Management: Sahakaranagar Layout, Unpublished M.ScInt Environ Sci. Lund University, Lund, Sweden.
- Adewole AT (2009) Waste Management towards sustainable development in Nigeria: A Case study of Lagos. Int NGO J 4: 173-179.
- Appelo CAJ, Postma D (1999) Variable dispersivity in a column experiment containing MnO and FeOH-coated sand. J ContamHydrol 40: 95–106.
- Krishan G, Lohani AK, Rao MS, Kumar CP, Kumar B, et al. (2013) Studying Dynamics of the South West Monsoon in Indian Sub-Continent through Geospatial Correlation of Isotopes in Air Moisture. International Journal of Earth Sciences and Engineering 7: 16-26.
- Ako BD, Adeniyi FI, Adepoju JF (1990) Statistical test and chemical quality of shallow groundwater from a metamorphic terrain, Ile-Ife / Modakeke, South Western Nigeria. J Afr Earth Sci 10: 602-613.
- Drever JI (1997) The Geochemistry of Natural Waters. [3rd Ed], Pearson Education Canada. Upper Saddle River (PrenticeHall), Michigan.
- Saleh A, Al-Ruwaih F, Shehata M (1999) Hydrogeochemical processes operating within the main aquifers of Kuwait. J Arid Environ, 42:195–209.
- Boboye OA (2008) Hydro-geochemical assessment of groundwater in Iddo area, South Western Nigeria. J Min Geol: 183-201.
- Rahaman MA (1976) Progressive polyphase metamorphism in pelitic schist around Aiyetoro, Oyo State, Nigeria. J Min Geol 13: 33-44.
- Tardy Y (1997) Petrology of Laterites and Tropical Soils. Masson and Armand Colin Editeurs, Paris.
- Tijani MN, Shinichi O (2009) Hydro-geochemical Assessment of Metals Contamination in an Urban Drainage System: A Case Study of Osogbo Township, SW-Nigeria. J Water Resour Protection 3: 164-173.
- Tijani MN, Shinichi O, Adeleye MA (2005) Environmental implications of absorbed and total trace concentrations in bottom-sediments of an urban drainage network in a developing country. Materials and Geo-environments 5: 127-130.
Citation: Ojo AO , Oyelami CA and Adereti AO (2014) Hydro-geochemical and Geophysical Study of Groundwater in the Suburb of Osogbo, South Western Nigeria. J Earth Sci Clim Change 5: 205. DOI: 10.4172/2157-7617.1000205
Copyright: © 2014 Ojo AO, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 15797
- [From(publication date): 8-2014 - Jul 12, 2025]
- Breakdown by view type
- HTML page views: 11147
- PDF downloads: 4650