Research Article Open Access
A Comparison of Red Pigments in Different Lipsticks Using Thin Layer Chromatography (TLC)
Bhawana Joshi1, Kapil Verma1* and Jyoti Singh2
1Forensic Science, Amity Institute of Forensic Sciences (AIFS), Amity University, India
2Amity Institute of Forensic Sciences (AIFS), Amity University, India
- *Corresponding Author:
- Kapil Verma, M.Sc Forensic Science
Amity Institute of Forensic Sciences (AIFS)
B-Block, Lower Ground Floor
Amity University, Uttar Pradesh
Sec-125, Noida-201303
Uttar Pradesh, India
E-mail: forensic.kapilalert@gmail.com
Received date: November 22, 2012; Accepted date: December 17, 2012; Published date: December 24, 2012
Citation: Joshi B, Verma K, Singh J (2013) A Comparison of Red Pigments in Different Lipsticks Using Thin Layer Chromatography (TLC). J Anal Bioanal Tech 4:157. doi: 10.4172/2155-9872.1000157
Copyright: © 2013 Joshi B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
The main aim of present work is chromatographic analysis of red pigment in different well known and local brands of lipsticks. Lipstick samples of different brands of similar color were selected for this study. Coloring agent was analyzed by thin layer chromatography (TLC). Using different solvent systems [Toluene/Benzene] (4:12), Toluene/Benzene/Cyclohexane (4:12:4), Toluene/Benzene/Diethyl ether (4:4). It is hypothesized that through thin layer chromatography analysis of the red pigment in these different brands will provide no characteristic data to distinguish among lipstick sources. There is no significant difference in the hRf values among the local and well known brands of lipsticks which can be used as unique feature.
Keywords
Chromatographic analysis; Red pigments; Lipsticks; Lipstick samples; TLC; Solvent system; hRf value
Introduction
In forensic science, comparative examinations are usually based on the physical or chemical nature of a substance, or both. Lipsticks are composed of waxes, oils, organic dyes [1], and inorganic pigments [2,3]. Color matching can do identifying the lipstick responsible for leaving a smear. This color analysis may be used to identify the lipstick found at the crime scene [4].
The colors of lipstick are often due to a mixture of several pigment compounds. These pigments can be separated using thin layer chromatography. Depending on the type of pigment, the mobile phase will vary. Lipsticks are soluble in toluene, so toluene serves as the mobile phase. After separation, the chromatogram is complete and illustrates the different pigments that make up a particular color of lipstick [2,3,5-10].
Traces of Lipsticks, cosmetics, nail polish, or other smears could be found left on drinking cups, glasses, cigarette butts, and tissue papers and may all be significant forensic evidence [11] in the investigation of a crime, especially in cases such as a sexual assault or a homicide [12]. This physical evidence may be found on clothing, parts of the body, a tissue, or cigarette [5,13]. By comparing the composition of a lipstick smear with that of a victim, forensic scientists can demonstrate indirect proof of contact or a relationship between victim and suspect. Also, it is sometimes possible to extract saliva DNA from the print and may link a suspect to a crime scene [2].
Various methods of forensic lipstick analysis were reported [6,8,14-17]. Small amount of lipstick (approximately 10 μg) could lead to good comparisons in TLC [3]. Thin Layer Chromatography (TLC) is a widelyused chromatography technique used to separate chemical compounds. Thin-layer chromatography incorporates a solid stationary phase and a moving liquid phase to cause a separation of the constituents of a mixture. Although simple test may be run by simply allowing a solvent to pick up a piece of porous paper, a more revealing test requires the preparation of a plate. Because most compounds are colorless, no separation will be noticed after development unless the materials are visualized. This is may be done by:
• Exposing to UV light
• Exposing to fluorescent dyes
• Exposing to iodine
• Spraying with a reagent
These procedures may be used alone or in conjunction to make the components of a sample visible. The distance a component has traveled up a plate can be assigned a numerical value known as the Rf value. Rf is defined as the distance traveled by the component divided by the distance traveled by the solvent. In the example to the right, the solvent was allowed to travel 10 cm up the plate before the plate was removed from the chamber and dried.
Materials and Methods
Jar with lid, lipstick samples (Branded and Local), TLC plates, mobile phase/solvent phase, clean cotton piece, sterilized scissor, fine capillaries, ruler, pencil, todine pellets, titer plates, graduated cylinder, gloves, protective mask and thirty samples of lip impressions bearing lipstick were obtained on cloth using various brands and types of lipsticks, which were available from volunteers [5,13,18-20].
Thin-Layer Chromatography (TLC)
A thin-layer plate is prepared by coating a glass plate with a thin film of a granular material. Commonly, silica gel or aluminum oxide or cellulose immobilized onto a flat, inert carrier sheet is used, but paper may suffice in simpler experiments. These serve as the solid stationary phase. If the sample to be analyzed is a solid, it must first be dissolved in a suitable solvent. A few micro liters of the solution are then applied to the lower edge of the plate. A liquid sample may be applied directly to the solid state in the same manner. The plate or paper is then placed upright into a closed chamber that contains a selected solvent.
The solvent will slowly begin to pick up the plate by capillary action. It is the rising solvent that serves as the moving phase in thin-layer chromatography. As it moves past the sample spot, the components of the sample will become distributed between the stationary solid phase and the moving liquid phase. Those components with the greatest affinity for the moving phase will travel up the plate at a faster speed as compared to those that have greater affinity for the stationary phase.
Thin-Layer Chromatography (TLC) is a method use to separate components from each other in a mixture. It is normally experimented on glass, aluminum foil or plastic which is coated by some kind of absorbent material (ex: silica gel, aluminum oxide). It takes the Rf values of each color component and compares them; Rf values are experiments that depend on the polarity of the substance on the paper chromatography [21];
1. A piece of cotton cloth was washed in a detergent solution, immersed in a hot water, and dried.
2. This cloth was cut into small pieces (2 cm×2 cm) and lipstick smears were rubbed onto these pieces.
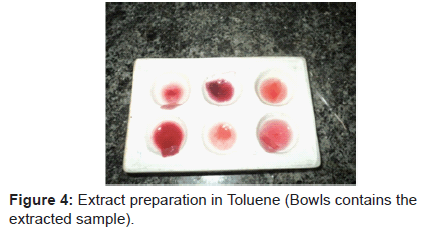
3. The stained areas from the cloth pieces were cut and transferred into serially marked small bowls.
4. These samples were mixed with an extracting solution and each bowl was then shaken for about 5-10 minutes to remove the stain from the cloth piece.
5. Cloth piece is taken out, and the extract was used for further examination with TLC.
6. Obtain jar with lid, piece of filter paper and a TLC plate. Handle TLC plate by the edges only, avoid touching the white silica layer.
7. With a pencil and ruler, gently draw a line across the short side of the TLC plate about 1.5 cm from the bottom of the plate. At even intervals label the top of the plate with the letters: 1B, 2B, 3B, 4B, 5B, 6B for six different well known brands of red lipsticks on a single TLC plate. Similarly 1L, 2L, 3L, 4L, 5L, 6L for six different local brands of red lipsticks.
8. Using a capillary, place a dot of each lipstick sample along the bottom penciled line directly under the corresponding label on the top of the plate. The dots should be about 0.2 cm in diameter and dark enough to be clearly visible.
9. Using a pipette dispenses mobile phase/solvent in the ratio (4:12:4) into the jar. The mobile phase/solvent should be about 0.5 cm deep.
10. Carefully insert the TLC plate into the jar, sample end down. The lipstick dots must be above the mobile phase/solvent. Secure the lid.
11. Allow the mobile phase/solvent to rise to within one cm of the top of the plate (5-10 minutes). Watch–do not allow the mobile phase to rise to the very top of the plate. Remove the TLC plate and mark the solvent front with a pencil.
12. Measure the distance the mobile phase/solvent moved in cm (the distance from the spotted pencil line to the solvent front end). Also measure the distance in cm each component of the lipsticks moved from the spotted pencil line. Some lipsticks have only two or three components, and some have more. Enter these measurements on the Data Table.
13. Determine the Rf for each lipstick component of all lipstick samples. Enter those values on the Data Table. To calculate the Rf value, divide the distance traveled by each lipstick component by the distance traveled by the solvent.
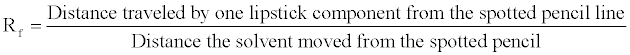
Samples are run for both the samples of branded and local lipstick. Use hood if possible. Self life is about one month.
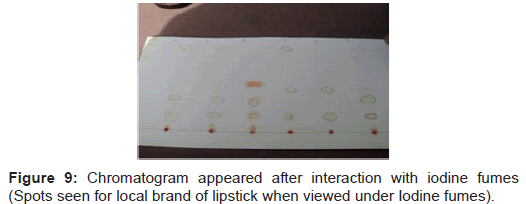
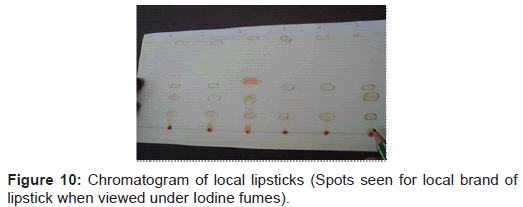
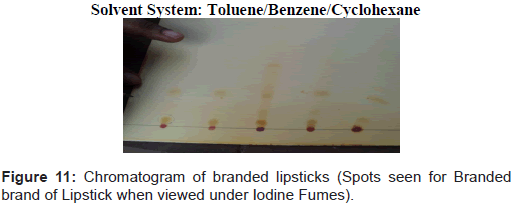
Steps followed are shown in figures 1-11.
Results
The data collected from the chromatograms were recorded, and the Rf values were calculated using distance solute travelled/distance solvent travelled (dsu/dsv). All of these values are shown in the table. The Rf values of the red dyes in the local brands of lipsticks are given in table 1 and in the well known brands are given in table 2.
| Column 3 | Column 4 | |||
|---|---|---|---|---|
| Lipstick Samples | No. of Spots | Distance Lipstick Components Moved (cm) | Distance Mobile Phase Moved (cm) | hRf Values for Each Colored Component |
| 1L | 1 | 0.7 | 10 | 7 |
| 2 | 1.2 | 10 | 12 | |
| 3 | 2.7 | 10 | 27 | |
| 4 | 3.7 | 10 | 37 | |
| 5 | 4.7 | 10 | 47 | |
| 6 | 7.6 | 10 | 76 | |
| 2L | 1 | 1.3 | 10 | 13 |
| 2 | 2.7 | 10 | 27 | |
| 3 | 3.7 | 10 | 37 | |
| 4 | 7.6 | 10 | 76 | |
| 3L | 1 | 0.3 | 10 | 3 |
| 2 | 1.3 | 10 | 13 | |
| 3 | 2.5 | 10 | 25 | |
| 4 | 4.2 | 10 | 42 | |
| 5 | 7.9 | 10 | 79 | |
| 4L | 1 | 0.3 | 10 | 3 |
| 2 | 1.3 | 10 | 13 | |
| 3 | 3.7 | 10 | 37 | |
| 4 | 7.9 | 10 | 79 | |
| 5L | 1 | 1.3 | 10 | 13 |
| 2 | 3.6 | 10 | 36 | |
| 3 | 7.8 | 10 | 78 | |
| 6L | 1 | 0.3 | 10 | 3 |
| 2 | 1.3 | 10 | 13 | |
| 3 | 2.9 | 10 | 29 | |
| 4 | 3.6 | 10 | 36 | |
| 5 | 8 | 10 | 80 | |
Table 1: Rf values of the red dyes in the local brands of lipsticks.
| Column 3 | Column 4 | |||
|---|---|---|---|---|
| Lipstick Samples | No. of Spots | Distance Lipstick Components Moved (cm) | DistanceMobile Phase Moved (cm) | Rf Values for Each Colored Component |
| 1B | 1 | 0.4 | 10 | 4 |
| 2B | 1 | 0.35 | 10 | 3.5 |
| 2 | 1.3 | 10 | 13 | |
| 3B | 1 | 0.25 | 10 | 25 |
| 2 | 1.55 | 10 | 15.5 | |
| 4B | 1 | 0.2 | 10 | 2 |
| 2 | 1.3 | 10 | 13 | |
| 5B | 1 | 0.25 | 10 | 2.5 |
| 2 | 1.5 | 10 | 15 | |
| 6B | 1 | 0.25 | 10 | 2.5 |
| 2 | 1.5 | 10 | 15 | |
Table 2: Rf values of the red dyes in the well known brands of lipsticks.
For local brands
Table 1 shows the result obtained from the local brands of lipsticks. In which sample marked as 1L shows six pigments having hRf values (7,12,27,37,47,76), sample 2L shows four pigments having hRf values (13,27,37,76), sample 3L shows five pigments having hRf values (3,13,25,42,79), sample 4L shows four pigments having hRf values (3,13,37,79), sample 5L shows three pigments having hRf values (13,36,78) and sample 6L shows five pigments having hRf values (3,13,29,36,80). This shows that there is no significant difference in the hRf values among the different local brands of lipsticks under study.
For well known brands
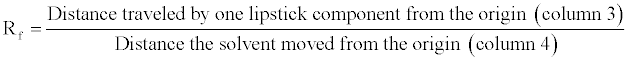
Table 2 shows the result obtained from the local brands of lipsticks. In which sample marked as 1B shows one pigment having hRf value (4), sample 2B shows Two pigments having hRf values (3.5,13), sample 3B shows two pigments having hRf values (25,15.5), sample 4B shows two pigments having hRf values (2,13), sample 5B shows two pigments having hRf values (2.5,15) and sample 6B shows two pigments having hRf values (2.5,15). This shows that there is no significant difference in the hRf values among the different well known brands of lipsticks under study.
Discussion and Conclusion
The conclusion is that through thin layer chromatography analysis of red dye concentrations in these different (well known) and (local) brands of lipsticks, there is no characteristic data to distinguish among the lipstick sources, and therefore the concentrations of the red dye is not be a unique identifier for the lipstick sources. The concentration of red pigment differs among these “well known” and “local” brands within a small constant sample of red lipsticks.
The chromatogram of local lipsticks (Table 3) shows that majority of the local brands contains more than 2 dyes pigments given in table 4. While branded lipsticks contains only 1 or 2 dyes pigments given in table 5. There is no significant difference in the hRf values among the local and well known brands of lipsticks which can be used as unique feature.
| Sample No. | Name & Number | Brand | Color |
|---|---|---|---|
| 1L | biros 14 | local | pink |
| 2L | biros 8 | local | rosy red |
| 3L | matte lip color 293 | local | red |
| 4L | aver matte 147 | local | light red |
| 5L | iffy super soft 15 | local | red |
| 6L | iffy matte soft 17 | local | maroon |
Table 3: LOCAL brand lipstick examined.
| Sample No. | Solvent System |
|---|---|
| 1 | Toluene/Benzene (4:12) |
| 2 | Toluene/Benzene/Cyclohexane (4:12:4) |
| 3 | Toluene/Benzene/Diethyl ether (4:12:2) |
Table 4: Mobile or Solvent phase.
| Sample No. | Name & Number | Brand | Color |
|---|---|---|---|
| 1B | bridal dream 104 | revlon | spunky red |
| 2B | cherry sparkles 57 | revlon | bright red |
| 3B | red flame 13 | revlon | bright red |
| 4B | red spice | street wear | light red |
| 5B | crystal shine | street wear | dark red |
| 6B | color burst lipstick plum | revlon | maroon |
Table 5: REVLON brand lipstick examined.
References
- Sjöberg AM, Olkkonen C (1985) Determination of synthetic organic colours in lipsticks by thin-layer and high-performance liquid chromatography. J Chromatogr 318: 149-154.
- Webb LG, Egan SE, Turbett GR (2001) Recovery of DNA for Forensic Analysis from Lip Cosmetics. J Forensic Sci 46: 1474-1479.
- Russell LW, Welch AE (1984) Analysis of lipsticks. Forensic Science International 25: 105-116.
- Alvarez Segui M, Miquel Feucht M, Castello Ponce A, Verdu Pascual F (2000) Persistent lipsticks and their lip prints: new hidden evidence at the crime scene. Forensic Sci Int 112: 41-47.
- Andrasko J (1981) Forensic Analysis of Lipstick. Forensic Science International 17: 235-251.
- Ehara Y, Marumo Y (1998) Identification of lipstick smears by fluorescence observation and purge-and-trap gas chromatography. Forensic Science International 96: 1-10.
- Scalia S, Simeoni S (2001) Assay of xanthenes dyes in lipsticks by inverse supercritical fluid extraction and HPLC. Chromatographia 53: 490-494.
- Misra G, Mittal VK (2004) Neutron Activation Analysis of Lipsticks Using g-Ray Spectrometry. J Appl Spectrosc 71: 270-274.
- Cho L, Hsui KC (2006) Analysis of lipstick smears by ATR micro spectroscopy.
- Wegener JW, Klamer JC, Govers H, Brinkman UATh (1987) Determination of organic colorants in cosmetic products by high-performance liquid chromatography. Chromatographia 24: 865-875.
- Castelló A, Alvarez-Seguí M, Verdú F (2005) Luminous lip prints as criminal evidence. Forensic Sci Int 155: 185-187.
- Castello A, Alvarez M, Miquel M, Verdu F (2002) Long-lasting lipsticks and latent prints. Forensic Sci Comm 4.
- Barker AM, Clarke PD (1972) Examination of Small Quantities of Lipstick. J Forensic Sci Soc 12: 449-451.
- Reuland DJ, Trinler WA (1980) A comparison of lipstick smears by high performance liquid chromatography. J Forensic Sci Soc 20: 111-120.
- Reuland DJ, Trinler WA (1984) A Comparison of Lipstick Smears by High Performance Liquid Chromatography. Part II. The Effects of Wear-Time and Subject on the Chromatograms. J Forensic Sci Soc 24: 509-518.
- Choudhry MY (1991) Comparison of Minute Smears of Lipstick by Microspectrophotometry and Scanning Electron Microscopy/EnergyDispersive Spectroscopy. JFSCA 36: 366-375.
- Rodger C, Broughton D (1998) The in-situ analysis of lipsticks by surface enhanced resonance Raman scattering. Analyst 123: 1823-1826.
- Lucas DM, Eijgelaar G (1961) An evaluation of a technique for the examination of lipstick stain. J Forensic Sci 6: 354-362.
- Abdullah AFL, Marimuthu Y, Haw CK, Mohamad Said NF, Md Muslim NZ, et al. (2011) Forensic Discrimination of Lipsticks by Thin Layer Chromatography and Gas Chromatogrphy - Mass Spectrometry. Malaysian Journal of Forensic Sciences 2: 22-28.
- Srivastava S, Verma K, Singh J (2012) To Identify the Concentration Level of Various Pigments & to Determine Suitable Solvent System for Different Lipstick Samples by Using TLC. J Chromat Separation Techniq 3: 146.
- Wall PE (2005) Thin-layer Chromatography: a Modern Practical Approach. Volume 12 of RSC Chromatography Monographs, Royal Society of Chemistry, Cambridge.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 27809
- [From(publication date):
February-2013 - Dec 23, 2025] - Breakdown by view type
- HTML page views : 22294
- PDF downloads : 5515