Make the best use of Scientific Research and information from our 700+ peer reviewed, Open Access Journals that operates with the help of 50,000+ Editorial Board Members and esteemed reviewers and 1000+ Scientific associations in Medical, Clinical, Pharmaceutical, Engineering, Technology and Management Fields.
Meet Inspiring Speakers and Experts at our 3000+ Global Conferenceseries Events with over 600+ Conferences, 1200+ Symposiums and 1200+ Workshops on Medical, Pharma, Engineering, Science, Technology and Business
Special Issue Article Open Access
Differentiation Capacity of Chondrocytes in Microtissues Depends on TGF-Beta Subtype
| Frank Martin1,2, Mario Lehmann1,2, Peter Schläger1, Ulrich Sack2,3 and Ursula Anderer1* | |
| 1Department of Cell Biology and Tissue Engineering, Lausitz University of Applied Sciences, Senftenberg, Germany | |
| 2Institute of Clinical Immunology, Medical Faculty, University of Leipzig, Leipzig, Germany | |
| 3Translational Centre for Regenerative Medicine (TRM), University of Leipzig, Leipzig, Germany | |
| Corresponding Author : | Prof. Dr. Ursula Anderer Department of Cell Biology and Tissue Engineering Lausitz University of Applied Sciences Faculty of Science, Großenhainer Str.57 D-01968 Senftenberg, Germany Tel: +49-3573-85 916 (-800) Fax: +49-3573-85 809 E-mail: ursula.anderer@hs-lausitz.de |
| Received October 12, 2012; Accepted November 07, 2012; Published November 09, 2012 | |
| Citation: Martin F, Lehmann M, Schläger P, Sack U, Anderer U (2012) Differentiation Capacity of Chondrocytes in Microtissues depends on TGF-ß Subtype. J Biochip Tissue chip S2:002. doi:10.4172/2153-0777.S2-002 | |
| Copyright: © 2012 Martin F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Bioengineering and Bioelectronics
| Introduction |
| Today, people get the chance to reach a higher age and therefore, an increasing number of age-related degeneration of various tissues and organs like heart or articular joints will emerge. A 2005 study demonstrated that every second person at an age above 60 years has osteoarthritically modified joints [1]. Concomitant with these aging problems, the number of active living people doing all kinds of activities including sports with high risk of injuries, such as downhill skiing, skateboarding, or even playing football is increasing. The resulting higher frequency of traumatic injuries, especially in the musculoskeletal system made it necessary to develop and improve procedures to repair, or even better regenerate diseased or damaged tissues. Furthermore, the regenerative capacity of human hyaline cartilage is very limited and lesions in the adult tissue might induce degenerative processes, and finally result in osteoarthritis [2]. These changes in the tissue homeostasis are accompanied by fibrillation and cartilage matrix degeneration, and could be visualised by loss of Proteoglycans (PG) and Type II Collagen [3]. Taking these prerequisites of intrinsic articular cartilage repair and the high number of patients suffering from osteoarthritis into account, suitable and effective therapies are in the focus of interest. Besides the well-established orthopaedic interventions like Pridie drilling, microfracture, or the replacement by osteochondral crafts, new cell based strategies were developed [4,5]. By using this approach, the formation of fibrocartilage tissue lacking the mechanical properties of normal hyaline cartilage [6,7] should be avoided. One important step towards cartilage regeneration was the implementation of the Autologous Chondrocyte Transplantation (ACT) to the clinical practice [8,9]. Further improvements of cell based techniques led to the generation of in vitro cartilage tissue by using scaffold materials [10,11], or using a scaffold free approach [12,13]. With respect to the treatment of osteoarthritical defects, 3D cartilage constructs with a suitable amount of extracellular matrix are essential. Thereby, the ECM plays an important role for the fixation of the implant within the area of defect. As already known, the isolation of chondrocytes from their natural environment and subcultivation in monolayer caused a reduced synthesis of ECM specific molecules, like type II collagen or proteoglycans. The cells start to dedifferentiate resulting in a shift from collagen type II to collagen type I expression [14,15]. However, cultivation of the cells in a three-dimensional environment initiates the redifferentiation back to a cartilage phenotype. This redifferentiation process is most times positively influenced by adding soluble growth factors. Different experimental approaches identified growth factors and their related signalling pathways as important players in cartilage tissue development and homeostasis [16]. In this context, most of the studied classes of growth factors are the TGF-β family, the fibroblast growth factor family, and insulin-like growth factors [17,18]. Besides the involvement in developmental processes of cartilage, the mentioned factors are also players during pathogenic alterations of cartilage tissue seen in osteoarthritis [19,20]. With respect to their potential in stimulating the repair of tissue injury and the role in chondrocyte differentiation, members of the TGF-β family might be valuable candidates for the tissue engineering of cartilage, and the treatment of cartilage defects [21-23]. The TGF-β superfamily consists of more than 35 different members and they are characterized by the formation of active dimers [24]. In case of cartilage tissue engineering, the three TGF-β isoforms β1, β2, and β3 are in the focus of scientific interest. For TGF-β1 and β2 treatment, an increased expression of cartilage specific collagen type II and an accumulation of proteoglycans were shown [25-28]. Additionally, these proteins are involved in chondrocyte maturation and are important interaction partners for other growth factors [25,29]. From this point of view, the Bone Morphogenic Proteins (BMPs) are the most important ones, with respect to chondrocyte redifferentiation. Basically, TGF-β and BMP are members of the same family of cytokines, and are involved in embryonic development and tissue homeostasis. Furthermore, strong interconnections to other signalling pathways are known [30]. One interesting candidate from the above mentioned BMP-family is BMP- 7. The latter protein is known to positively influence cartilage repair and stimulate proteoglycan and collagen synthesis [31]. Furthermore, chondrocytes from osteoarthritic cartilage tissue showed up-regulated receptors for BMP-7. Administration of this growth factor displayed a stimulating effect on extracellular matrix synthesis [32]. Recently, a phase one study was published, administering BMP-7 to osteoarthritic knee joints to determine the effect on symptomatic responses [33]. |
| Based on these findings, the present study deals with the engineering of scaffold-free cartilage-like microtissues, and focus on possible influences of individual members of the TGF-β-superfamily on the process of redifferentiation. The inductive effect of TGF-β1 and TGF-β2 on the process of chondrocyte differentiation in a 3D-model was investigated. Taking previous experiments of the group with TGF-β1 into account, the possible stimulating effect of BMP-7 on cartilage differentiation was analyzed. |
| Materials and Methods |
| Cell source and monolayer culture of human chondrocytes |
| Human articular cartilage samples were obtained from human femur condyles of patients undergoing knee surgery. Results were generated, performing three independent experiments with cartilage from three different patients with informed consent. Condyles were collected in alpha-medium and HAM’S F12 (1:1) (Biochrom, Berlin, Germany) supplemented with an antibiotics mixture. |
| Cartilage tissue was peeled from the condyles with a sterile scalpel. Chondrocytes were isolated from the surrounding matrix by mechanical mincing of the tissue with scalpels, followed by enzymatic treatment with collagenase (350 U/mL; Biochrom) at 300 rpm interval mixing (EppendorfThermomixer comfort) for 20 h at 37°C. The chondrocytes were centrifuged at 300 × g for 5 min. The supernatant was removed and the cell pellet was resuspended with 10 ml of alpha medium plus HAM’s F12 (1:2) enriched with 1% L-glutamine, 10% human serum (serum pool from voluntary donors), further designated as Basal Medium (BM). The chondrocytes were plated at a cell density of 2×104 cells/cm² and expanded in monolayer at 37°C and 5% CO2. After a period of approximately 8-10 days, the cells were detached using 0.05% trypsin and 0.02% EDTA (Biochrom), and replated with a defined ratio (1:3). After another week in culture when cells reached confluence, second passage cells (P2) where trypsinized and subsequently cultured in a 3D-promoting environment, as described below. During the expansion stage, chondrocytes were cultured in basal medium without the addition of growth factors. |
| Generation of cartilaginous microtissues |
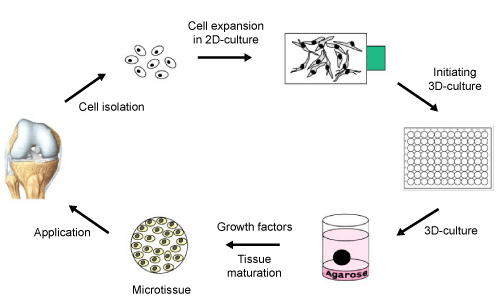
| To induce microtissue formation, P2 chondrocytes were suspended in basal medium and seeded in agarose-coated 96-well plates at a concentration of 3×105cells/well. Stable chondrocyte aggregates were already formed after two days. After this first aggregation step, the spheroid shaped constructs were cultivated in agar overlay up to four weeks (Figure 1). Following two to four weeks of culture, the resulting pellets were prepared for histology and subsequently examined as described below. |
| Chondrogenic redifferentiation conditions |
| Cultivation of chondrocytes in an environment enabling the formation of cell aggregates is one important step to stimulate cartilage redifferentiation in vitro. To enhance the redifferentiation of chondrocytes, spheroids were cultivated with four different media compositions concerning the presence of bioactive molecules. These include Basal Medium (BM), BM supplemented with 5 ng/ml TGF-β1 (MiltenyiBiotec, BergischGladbach, Germany), BM plus 5 ng/ml TGF-β2 (MiltenyiBiotec), or BM supplemented with 5 ng/ml TGF-β1 and 100 ng/ml BMP-7 (MiltenyiBiotec). Spheroids were cultured for two or four weeks on agar coated plates. Medium change was performed three times a week. |
| Sizing and preparation of in vitro cartilage for analysis |
| Within the culture time of four weeks, the size of the spheroids was assessed by measuring the diameter using an inverted phase contrast microscope (OLYMPUS CKX41, OLYMPUS, Hamburg, Germany), a DP-71 digital camera (Soft Imaging Systems, Münster, Germany), and the imaging software CellD (Soft Imaging Systems). After two and four weeks of maturation in vitro, tissue constructs were harvested and prepared for further analyses. The spheroids were rinsed in PBS, embedded in Neg-50 frozen section medium (Richard Alan scientific, Kalmazoo, USA), and sectioned using a cryomicrotom (Microm GmbH, Walldorf, Germany). The 7 μm thick cryosections on glass slides (Superfrost Plus; MenzelGläser, Braunschweig, Germany) were air-dried and directly analyzed or stored at -20°C. |
| Histology and immunohistochemistry |
| Prior to analyses, tissue cryosections on glass slides were fixed in a two-step process. The sections were fixed with 4% formalin (AppliChem, Darmstadt, Germany) at 4°C for 10 min, followed by an incubation in a 1:1 mixture of methanol/acetone (Roth, Karlsruhe, Germany) at -20°C for 10 min After this fixation procedure, the slides were rinsed in PBS for 3 to 5 min. |
| Safranin-O-Fast Green (AppliChem) was used as histological staining to detect Glycosaminoglycans (GAGs). Fixed cryosections were subjected to indirect immunohistochemistry for human collagen type I, type II, and S100B to assess the degree of redifferentiation. Sections were rinsed with PBS and incubated for 20 min at Room Temperature (RT), with normal goat serum (Dianova, Hamburg, Germany) diluted 1:50 in PBS/0.1% BSA (Roth). Primary antibodies were diluted in PBS/0.1% BSA as follows: mouse anti-collagen type I and anti-collagen type II (1:1000, MP Biomedicals, Ohio, USA), and rabbit anti-S100 (1:400, DakoCytomation, Glostrup, Denmark). The latter antibody displays a strong affinity to S100B and a weak interaction for S100A1. The cryosections were incubated with primary antibodies in a humified chamber overnight (o.n.) at 4°C. Subsequently, slides were washed three times with PBS and then incubated in a humidified chamber for 1 h in the dark, with Cy3-conjugated goat anti-mouse (collagen type I and II) and goat anti-rabbit (S100B) antibody (Dianova) diluted 1:600 in PBS/0.1% BSA, including DAPI (1 μg/ml; Fluka, Seelze, Germany) to stain cell nuclei. Slides were washed three times with PBS and sections were subsequently mounted with a fluorescent mounting medium (DakoCytomation). Slides were stored in the dark at 4°C until analysis by fluorescence microscopy. Cryosections of native human articular cartilage were used as positive control for collagen type II and S100B, and as negative control for collagen type I. In addition, all experiments included a control for unspecific binding of the secondary antibody applying PBS, instead of the primary antibody. |
| Microscopy |
| Fluorescence of Cy3-conjugated antibodies and DAPI-labelled cell nuclei was detected using the fluorescence microscope OLYMPUS IX81, with the xenon burner OLYMPUS MT20 (OLYMPUS). Image documentation and evaluation was performed using the digital camera F-View II (Soft Imaging Systems) and OLYMPUS CellR-Imaging Software for Life Science Microscopy. Coloured preparations of histological staining like Safranin O were documented using a BX41 microscope (Olympus) connected to an F-View I camera (Soft Imaging Systems). |
| Results |
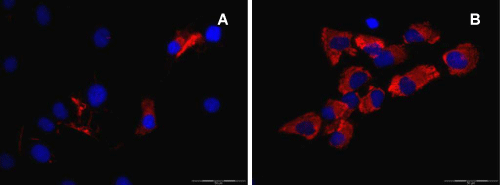
| In order to investigate the influence of specific growth factors on the formation and differentiation of cartilage microtissues, cells were isolated from human condyles, expanded in monolayer culture and subsequently transferred to a 3D culture system. During the expansion phase, the cells changed their morphology as well as their phenotype. They start to dedifferentiate, visualized via immunofluorescence by a clear down regulation of collagen type II, accompanied by an up regulation of collagen type I. This switch in the expression of the collagen proteins is visible after two passages, as shown in figure 2. |
| Transferring the expanded chondrocytes into the described 3D-promoting environment (Figure 1), the cells start to form small microtissues with a spherical shape. After a short aggregation phase, the spheroids were cultured in four different culture media referred as Basal Medium (BM), BM plus TGF-β1, BM supplemented with TGF-β2, and BM combined with BMP7 and TGF-β1. The monitoring of the size of these differentially cultured spheroids for four weeks revealed differences, with regard to the medium additives (Figure 3). In case of the basal medium and the supplementation with TGF-β1, only marginal changes were detected. In contrast, the diameter of microtissues supplemented with TGF-β2 increased around 5% (max value 1522μm) versus 10% in the TGF-β1 plus BMP-7 situation (max value 1646 μm) (Figure 3). Comparing the calculated volumes of the microtissues from day 5 to day 25, there was an increase of 10% for spheroids in BM plus TGF-β1, 30% for spheroids in BM plus TGF-β2, and reaching a volume increase of 50% in spheroids treated with TGF-β1 and BMP-7. Basal medium alone did not result in noteworthy volume expansion. The differentiation state of the generated spheroids was analyzed after two and four weeks of tissue maturation. In this context, the histological and immunohistochemical examination revealed significant differences between the different culture media at one hand, and the duration of the culture on the other hand. Taking collagen type II and proteoglycans as main differentiation markers for hyaline cartilage into account, clearly distinguishable redifferentiation levels were obtained. |
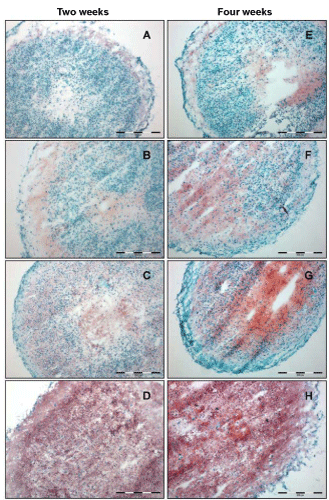
| All tested culture media induced the formation of compact spherical constructs. The morphology, however, shows clear differences concerning the cell arrangement inside the generated tissues. Cells in spheroids cultured in BM (2 and 4 weeks) are densely packed, with the exception of the inner core and a small outer rim (Figure 4A and C). Adding TGF-β1 resulted in matrix production in different parts in the spheroid, appearing red in Safranin O (SO) stained areas with cells widely separated from each other. After a culture time of 4 weeks, the cross section revealed an inhomogenously maturated tissue with foci of high cell density/less matrix, and foci with widely separated cells embedded in red stained matrix material (Figure 4F). Combining TGF-β1 and BMP-7 as culture medium additives resulted in a homogenous tissue morphology throughout the whole spheroid (Figure 4D and H). |
| In case of the proteoglycan synthesis visualised by SO staining, a more diverse picture is drawn. As shown in figure 4, differences in deposition and spatial arrangement of proteoglycans, with respect to the used media compositions are visible. After two weeks incubation in basal medium, a small amount of proteoglycans was detectable restricted to the outer rim of the microtissue (Figure 4A). The addition of TGF-β1 to the BM slightly increased the expression of proteoglycans, with the restriction to the central and the peripheral part of the spheroid (Figure 4B). In contrast to the TGF-β1 supplementation, treatment with TGF-β2 increased the proteglycan synthesis. The strongest expression of proteoglycans was induced by incubating microtissues with a combination of TGF-β1 and BMP-7, resulting in the staining of the whole section. Figure 4E-H shows the results for proteoglycan synthesis after four weeks in culture. In general, a time-dependent increase of proteoglycans after four weeks of culture was observed. Similar to the two week situation, the basal medium displayed the lowest amount of GAG. Nevertheless, after four weeks a moderate staining of the central part of the microtissue was detectable. In case of supplementation with TGF-β1, TGF-β2, and TGF-β1 plus BMP-7, the whole section was stained for proteoglycans (Figure 4F-H). For those three media compositions, an increase of GAG content was detected. Nevertheless, TGF-β2 and TGF-β1 plus BMP-7 resulted in an improved result, with a robust signal and a distribution to the whole section. Comparing all used media, cryosections of microtissues incubated with TGF-β1 and BMP-7 showed the most homogenous and intense SO-staining. |
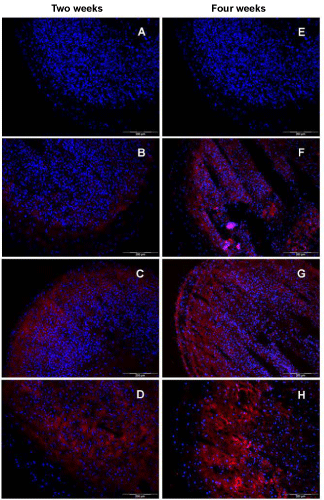
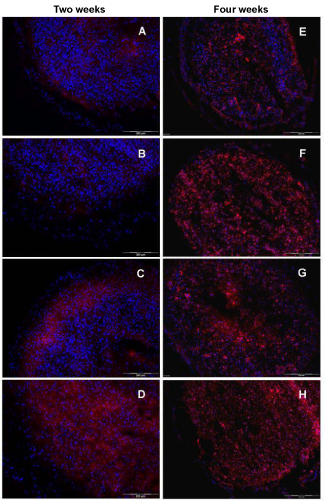
| In order to examine the effect of the used culture media on the expression of hyaline cartilage specific proteins, an immunohistochemical analysis was done. The expression of the cartilage-specific differentiation markers, collagen type II and S100B, as well as the dedifferentiation marker collagen type I in spheroids revealed clear differences between the used media. As visualized in figure 5A-D, an increase in collagen type II expression starting from the basal medium (Figure 5A) up to TGF-β1 plus BMP-7 (Figure 5D) is seen after two weeks of cultivation. Microtissues cultivated in basal medium did not express collagen type II, whereas the addition of TGF-β1 resulted in a marginal increase of that protein (Figure 5A,B). In contrast, supplementation with TGF-β2 revealed a high expression in the peripheral part of the tissue section, whereas the inner part is only slightly stained (Figure 5C). In case of TGF-β1 plus BMP-7, an adverse spatial organisation for collagen type II was obtained with a clear restriction to the central part of the microtissue (Figure 5D). Analysis of specimens cultured for four weeks displayed an augmentation in the expression of collagen type II. The only exception was spheroids cultivated in BM. Here, no collagen type II was detectable via immunohistochemistry, similar to the two weeks measurement (Figure 5E). For microtissues in medium supplemented with TGF-β2, a more intense fluorescence was obtained spreading all over the cross section with a higher intensity in the outer regions. Tissue specimens cultured in TGF-β1 plus BMP-7 showed a robust expression of collagen type II, with a spatial restriction to the middle area of the section (Figure 5H). |
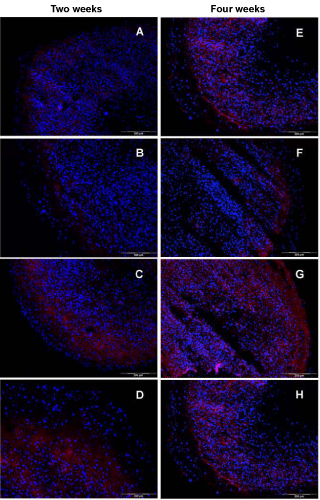
| The analysis for the small protein S100B that is expressed intracellularly by chondrocytes illustrates in principle a similar result compared to the collagen type II findings. Culturing microtissues in basal medium for two weeks resulted in a marginal expression of S100B in few cells in the outermost cell layer (Figure 6A). By adding TGF-β1, no significant change was observed (Figure 6B). In contrast, cells in microtissues supplemented with TGF-β2 were able to accumulate significant amounts of S100B in the peripheral part of the construct, as indicated in figure 6C. Treating spheroids with TGF-β1 plus BMP-7 was superior after two weeks of cell culture. With the exception for the peripheral region, nearly all cells in the whole cryosection were stained for S100B (Figure 6D). After four weeks, a more uniform expression of S100B protein for the tested media compositions was detected (Figure 7E-H). For all sectioned microtissues, a fluorescence signal distributed to the whole section was obtained. Nevertheless, TGF-β1 plus BMP- 7 displayed the most intense fluorescence, followed by TGF-β2 and TGF-β1. Incubation in basal medium resulted in a moderate induction of S100B expression. |
| The immunohistochemical analysis for the dedifferentiation marker collagen type I showed comparable outcomes for two and four weeks of culture. In general, the intensity of the fluorescence signal on cryosections was quite low, compared with the signals for collagen type II. The presence of TGF-β2 and TGF-β1 plus BMP-7 led to a moderate expression of collagen type I (Figure 7C,D,G and H), in comparison to the basal medium or TGF-β1 administration. After two weeks cell culture, spheroids incubated with TGF-β2 showed an expression of collagen type I, restricted to the peripheral part of the section (Figure 7C), whereas in the TGF-β1 plus BMP-7 situation, the central part is slightly positive stained (Figure 7D). In contrast, cryosection of spheroids cultivated in basal medium and supplementation with TGF-β1 revealed no signal for collagen type I at all (Figure 7A and B). After four weeks in culture, the cryosections showed a slight increase of the fluorescence signal for all media compositions. In case of the basal medium and the TGF-β1 supplementation, a weak expression signal was visible in the peripheral part of the section (Figure 7E and F). A similar distribution with an increased intensity of the fluorescence signal was detected for TGF-β1 plus BMP-7 (Figure 7H). Microtissues cultivated with TGF-β2 displayed the highest expression of collagen type I after four weeks with a staining of the whole section (Figure 7G). |
| Discussion |
| Traumatic as well as degenerative defects of articular cartilage tissue are increasing. Different therapeutic strategies are introduced to the clinic. However, the outcomes are far away from being satisfactory, meaning the regenerating of the specific morphological and physiological qualities of hyaline cartilage by therapies like microfracture, mosaicplasty or ACT [4,5]. More recent approaches try to circumvent existing problems using cell based techniques, which create already three-dimensional tissue constructs outside the body.Among a variety of parameters influencing the quality of the cell based transplants, proper redifferentiation of the generated microtissues is essential. In this present work, the differentiation capacity of monolayerexpanded chondrocytes in scaffold-free self-aggregated microtissues was analyzed, supported by members of the TGF-β superfamily. Special attention was directed to the expression and localization of collagen type II, S100B, and proteoglycans inside the in vitro tissues. Distinct expression patterns of the proteins, collagen type II and S100B via immunohistochemistry was showed. Furthermore, specific zonal arrangements of noncollagenous matrix components were formed. The microscopical analyses revealed significant differences, with respect to the used growth factors TGF-β1, TGF-β2, and TGF-β1 in combination with BMP-7. |
| Well known is the phenotypic shift of chondrocytes transferred from their in vivo environment to monolayer culture. This dedifferentiation process is characterized by a change of the cells from a hyaline cartilage chondrocyte to a more fibroblastic behaviour, indicated by a downregulation of collagen type II and proteoglycans, accompanied by an upregulation of collagen type I [14]. In contrast, the S100B protein could be detected even after several population doublings. Thirty years ago, the first description of S100 in human chondrocytes was published [34]. Among the S100 proteins, the S100B subtype is specifically expressed in chondrocytes [35]. S100B seems to be expressed at an earlier time point along the process of differentiation or redifferentiation, respectively and may therefore act as an indicator for early chondrocyte differentiation. This assumption is supported by the observation that S100B is a target of SOX9 and its co-activators, SOX5 and SOX6 [35]. The SOX trio are early expressed transcription factors during the process of cartilage differentiation and play an important role for the underlying signalling pathway [35,36]. The results have confirmed this typical behaviour of primary chondrocytes in proliferating monolayer culture (Figure 2). After two passages, only few cells expressed collagen type II, whereas increased Immunofluorescence (IF) signals for collagen type I were obtained. However, S100B was still expressed in all cells. In order to reverse the dedifferentiation process, self-aggregation was induced by cultivating the cells in a 3D-promoting environment, using the agar overlay technique [12] (Figure 1). This autonomous aggregation of cells is a kind of mimicry of the initial step of in vivo chondrogenesis. Using a scaffold-free system should avoid any negative aspects arising from scaffolds, like a change in environmental conditions by degradation of artificial matrix materials (e.g. acidic pH) [37]. |
| As initially stated, the present study was focusing on the influence of different members of the TGF-β superfamily on the redifferentiation potential of isolated monolayer-expanded chondrocytes in a 3D-model. Davies et al. [38] have placed emphasis on the role of TGF-β1 and other growth factors as mediators in promoting tissue repair by stimulating the production of major articular cartilage matrix components. In addition, corresponding receptors for the above mentioned growth factors were analyzed for young articular cartilage cells and monolayer expanded chondrocytes [39,40]. However, the effect of TGF-β on matrix metabolism in chondrocytes is controversial. Conflicting reports have demonstrated increase and decrease in proteoglycan synthesis in monolayer chondrocyte cultures [41,42]. Additional studies have demonstrated that TGF-β can either enhance differentiation and proliferation [43,44], or inhibit growth, which is believed to be dependent on the cell cycle phase of the chondrocytes [45]. Several studies have demonstrated that the response of chondrocytes to growth factors, including IGF-1, TGF-β1 and TGF-β2 is diminished with age and OA [38,46]. During the study, media supplementation with TGF-β1 and TGF-β2 resulted in unequal responses of the chondrocytes subjected to identical 3D culture conditions. The presence of TGF-β2 stimulated the synthesis of noncollagenous components in the extracellular matrix, visualized by a positive staining for proteoglycans in the whole microtissues (Figure 4C and G), whereby a longer culture time (four weeks) resulted in an increased deposition of proteoglycans indicated by a strong red color in the SO-staining. In contrast, addition of TGF-β1 to the culture media induced a zonal distribution of proteoglycans, restricted to the central and the peripheral part of the spheroid sections (Figure 4B and F). The intensity of the SO staining after TGF-β1 stimulation is always lower compared to the signal after TGF-β2 stimulation. The lowest SO signal is shown in cryosections of microtissues cultivated in basal medium (Figure 4A and E). With respect to proteoglycan synthesis visualized via SO staining, the microtissues displayed a gradual redifferentiation, starting from BM via adding TGF-β1 up to the supplementation of TGF-β2. Since limited effects of TGF-β1 on the redifferentiation of three-dimensional cartilage constructs in earlier experiments was observed, the impact of combining TGF-β1 with other chondrogenic growth factors, preferentially BMPs, should be tested. Shintani and Hunziker [47] reported the superior potency of BMP-7 over BMP-2 to induce chondrogenesis in bovine synovial chondrocytes. The analyses of Kim and Im [48] revealed that the chondrogenic potency of TGF-β2 in combination with BMP-7 is most effective compared with the BMPs 2, and 6. Thereby, a possible synergistic effect of both growth factors, TGF-β1 and BMP-7, should be examined. The answer to this question was positive as shown in Figure 4D and H. Already after two weeks, a dark red staining of the whole section for PG was detectable, which was even more intensive after four weeks. |
| Comparing the results for collagen type II expression (Figure 5) as typical cartilage matrix protein with the red staining pattern in SO-histology for proteoglycans reveals differences with respect to the onset of synthesis, and deposition of these two extracellular matrix components during the redifferentiation process initiated via cell aggregation, and additionally stimulated via different growth factors. Collagen type II synthesis was not able to be detected via antibodies in microtissues cultivated in basal medium up to an entire culture time of four weeks, whereas BM is sufficient to stimulate locally restricted PG synthesis. Naumann et al. [49] reported on the expression of cartilage specific matrix molecules, like collagen type II and GAG in macroaggregate cultures derived from monolayer expanded chondrocytes, even without adding any growth factors or cytokines. However, in contrast to the experiments, chondrocytes isolated from nasal septum and auricular cartilage was used. These particular differentiation characteristics of scaffold free in vitro tissues are often observed, using hyaline nasal cartilage or elastic auricular cartilage as cell source. This typical behavior was also observed in the lab, working with chondrocytes isolated from hyaline nasal cartilage [50]. Supplementation with TGF-β1 resulted in traces of fluorescence signals for collagen type II in a small peripheral rim of the spheroids after a two-week culture and a substantial accumulation of collagen type II with an enhancement in the outer part of the microtissue after four weeks. Here, the distribution is similar to the SO-staining, but less intensive. Supplementation with TGF-β2 did induce the expression of collagen type II in microtissues, already after an incubation time of two weeks, with increased signal intensity towards the peripheral part of the cryosection. Here and also after a culture time of four weeks, the collagen type II expression and the red SO stainings for PG are comparable with regard to patterning and intensity. These results may give first hints for TGF-β2 being a main differentiation stimulator in the used 3D cartilage tissue model. Adding BMP-7 to the TGF-β1 media to circumvent the limited differentiation of chondrocytes in media with TGF-β1 alone resulted in a remarkable increase in collagen type II deposition. Nevertheless, the expression pattern did not match with the results for PG. Collagen type II is mainly deposited inside the microtissues omitting several cell layers in the peripheral rim, whereas the proteoglycans were distributed all over the constructs. In case of four week cultivation, an increase of collagen type II expression was obtained in each medium composition with the exception of BM. Chondrocytes in microtissues cultivated in basal medium did not express the collagen type II protein. |
| Adding the S100B protein expression profile (Figure 6) to the above described and discussed pattern of cartilage specific markers, especially collagen type II, may give hints for the relevance of S100B in the (re-)differentiation process of (in vitro) cartilage tissue. Cultivation of spheroids only in BM (4 weeks) resulted already in the expression of the S100B protein in remarkable amounts. Adding TGF-β1 resulted in the fluorescence-based detection of the S100B protein in nearly all cells in the constructs after four weeks in culture, visible via the colocalization of blue (cell nuclei) with red (intracellular S100B protein) fluorescence signals (Figure 6F). Media supplementation with TGF-β2 or TGF-β1 plus BMP-7 revealed a robust expression of S100B on cryosections of the constructs. Remarkably, the observed expression patterns for this protein correlate with the results for collagen type II. A four-week maturation time of cell aggregates in basal medium with/without the additives TGF-β1, TGF-β2, or TGF-β1 plus BMP-7 resulted in the expression of S100B. However, collagen type II was not expressed in microtissues cultivated in BM and was expressed only in low amounts in microtissues cultivated in the presence of TGF-β1. As already shown for collagen type II, a clear time dependence of the S100B expression was revealed. All tested media were able to increase the amount of S100B in the analysed hyaline cartilage constructs, comparing the expression results for two weeks and four weeks culture. Taken together, these results may strengthen the potential role of the S100B protein in the temporal pattern of cartilage (re-)differentiation processes. |
| In order to draw a conclusion regarding the redifferentiation potential of engineered hyaline cartilage constructs, the analysis of typical dedifferentiation markers was of major interest. Due to the phenomenon that chondrocytes originating from knee joint tend to shift their expression from collagen type II to collagen type I in cell culture, mainly in monolayer culture, the latter protein is suitable for a characterization with respect to dedifferentiation. Cultivation of cartilage tissue constructs in basal and TGF-β1 enriched medium did not show any fluorescence signals related to collagen type I. That means, the three-dimensional arrangement of collagen type I expressing chondrocytes itself was an initial event to start the redifferentiation process, indicated by a down regulation of collagen type I in spheroids. Nevertheless, supplementation with TGF-β2 or TGF-β1 plus BMP-7 resulted in a moderate deposition of collagen type I inside the microtissues. Thereby, the area of accumulation is similar to the collagen type II expression. A possible co-expression of both collagen proteins might result from a general stimulation of collagen synthesis by the used growth factors. |
| Summarizing the results, it was stated that addition of the growth factors, TGF-β1 or TGF-β2 is positively affecting the process of redifferentiation during the generation of hyaline cartilage microtissues, initiated with cells augmented in monolayer culture. Furthermore, a clear time dependency of this process was obvious. The best redifferentiation state of the microtissues was obtained after four weeks in culture. With respect to proteoglycan synthesis and collagen type II expression, a growth factor specific pattern was obtained. Based on the results of SO-staining and immunohistochemistry for collagen type II and S100B TGF-β2, S100B TGF-β2 was superior in comparison to TGF-β1. This observation is surprising, since both ligands (TGF-β1 or TGF-β2) use a similar signal transduction pathway. In general, the signalling is accompanied by the assembly of a heterotetrameric complex of paired type I (TGF-βRI) and type II (TGF-βRII) receptors. Nevertheless, activation of the receptors depends on the isoform of the ligand. Taking this prerequisite into account, a different binding affinity and kinetic of the used ligands, with respect to their corresponding receptors is thinkable. This may lead to a specific pattern of activation. Moreover, signalling via TGF-β2 requires an additional co-receptor (TGF-βRIII or betaglycan), and is therefore, distinguishable from TGF- β1-signalling [51]. Depending on the expression of this co-receptor in the used chondrocytes, a preferred TGF-β2-signalling could be possible, which might explain the stronger differentiation promoting effect of TGF-β2. |
| Otherwise, in case of TGF-β2 treatment, the increased expression of hyaline cartilage markers was accompanied by an increasing level of collagen type I, indicating an unwanted development towards cartilage with reduced hyaline quality. However, the reduced ability of TGF-β1 to induce the redifferentiation process in cartilage microtissues observed in previous experiments was rescued by the application of BMP-7. In addition to the above described results, microtissues were cultivated in basal medium supplemented only with BMP-7. Thereby, a re-differentiation pattern similar to the TGF-β1 situation was observed. Due to the promising results of the TGF-β2 treatment on the redifferentiation of articular chondrocytes in the presented 3D-model, the combination of TGF-β2 and BMP-7 was not tested. But, a further increase in collagen type II and proteoglycan expression was assumed. Consequently, the differentiation promoting effect of the TGF-β superfamily member BMP-7 should be tested in future experiments, in combination with TGF-β2 for a further improved generation of cartilaginous microtissues. |
| Acknowledgment |
| The authors acknowledge the financial support of the project by the Federal Ministry of Education and Research, Germany (support code 1787X08). |
| References |
|
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
 |
 |
 |
| Figure 5 | Figure 6 | Figure 7 |
Post your comment
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14368
- [From(publication date):
specialissue-2013 - Dec 19, 2025] - Breakdown by view type
- HTML page views : 9611
- PDF downloads : 4757
