Research Article Open Access
Dot-ELISA Affinity Test: An Easy, Low-Cost Method to Estimate Binding Activity of Monoclonal Antibodies
Svobodova Z1*, Jankovicova B1 , Horak D2 and Bilkova Z1
1Department of Biological and Biochemical Sciences, Faculty of Chemical Technology, University of Pardubice, Studentska 573, 532 10 Pardubice, Czech Republic
2Institute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, Heyrovsky Sq. 2, 162 06 Prague 6, Czech Republic
- *Corresponding Author:
- Svobodova Zuzana
Department of Biological and Biochemical Sciences
Faculty of Chemical Technology, University of Pardubice
Studentska 573, 532 10 Pardubice, Czech Republic
Tel: +420 46 6037700
E-mail: zuzana.svobodova@upce.cz
Received date: June 07, 2013; Accepted date: June 26, 2013; Published date: June 28, 2013
Citation: Svobodova Z, Jankovicova B, Horak D, Bilkova Z (2013) Dot-ELISA Affinity Test: An Easy, Low-Cost Method to Estimate Binding Activity of Monoclonal Antibodies. J Anal Bioanal Tech 4:168. doi: 10.4172/2155-9872.1000168
Copyright: © 2013 Svobodova Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Selecting the “right” monoclonal antibody (mAb) for an immunoaffinity-based application can be tricky, as many mAb producers offer a wide range of mAb clones against molecular structures of interest. Since there are significant differences in the quality of mAb clones, and particularly in their binding activity, an easy method for quick and low-cost comparison of various mAb clones was developed. The dot-ELISA affinity test is a simple, versatile and instrumentally no demanding technique, since it requires no expensive equipment (such as an ELISA reader or chemiluminescence/fluorescence imaging system) and can be performed in any biochemical laboratory. This method is based on a previously described dot-ELISA technique that is improved with a chaotropic step using different concentrations of ammonium thiocyanate in the range 0-2 M. In this work, the dot-ELISA affinity test was optimized on Aβ peptide as antigen and anti-Aβ mAb. Such protocol was then applied to a panel of eight anti-EpCAM (epithelial cell adhesion molecule) mAbs which should be subsequently used for preparation of magnetic immunosorbent to capture circulating tumor cells (CTCs).
Keywords
Dot-ELISA; Affinity; Monoclonal antibody; Dot blot; Chaotropic elution; EpCAM
Abbreviations
Ag: Antigen; BSA: Bovine Serum Albumin; CTC: Circulating Tumor Cell; ELISA: Enzyme-Linked Immunosorbent Assay; EpCAM: Epithelial Cell Adhesion Molecule; HRP: Horse Radish Peroxidase; IC50: 50% Inhibitory Concentration; IntDen: Integrated Density; mAb: Monoclonal Antibody; PBS: Phosphate-Buffered Saline; PVDF: Poly Vinylidene Difluoride
Introduction
Monoclonal antibodies (mAbs) are used today in a wide range of research and clinical applications, including as diagnostic or research reagents in biotechnologies as well as in human therapy. The major turning point in the use of mAbs occurred with the hybridoma technique for mAb production described by Köhler and Milsteinin [1]. Since that time, mAbs have been produced on a large scale. The market now offers an immense number of different mAb clones reacting with specific antigen (Ag) epitopes. When searching for a suitable mAb clone, therefore, we usually obtain a list of multiple companies selling antibodies of particular specificity (regarding species, antigen, type [primary or secondary], etc.) [2]. However, information about Ab affinity for the specific Ag is usually not provided. One reason for this could be the fact that affinity might be influenced by the conditions of the immunoassay methodology and/or antigen used [3]. Our experience has been that even the same antibody clones with the same epitope specificity coming from various providers differ not only by price, transport medium additives (e.g., BSA, trehalose, sodium azide) and storage stability but most importantly by their affinity for the target antigen. Hence, if we are not interested in absolute numeric determination of affinity constant but need only to know whether particular mAb clones really react with the antigen, then to select one with the desired affinity an easy and affordable technique known as the dot-ELISA affinity test may be applied. This simple, semi-quantitative method combines the principle of dot-ELISA technique with a modified ELISA test using a chaotropic reagent for determining the avidity index of a polyclonal antibody [4,5] and/or the affinity index of the mAb [6,7].
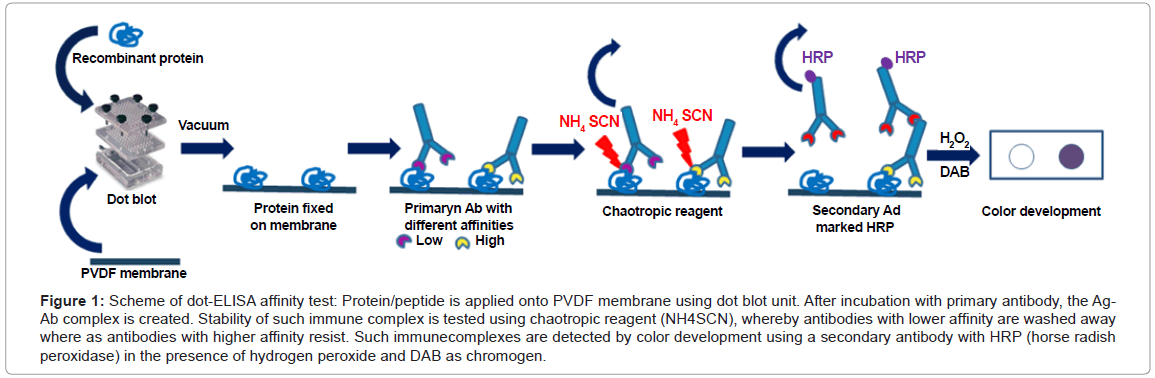
Conventional dot-ELISA technique [8,9], also known as dotimmunobinding assay [10,11] is a highly versatile solid-phase immunoassay for antibody or antigen detection on blotting membrane. Protein samples to be identified are spotted through circular templates of dot blot manifold using vacuum directly onto the blotting membrane and immunoblotting detection follows [8,12,13]. In the newly developed dot-ELISA affinity test, a chaotropic step is implemented into a conventional dot-ELISA protocol and coming immediately after the creation of immune complexes (Ag-Ab) on the blotting membrane. It consists in incubating membrane squares with increasing molarity of the chaotropic reagent ammonium thiocyanate from 0 to 2 mol/L. The resistance of the immune complex to the action of ammonium thiocyanate correlates with the affinity index of the mAb clone. According to MacDonald et al. [6], the affinity index is expressed as the molarity of chaotropic reagent causing 50% reduction in the initial optical density, known as the 50% inhibitory concentration (IC50). The higher mAb’s affinity index the higher the concentration of ammonium thiocyanate the immune complex is able to resist. Presence of immune complexes on the membrane was detected directly using enzymeconjugated immunoglobulins and substrate (Figure 1).
Figure 1: Scheme of dot-ELISA affinity test: Protein/peptide is applied onto PVDF membrane using dot blot unit. After incubation with primary antibody, the Ag- Ab complex is created. Stability of such immune complex is tested using chaotropic reagent (NH4SCN), whereby antibodies with lower affinity are washed away where as antibodies with higher affinity resist. Such immunecomplexes are detected by color development using a secondary antibody with HRP (horse radish peroxidase) in the presence of hydrogen peroxide and DAB as chromogen.
First, we developed and validated the dot-ELISA affinity protocol using a biospecific pair of anti-AβmAb and Aβ 1-42 peptides. The verified protocol was subsequently applied on a panel of eight anti- EpCAM (epithelial cell adhesion molecule) mAb clones from five different suppliers to estimate their binding activity. Our goal was to use this innovated method for selecting an anti-EpCAM mAb clone to prepare magnetic immunosorbent for capture of circulating tumor cells (CTCs), because one of the relevant markers of CTC is high density of EpCAM structure located on their surface. Next, three anti-EpCAM clones with parameters in compliance with our requirements were used in preparing such magnetic immunosorbent. Their ability to capture EpCAM-positive cells was proven and compared with results from the dot-ELISA affinity test. Final observations and possible applications of the dot-ELISA affinity test are discussed here.
Materials and Methods
Materials
Bovine serum albumin (BSA), phosphate-buffered saline (PBS), Tween 20, NiCl2, N-(3-dimethylaminopropyl)-N′-ethyl carbodiimide hydrochloride (EDC), MES [2-(N-morpholino) ethane sulfonic acid], goat anti-mouse IgG with HRP (horseradish peroxidase), and anti- Aβ mAb (clone NAB228) were purchased from Sigma-Aldrich (St Louis, MO, US).Mouse monoclonal anti-human EpCAM IgG1 clones were acquired as follows: 323/A3 was from Abcam (Cambridge, UK); HEA-125 from BioPrime (Aachen, Germany); C-10, 323/A3, KS/1 and HEA-125; from Santa Cruz Biotechnologies (St. Cruz, CA, US); 4A8H7D12 from Sino Biological (Beijing, China) and HEA-125from Progen Biotechnik (Heidelberg, Germany). NH4SCN and H2O2 were provided by Penta (Chrudim, Czech Republic), recombinant EpCAM protein was from Sino Biological (Beijing, China), and antigen Aβ 1-42 was from Apronex (Jesenice, Czech Republic). 3,3′-Diaminobenzidine tetrahydrochloride (DAB) and PVDF membrane (ImmunoBlot™ PVDF Membrane, 0.2 μm porosity) were purchased from BioRad (Hercules, CA, US) and Falcon-Multiwell tissue culture polystyrene plate with flat bottom was from Becton Dickinson (Lincoln Park, NJ, US). Magnetic monodisperse macroporous poly(glycidyl methacrylateco- 2-[(methoxycarbonyl)methoxy]ethyl methacrylate-co-ethylene dimethacrylate) microspheres, 4 μm in diameter-for short, P(GMAMOEAA- EDMA)-NH2 microspheres with carboxyl group-were provided by the Institute of Macromolecular Chemistry AS CR (Prague, Czech Republic). Dulbecco’s modified Eagle’s medium (DMEM), fetal calf serum, penicillin-streptomycin antibiotics and insulin were all purchased from Life Technologies (Carlsbad, CA, USA). The human breast adenocarcinoma MCF7 cell line was purchased from Health Protection Agency Culture Collections (Salisbury, UK).
Dot-ELISA affinity test
Recombinant antigen (1-8 μg/dot) was applied on PVDF membrane using a DHM-96 dot blot manifold (Scie-Plas, Cambridge, UK) providing 96 dots, 3 mm in diameter. This was connected to a vacuum pump rated at 600 mm Hg (0.8 Bar). After dotting onto the membrane, the Ag was left to dry for several minutes. Subsequently, the membrane was blocked using 5% BSA in PBS-T (PBS with 0.05% Tween 20) for 1h at room temperature (RT) under gentle orbital shaking. The blocking reagent was then poured off and the membrane was cut into small squares, with one dot per square of membrane. These squares were inserted into a 20-well tissue culture plate with flat bottom, where they were incubated with 0.5 ml of tested antibody (13 μM of IgG in PBS-T with 0.25% BSA) at RT under gentle orbital shaking for 1 h. The membrane squares were washed with PBS-T 3X (quick wash) and 3× incubated with PBS-T for 5 min at RT under gentle orbital shaking. Then the membrane with Ag-Ab complexes on its surface was subjected to the chaotropic reagent treatment. NH4SCN at different molarities (0-2 M) was added to the membrane and precise incubation at RT and mild shaking for 5 min followed. Immediately after incubation, the reagent was replaced by PBS-T and a washing step similar to the previous one was performed. Finally, the secondary antibody (goat anti-mouse IgG with HRP) diluted 1:10,000 with PBS-T was incubated with the membrane squares for 1 h at RT under gentle orbital shaking. The DAB substrate solution was prepared: 5 mg of DAB was diluted in 10 ml of tris-buffered saline and then 10 μl of 3% H2O2 and 90 μl of solution NiCl2 (80 mg/ml) were added. After short incubation, development of a purple-pink color of insoluble substrate product on the dot was observed by naked eye or camera. Images were taken and examined using the free software Image J (Wayne Rasband, Research Services Branch, National Institute of Mental Health, Bethesda, MD, US) and the integrated density of spots was determined.
Magnetic anti-EpCAM immunosorbent preparation
The magnetic P (GMA-MOEAA-EDMA)-NH2 microspheres with carboxyl group were 3× washed with binding buffer (10 mM MES with 50 mM NaCl; pH 6). Then the microspheres were activated by 0.06 M EDC solution in binding buffer and the appropriate amount of anti- EpCAM antibody (50 μg/mg of magnetic microspheres) in binding buffer was added. The coupling proceeded at room temperature for 2 h under stirring. The magnetic microspheres with immobilized anti- EpCAM antibody were 5× washed with binding buffer and stored at 4-8°C in PBS (pH 7.4) containing 0.05% sodium azide.
Immunomagnetic capture of EpCAM-positive cells
The magnetic anti-EpCAM immunosorbents (0.5 mg per tube) were 3× washed with 1 mL of PBS (pH 7.4) containing 0.1% BSA using a magnetic separator from Life Technologies (Carlsbad, CA, USA). They were then mixed with 2×106 of EpCAM-positive cells (MCF7 cell line) and rolled in a tube rotator for 30 min at RT. In the next step, the microspheres with immuno captured MCF7 cells were 5× washed with PBS (pH 7.4) containing 0.1% BSA to remove all unbounded cells, always using a new Eppendorf tube. The captured cells were subsequently observed and counted in a Bürker’s chamber using a Nikon Eclipse 80i microscope (Tokyo, Japan) equipped with Nikon Plan Fluor 10×, 20×, 40× and 60× objective lenses and a Nikon digital sight DS-MS camera. The images were acquired and processed using NIS-Elements AR Analysis 3.2 software (Nikon, Tokyo, Japan) and a procedure described by Horak et al. [14]. The MCF7 cells used in immunomagnetic separation experiments (expressing EpCAM molecules) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum, 1% penicillin-streptomycin antibiotics and 0.01 mg/ml insulin at 37°C in a humidified 5% CO2 atmosphere.
Results and Discussion
The aim of our study was to develop a simple method capable semiquantitatively to predict which of several tested clones are able to satisfy our demands for affinity of mAb clone. The method was based upon dot-ELISA for several reasons. First, this assay allows simultaneous processing of multiple mAb clones and the entire operational procedure requires just 4-6 h, as reported also by other authors [15]. Second, it enables easy loading of protein directly onto membrane which is matter of minutes, and thus it saves time compared to conventional ELISA where loading of protein to well wall is a matter of (often several) hours. Moreover, according to Bakkali et al. [16], a method based on dot-ELISA does not require a purified Ag due to the higher binding capacity of blotting membrane. Moreover, it has lower background and higher sensitivity compared to conventional ELISA.
Finally, the technical steps involved in the assay and required equipment are much simpler than in other immunoassays. An ELISA reader or chemiluminescence imager is not required, for example, as we need only a common camera and readily accessible software for processing of images. If a chemiluminescence imager is at hand in the laboratory, on the other hand, then the sensitivity of the test could be increased and therefore consumption of Ag and Ab decreased.
The dot-ELISA affinity test conditions were optimized over a series of experiments on an Aβ peptide-anti-Aβ mAb system to obtain the ideal ratio of antigen and antibodies as well as concentration range of chaotropic reagent (ammonium thiocyanate). The measured data and their statistics are presented herein. The selection of procedure conditions is first described, and then the potential influence of chaotropic reagent on the Ag adsorbed on the blotting membrane is discussed. Finally, the affinity index of the model Aβ-anti-Aβ system is determined and selection of an anti-EpCAM clone with high affinity is made.
Optimization of dot-ELISA affinity test conditions
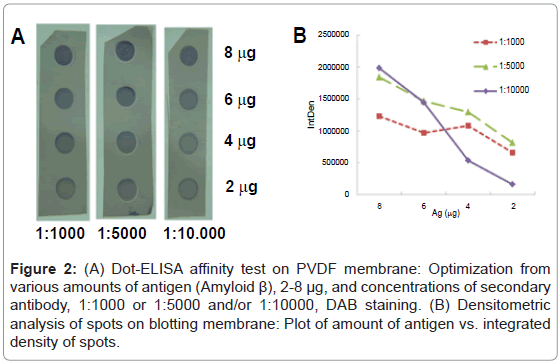
First, a suitable concentration of mAb was selected. Two concentrations of anti-AβmAb-1:1000 (13 μM in PBS-T with 0.25% BSA) and/or 1:4000 (53 μM)-were tested using dot-ELISA affinity test. The chaotropic treatment was applied at levels 0, 0.5, 1.0, 1.5 and 2.0 M of ammonium thiocyanate. The concentration of primary Ab (1:4000) was too low to detect the differences between integrated densities of spots, and hence it was concluded that the concentration 1:1000 would afford better sensitivity and such dilution of primary Ab was used in the following experiments. The main issue was to prepare spots with sufficient density of immuno complexes to monitore the unfolding effect of chaotropic agent applied after but also to avoid the multilayering effect in case of overdosed of secondary antibodies. Therefore, various amounts of antigen Aβ 1-42 (2, 4, 6 and 8 μg) were spotted onto membrane in triplicate. Subsequently, it was incubated with primary antibody and after a washing step the membrane was cut into 3 squares and each was incubated with a different concentration of secondary antibody (1:1000, 1:5000 and 1:10,000). The chaotropic step with NH4SCN was omitted in this experiment. The membrane developed with DAB is shown in Figure 2A. The graph of densitometric analysis of stained spots on the membrane (Figure 2B) shows their large differences in integrated densities. For the 8 μg of Ag and antibody titer 1:10,000 provided the highest sensitivity, it means big difference in the values of the measured signal at small change of input parameters. Hence, the probability of multilayering or nonspecific adsorption is minimized under these conditions.
Figure 2: (A) Dot-ELISA affinity test on PVDF membrane: Optimization from various amounts of antigen (Amyloid β), 2-8 μg, and concentrations of secondary antibody, 1:1000 or 1:5000 and/or 1:10000, DAB staining. (B) Densitometric analysis of spots on blotting membrane: Plot of amount of antigen vs. integrated density of spots.
Testing of antigen stability on membrane after chaotropic treatment
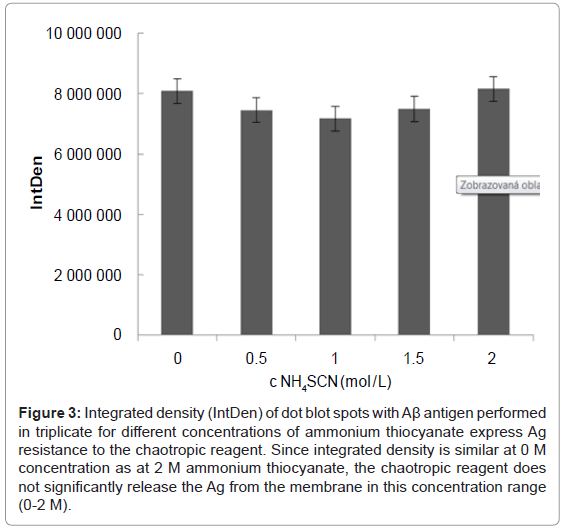
Since Ag on the blotting membrane is only adsorbed and is not fixed covalently, there could be uncertainty as to whether the chaotropic reagent might release not only the antibody from the immune complex but the entire immune complex from the PVDF membrane. This could lead to distorted results, and therefore this effect was studied. An identical amount of recombinant Ag (8 μg) was spotted onto the membrane in triplicate for each concentration of ammonium thiocyanate (0, 0.5, 1, 1.5 or 2 M). In addition, three dots without spotted Ag were used as blank. To observe the influence of Ag on the membrane, the chaotropic treatment preceded incubation of the membrane squares with primary and secondary Ab. Figure 3 shows a densitometric analysis for each of the 5 tested concentrations of ammonium thiocyanate performed in triplicate. Within given triplicates, the standard deviation was 0.8-3.08% (average 1.28%) while the standard deviation between the different concentrations of ammonium thiocyanate was only 5.4%. Therefore, we concluded that the chaotropic reagent in the concentration range of 0-2 M NH4SCN does not significantly influence the stability of the adsorbed Ag.
Figure 3: Integrated density (IntDen) of dot blot spots with Aβ antigen performed in triplicate for different concentrations of ammonium thiocyanate express Ag resistance to the chaotropic reagent. Since integrated density is similar at 0 M concentration as at 2 M ammonium thiocyanate, the chaotropic reagent does not significantly release the Ag from the membrane in this concentration range (0-2 M).
Evaluation of anti-Aβ antibody affinity
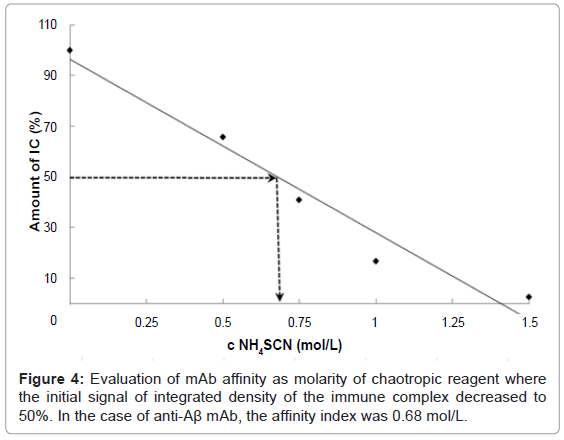
The dot-ELISA affinity test was optimized on an Aβ and anti-Aβ mAb model system. The affinity of mAb was expressed as affinity index, meaning the molarity of chaotropic reagent such that the signal of the immune complex decreased to 50% of the initial integrated density, also known as the 50% inhibitory concentration (IC50%). The solution of recombinant Ag (8 μg/dot) was spotted onto PVDF membrane. After incubation with anti-Aβ mAb, there followed the chaotropic step using ammonium thiocyanate at different concentrations (0, 0.25, 0.5, 0.75, 1.0, 1.25, 1.5 M). In addition, three dots were filled only with PBS-T (without Ag) to evaluate the nonspecific adsorption of the PVDF membrane and the integrated density of such dots was used as blank. Repeatedly measured, we observed correlation between the concentration of chaotropic reagent and integrated density of dots on the membrane. The standard deviation between the triplicates of integrated densities was in the range 0.2% to 1.17%. The affinity index of the anti-Aβ mAb was estimated as 0.68 mol/L (Figure 4). In other words, 0.68 M ammonium thiocyanate was sufficiently strong to release 50% of the initial amount of the created immune complexes of Aβ (1- 42) and anti-Aβ mAb from the membrane.
Evaluation of anti-EpCAM antibodies affinity
In our research, we were considering the question of how to select a high-affinity anti-EpCAM mAb clone for preparing magnetic immunosorbent to capture EpCAM-expressing cells from among the huge number of anti-EpCAM clones available on the market. We first reduced the number of possible mAb clones by selecting desired epitope specificity. In our case, this was an extracellular part of the EpCAM transmembrane protein and specifically a region among the first 59 aminoacids and which should be easily accessible for antibodies immobilized on magnetic carrier. The next criteria for clone selection were whether it already had been used in some published work and/ or originated from a well-known antibody producer. Finally, we considered the factors of price, presence of detergents, preservatives, stabilizing additives, and also availability. Thus, we ended up with 8 mAb clones from 5 different suppliers which would be worthwhile to evaluate by our dot-ELISA affinity test (Table 1). Since clones 323/ A3 and HEA-125 seemed promising according to published papers [17,18], those from various providers were tested. The results of the dot-ELISA affinity test are presented in the same table. To simplify the results, the affinity index was expressed as (+), (++) or (+++). Antibody clones rated (+) did not resist 1M ammonium thiocyanate, and thus integrated density decreased significantly. Clones rated (++) resisted 1M ammonium thiocyanate but not 2M, and those rated (+++) were designated as clones for which integrated density did not decrease even when 2M ammonium thiocyanate was applied. All three tested HEA- 125 clones from different providers showed the highest resistance to the chaotropic reagent. We based our final clone selection among the three providers based upon their composition of transporting buffer, because such storage additives as BSA or gelatin in the bulk of mAb are undesirable for magnetic immunosorbent preparation. Thus, we evaluated as most suitable the HEA-125 clone from Progen Biotechnik.
| Clone | Supplier | Storage additive | Dot-ELISA |
|---|---|---|---|
| HEA-125 | BioP | 0.5% BSA | +++ |
| C-10 | SC | Gelatin | ++ |
| 323/A3 | AbC | - | + |
| 323/A3 | SC | Gelatin | + |
| HEA-125 | SC | Gelatin | +++ |
| KS/1 | SC | Gelatin | +++ |
| 4A8H7D12 | SB | 5% trehalose | ++ |
| HEA-125 | PB | - | +++ |
BioP–BioPrime, SC–Santa Cruz Biotechnologies, AbC–Abcam, SB–Sino Biological, PB–ProgenBiotechnik.
Table 1: Tested clones of mouse monoclonal anti-EpCAM antibodies to extracellular part of EpCAM antigen.
Immunomagnetic capture of EpCAM-positive cells in batch arrangement
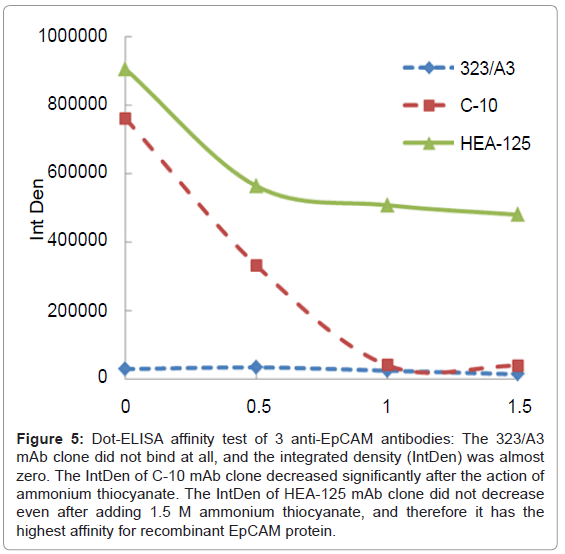
Three mAb clones with various affinities - HEA-125 (BP, +++), C-10 (SC, ++) and 323/A3 (AbC, +)-that had been previously tested using the dot-ELISA affinity test (Figure 5) were used for preparing magnetic immunosorbent. Their immuno capture efficiency of MCF7 cells was subsequently evaluated according to the procedure described in Immunomagnetic capture of EpCAM-positive cells. The results are presented in Table 2. The clone 323/A3 which showed no affinity for EpCAM antigen in the dot-ELISA affinity test also did not bind the MCF7 cells. The immunosorbent using mAb C-10 with the affinity index 0.50 mol/L demonstrated an immuno capture ability of just 20,000 cells/mg of carrier, whereas the immunosorbent with mAb HEA-125, which had the highest affinity index (1.02 mol/L), showed the highest number of immuno captured EpCAM+ cells (440,000 cells/ mg of immunosorbent). These results illustrate that the mAb capture ability was correlated with the results obtained by our dot-ELISA affinity test. We may conclude that the dot-ELISA affinity test is able to predict the reactivity of antibodies to target antigen and thus will save time and resources in case of such time-consuming or costly procedures as magnetic immunosorbent preparation. By pretesting mAbs before their implementation into our immunoassay, therefore, we may easily determine that any problems which might occur during its set-up were not caused by the mAb.
Figure 5: Dot-ELISA affinity test of 3 anti-EpCAM antibodies: The 323/A3 mAb clone did not bind at all, and the integrated density (IntDen) was almost zero. The IntDen of C-10 mAb clone decreased significantly after the action of ammonium thiocyanate. The IntDen of HEA-125 mAb clone did not decrease even after adding 1.5 M ammonium thiocyanate, and therefore it has the highest affinity for recombinant EpCAM protein.
| Clone | Supplier | Dot ELISA affinity test | Number of captured cells |
|---|---|---|---|
| HEA-125 | BioP | +++ | 440,000 |
| C-10 | SC | ++ | 20,000 |
| 323/A3 | AbC | + | 0 |
Table 2: Summary of results obtained from dot-ELISA affinity test and immunomagnetic separation of EpCAM-positive cells.
Conclusion
This dot-ELISA affinity test is intended for research groups searching for a suitable mAb clone for their immunoassays with a view to its affinity for target Ag that can be a recombinant or even nonpurified protein or peptide. This method can serve for easy, low-cost comparison of a panel of mAb clones and may be performed in any biotechnological laboratory since it requires only a dot blot manifold, orbital shaker, camera and PC. Using this method we may test quality of mAbs in relation to their affinity and/or specificity. This is especially useful for immunoanalytical methods where we need a certain level of sensitivity or selectivity, such as immunoaffinity-based or EIA methods and/or immunohistochemistry.
Acknowledgements
Financial support of the European Union’s projects CaMiNEMS (No. 228980) and NADINE (No. 246513) are gratefully acknowledged, as well as the grants from the Ministry of Education of Czech Republic No. 7E09080 and Czech Science Foundation No. P206/12/0381, and Ministry of Education, Youth and Sports of the Czech Republic Project CZ.1.07/2.3.00/30.0021 “Enhancement of R&D Pools of Excellence at the University of Pardubice”.
References
- Köhler G, Milstein C (1975) Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256: 495-497.
- Pepper DS (1992) Selection of Antibodies for Immunoaffinity Chromatography. Methods Mol Biol 11: 135-171.
- Schots A, Van der Leede BJ, De Jongh E, Egberts E (1988) A method for the determination of antibody affinity using a direct ELISA. J Immunol Methods 109: 225-233.
- Hall TJ, Heckel C (1988) Thiocyanate elution estimation of relative antibody affinity. J Immunol Methods 115: 153-155.
- van Dam GJ, Verheul AF, Zigterman GJ, de Reuver MJ, Snippe H (1989) Estimation of the avidity of antibodies in polyclonal antisera against Streptococcus pneumoniae type 3 by inhibition ELISA. Mol Immunol 26: 269-274.
- Macdonald RA, Hosking CS, Jones CL (1988) The measurement of relative antibody affinity by ELISA using thiocyanate elution. J Immunol Methods 106: 191-194.
- Romero-Steiner S, Holder PF, Gomez de Leon P, Spear W, Hennessy TW, et al. (2005) Avidity determinations for Haemophilus influenzae Type b anti-polyribosylribitol phosphate antibodies. Clin Diagn Lab Immunol 12: 1029-1035.
- Wang Z, Yu D, Li X, Zeng M, Chen Z, et al. (2012) The development and application of a Dot-ELISA assay for diagnosis of southern rice black-streaked dwarf disease in the field. Viruses 4: 167-183.
- Pappas MG (1988) Recent applications of the Dot-ELISA in immunoparasitology. Vet Parasitol 29: 105-129.
- Pappas MG, Hajkowski R, Hockmeyer WT (1983) Dot enzyme-linked immunosorbent assay (Dot-ELISA): a micro technique for the rapid diagnosis of visceral leishmaniasis. J Immunol Methods 64: 205-214.
- Piña R, Gutiérrez AH, Gilman RH, Rueda D, Sifuentes C, et al. (2011) A dot-ELISA using a partially purified cathepsin-L-like protein fraction from Taenia solium cysticerci, for the diagnosis of human neurocysticercosis. Ann Trop Med Parasitol 105: 311-318.
- Hawkes R, Niday E, Gordon J (1982) A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem 119: 142-147.
- Sulimenko T, Dráber P (2004) A fast and simple dot-immunobinding assay for quantification of mouse immunoglobulins in hybridoma culture supernatants. J Immunol Methods 289: 89-95.
- Horak D, Svobodova Z, Autebert J, Coudert B, Plichta Z, et al. (2013) Albumin-coated monodisperse magnetic poly(glycidyl methacrylate) microspheres with immobilized antibodies: Application to the capture of epithelial cancer cells. J Biomed Mater Res A 101: 23-32.
- Sumi S, Mathai A, Radhakrishnan VV (2009) Dot-immunobinding assay. Methods Mol Biol 536: 89-93.
- Bakkali L, Guillou R, Gonzague M, Crucière C (1994) A rapid and sensitive chemiluminescence dot-immunobinding assay for screening hybridoma supernatants. J Immunol Methods 170: 177-184.
- Balzar M, Winter MJ, de Boer CJ, Litvinov SV (1999) The biology of the 17-1A antigen (Ep-CAM). J Mol Med (Berl) 77: 699-712.
- Yaziji H, Battifora H, Barry TS, Hwang HC, Bacchi CE, et al. (2006) Evaluation of 12 antibodies for distinguishing epithelioid mesothelioma from adenocarcinoma: identification of a three-antibody immunohistochemical panel with maximal sensitivity and specificity. Mod Pathol 19: 514-523.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 25113
- [From(publication date):
August-2013 - Nov 30, 2025] - Breakdown by view type
- HTML page views : 19915
- PDF downloads : 5198