Case Report Open Access
Esophageal Obstruction Associated with Enteral Feedings with Nepro®: an Unreported Event
Nonihal Singh*, Suman BThapamagar and Shanker Mukherjee
Department of Internal Medicine, Easton Hospital, Easton, PA, USA
- Corresponding Author:
- Nonihal Singh, MD
Internal Medicine Residency Program
Easton Hospital, 250, South 21st Street
Easton, PA 18042, USA
Tel: 610-250-4515
Fax: 610-250-4833
E-mail: nonihal_singh@chs.net
Received Date: June 11, 2013; Accepted Date: July 24, 2013; Published Date: July 26, 2013
Citation: Singh N, Thapamagar SB, Mukherjee S (2013) Esophageal Obstruction Associated with Enteral Feedings with Nepro®: an Unreported Event. J Gastroint Dig Syst S1:006 doi: 10.4172/2161-069X.S1-006
Copyright: © 2013 Takahashi T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
We report a unique case of esophageal obstruction related to orogastric (OG) tube feedings with Nepro. The patient was an 84 year old male who was on mechanical ventilation for severe respiratory distress. He was started on Nepro® via an OG tube. Seven days later, while reinserting the OG tube, an esophageal obstruction was identified. On the same day, esophago-gastroduodenoscopy was done for esophageal disimpaction. Subsequently, we concluded that the Nepro® feed, which precipitated and solidified, caused obstruction of the entire esophagus. We report that Nepro® feeds have the property of solidifying and causing obstruction especially in recumbent patients who do not have the benefit of gravity for food propulsion. Such obstructions have been reported for other enteral nutrition formulations, but to the best of our knowledge, this is the first reported case for Nepro® and hence it should be added to the list of complications.
Keywords
Nepro®; Esophago-gastroduodenoscopy; Orogastric tube; Enteral feeds; Esophageal obstruction
Introduction
The superiority of enteral nutrition to parenteral nutrition in reducing morbidity in critically ill patients is well-known and documented [1]. Enteral feeding is administered into the gastrointestinal (GI) tract via a tube usually called a feeding tube, categorized as a nasogastric, gastrostomy or jejunal tube [2]. When started early in the course of hospitalization, enteral feeds help preserve the gut immune function and reduce inflammation [3]. The known complications of enteral feeding include: blocked tube, hyperglycemia, esophageal reflux, diarrhea and aspiration pneumonia [2]. We report a case of esophageal obstruction related to orogastric tube feedings with Nepro®.
Case Report
A 84-year-old male, with end-stage renal disease on hemodialysis, presented with shortness of breath. On admission, he was dyspenic and in severe respiratory distress. He was subsequently intubated and mechanically ventilated. On second day of mechanical ventilation, he was started on Nepro® via an OG tube.
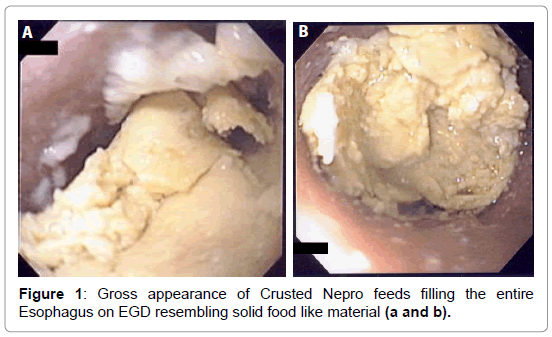
On day seven, a bronchoscopy was performed to evaluate for possible mucus plugs in the airway. During the procedure the OG tube was dislodged. Several attempts were made to replace the feeding tube with no success. Each attempted insertion met with resistance when pushing the tube down in the esophagus. A gastroenterology (GI) consult was requested for OG tube placement. The gastroenterologist was also unsuccessful and subsequently an EGD was performed. When the endoscope was inserted, a solid food-like material was seen just below the cricopharyngeus muscle which was initially thought to be impacted chicken-meat (Figure 1). Since the patient was intubated with acute respiratory failure for more than a week, this hypothesis was quite implausible.
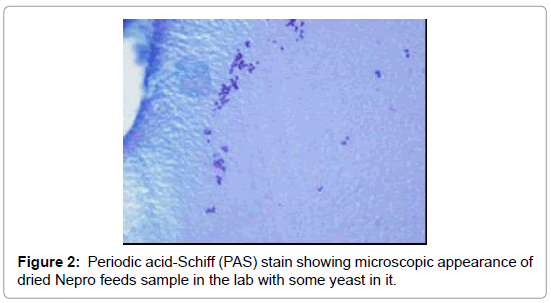
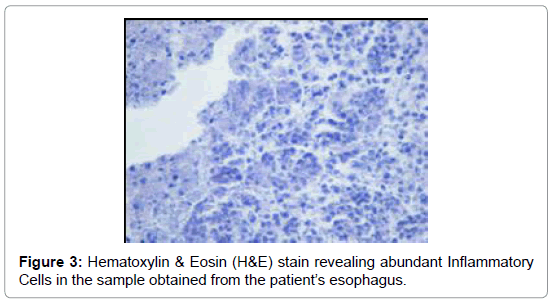
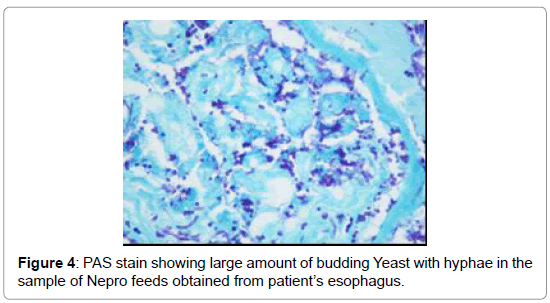
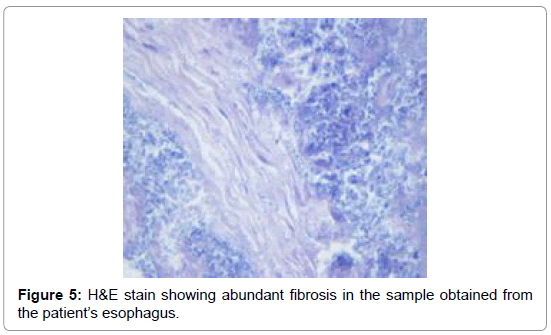
The esophageal disimpaction was successfully done using a Roth foreign body retriever basket. A sample was sent to the pathology lab for analysis. The crust was confirmed not to be muscle fibers or vegetable. It was assumed to be the Nepro® feeds which the patient was receiving. It was postulated that the Nepro® feeding became desiccated, crusted and eventually impacted the esophagus. To confirm our hypothesis, a sample of the Nepro® was allowed to dry in a Petri-dish. This dried material was studied by the same pathologist under a microscope (Figures 3 and 4). This sample had similar properties to the impacted material. Hence, it was proven that the Nepro® feeds which the patient was receiving accumulated in the es A B ophagus, desiccated, and caused obstruction of the esophagus. The impacted material also demonstrated a large amount of growing yeast with abundant inflammatory cells and fibroblasts (Figures 2-5).
It was assumed that the OG tube was either entirely above the lower esophageal sphincter (LES) or at least one of its proximal ports was above the LES during his feeding. The patient’s chest radiographs were reviewed with the radiologist and at no time it could be convincingly confirmed that the tube passed through into the abdominal side of the diaphragm. Since the location of OG tube could not be confirmed, there could only be two reasonable explanations. If the tube was entirely in the esophagus, continuous feeding was only possible due to the pump. In this case, the majority of the tube feeding was passively leaking through the gastero-esophageal (GE) junction in to the stomach and some of the feed stagnated in the esophagus and caused the obstruction. Whereas, if the tube was partially or completely below the GE junction, regurgitation of the Nepro® into the esophagus can explain the impaction. However, we believe that the obstruction was likely due to an earlier event than the latter. The other possibility would be proximal migration of the tube and failure to check the proper positioning of it in the stomach.
Discussion
Esophageal obstruction due to enteral formulations has been reported in a few case reports, most of which attributed casein as a factor responsible for the solidification. Other cases were attributed to co-administration of sucralfate. We were unable to find any reported cases of esophageal obstruction due to Nepro®. Our patient received Nepro® which contained casein, but there was no co-administration of any other agents such as sucralfate.
Garcia et al. reported three cases of esophageal obstruction with enteral feeds with sucralfate [4]. Lentsch et al. reported esophageal obstruction due to enteral feeds in the early postoperative period in a patient who underwent larygectomy [5]. These case reports have identified various risk factors for obstruction such as: esophageal stasis caused by esophageal dysmotility, protein precipitation by acidic gastric contents, tube damage, and concomitant use of sucralfate and other antacids [5]. In mechanically ventilated patients, some degree of gastroesophageal reflux is unavoidable. The nasogastric tube can lead to loss of sphincter action at the gastroesophageal junction with subsequent reflux of gastric acid and food contents from stomach [6]. Likewise, altered esophageal tone and motility will cause enteral feed stasis and precipitation [7]. Turner et al. [8] and Myo et al. [9] demonstrated that different formulations of enteral feed containing casein solidify in acidic medium (pH<4.6) in vitro. They also suggested that decreased pepsin and pancreatic enzyme secretions may be responsible for the solidification of casein containing enteral formulas [8,9].
Nepro® has properties of forming precipitates which in turn can solidify and cause significant obstruction. In our case, the entire esophagus was full of solidified Nepro®. Such obstruction can lead to bacterial and fungal overgrowth and a mucosal inflammatory response and hence should be recognized early. Awareness of this complication, which has not been reported in the product information sheet, will help health care professionals, including endoscopists to identify, treat and prevent morbidity promptly. We should also be aware of possible migration of feeding tubes during daily patient care to prevent esophageal feeding. We should also be aware of other predisposing factors such as mechanical ventilation, supine position, neurological diseases, diabetes mellitus, hypothyroidism, obesity and history of partial gastrectomy [10].
We recommend adding esophageal obstruction as complication of Nepro®. We also recommend daily assessment of feeding tube position, feeding in a semi-recumbent position, administration of prokinetic agents and proton pump inhibitors as preventative measures [10]. If feasible tube feeding should be done with nasoduodenal or nasojejunal tubes rather than orogastric or nasogastric tubes.
References
- Kreymann KG, Berger MM, Deutz NE, Hiesmayr M, Jolliet P, et al. (2006) ESPEN Guidelines on Enteral Nutrition: Intensive care. Clin Nutr 25: 210-223.
- Stroud M, Duncan H, Nightingale J; British Society of Gastroenterology (2003) Guidelines for enteral feeding in adult hospital patients. Gut 52 Suppl 7: vii1-1vii12.
- Marik PE, Zaloga GP (2001) Early enteral nutrition in acutely ill patients: a systematic review. Crit Care Med 29: 2264-2270.
- García-Luna PP, García E, Pereira JL, Garrido M, Parejo J, et al. (1997) Esophageal obstruction by solidification of the enteral feed: a complication to be prevented. Intensive Care Med 23: 790-792.
- Lentsch EJ, Bumpous JM (1999) Early postoperative esophageal obstruction caused by enteral feeding concretions in patients who have undergone laryngectomy. Otolaryngol Head Neck Surg 120: 617-618.
- Cremer SA, Gelfand DW (1996) Esophageal bezoar resulting from enteral feedings. JPEN J Parenter Enteral Nutr 20: 371-373.
- Algozzine GJ, Hill G, Scoggins WG, Marr MA (1983) Sucralfate bezoar. N Engl J Med 309: 1387.
- Turner JS, Fyfe AR, Kaplan DK, Wardlaw AJ (1991) Oesophageal obstruction during nasogastric feeding. Intensive Care Med 17: 302-303.
- Myo A, Nichols P, Rosin M, Bryant GD, Peterson LM (1986) An unusual oesophageal obstruction during nasogastric feeding. Br Med J (Clin Res Ed) 293: 596-597.
- Marcus EL, Arnon R, Sheynkman A, Caine YG, Lysy J (2010) Esophageal obstruction due to enteral feed bezoar: A case report and literature review. World J Gastrointest Endosc 2: 352-356.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 15040
- [From(publication date):
specialissue-2012 - Nov 19, 2025] - Breakdown by view type
- HTML page views : 10341
- PDF downloads : 4699