Review Article Open Access
Gastric Cancer: Aspects of Minimal Invasive Technique
Bruno Zilberstein1*, Björn LDM Brücher2,3, Brian Guilherme Marta Coimbra1, Carolos Eduardo Jacob1, Leandro Cardoso Barchi1, Hernique Giroud Joaquim1 and Ivan Ceccobello1
1Digestive Surgery Division, San Paulo University, Sao Paulo, Brazil
2INCORE, International Consortium of Research Excellence of the Theodor- Billroth-Academy®, Germany–Israel– Serbia-USA
3Bon Secours Cancer Institute, Richmond, VA, USA
- Corresponding Author:
- Bruno Zilberstein
Professor of Surgery, Digestive Surgery Division
San Paulo University, Sao Paulo, Brazil
Tel: +55-11-999408370
E-mail: brunozilb@uol.com.br
Received Date: September 26, 2013; Accepted Date: November 28, 2013; Published Date: December 05, 2013
Citation: Zilberstein B, Brücher BLDM, Coimbra BGM, Jacob CE, Barchi LC, et al. (2013) Gastric Cancer: Aspects of Minimal Invasive Technique. J Gastroint Dig Syst 3:158. doi:10.4172/2161-069X.1000158
Copyright: © 2013 Zilberstein B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Keywords
Gastric Cancer; Minimal invasive; Laparoscopy; D2- lymphadenectomy
Introduction
Gastric cancer is a burden in medicine with a male predominant gender relation [1,2]. Although the incidence of gastric cancer has decreased in the last few decades [3,4], an increase of females and elderly patients was also reported [5,6]. The standard of care for gastric cancer is radical surgical resection [7,8] with reported morbidity rates between 20 and 46% and a postoperative observed mortality rate between 0.8 – 10% [9], depending on the expertise,and in case of locally advanced tumor category, from multimodal therapy. The Cochrane review from 2010 investigated 5,726 patients and revealed that chemotherapy significantly improves survival in advanced gastric cancer patients compared to best supportive care [10]. The type of the surgical resection depends on the tumor location, size, cancer depth, clinical staging and the histological subtype as well as a lymphovascular invasion and contents of a radical subtotal or a total gastrectomy with D-I and/or D-II lymphadenectomy [7]. D-I lymphadenectomy contains the perigastric lymph nodes, while a D-II lymphadenectomy contains the D-I-lymphadenectomy plus the resection of the lymph nodes along the hepatic, left gastric, celiac and splenic arteries as well as those in the splenic hilus [11]. In western countries, the diagnosis of gastric cancer is mostly in advanced cancer tumor categories in contrast with eastern countries where most of the cases are of early cancer (about 50%) [12]. Until now, surgical treatment continues to be the most efficient treatment and other modalities like chemo- or radiotherapy are indicated mostly to improve surgical results or palliation.
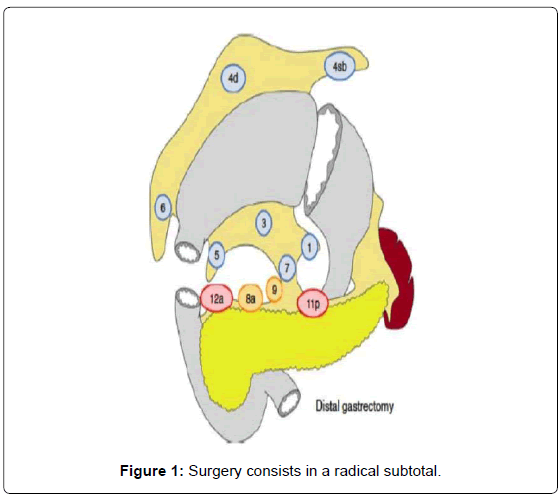
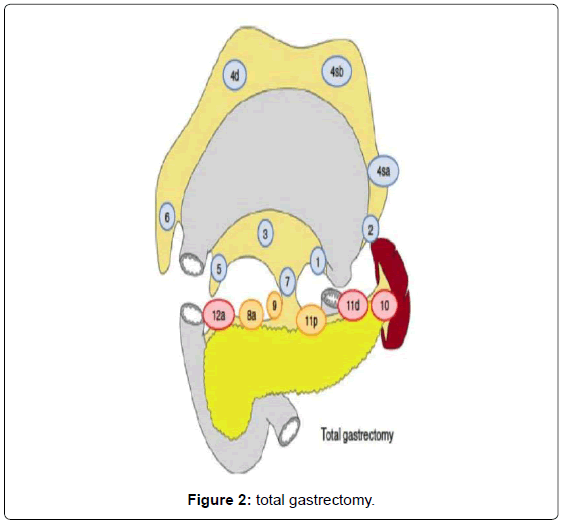
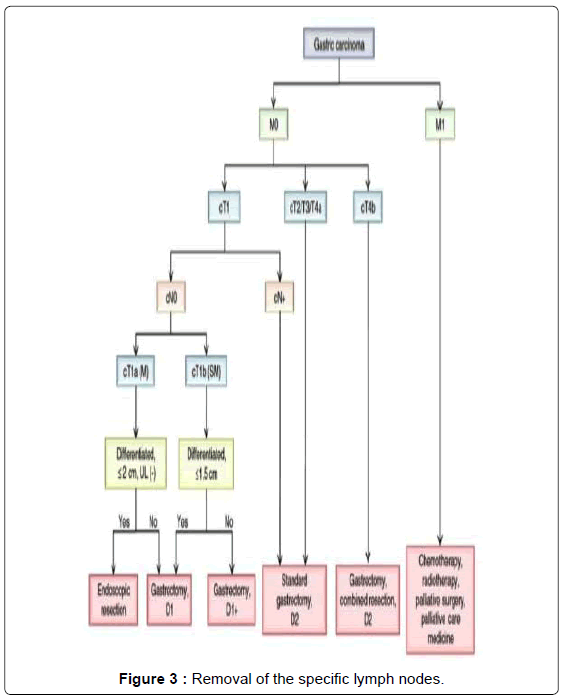
Surgery consists in a radical subtotal (Figure 1) or total gastrectomy (Figure 2) where lymphadenectomy is mandatory. The “gold standard” accepted worldwide is the D2 lymphadenectomy for advanced gastric cancer, which means removal of at least 16 lymph nodes in the AJCC recommendations and the removal of the specific lymph nodes stations according to the Japanese rules or guidelines in eastern and western services that follow this protocol (Figure 3) [13-15]. Although it is still discussed, D2-lymphadenectomy is recommended for surgeons with sufficient technical and anatomical experience [16].
For 20 years, when Goh et al. performed the first laparoscopicassisted gastrectomy and the reconstruction by a B-II gastrojejunostomy, there has been an ongoing discussion if and / or how the minimal invasive approach can and / or should be performed and if it has a positive and / or negative defined effect on survival and prognosis compared to open surgery [17]. After gastric and duodenal ulcers have been operated by the laparoscopic approach, this technique was increasingly used in gastric cancer but first in early carcinoma only because in these patients no D-2 lymphadenectomy is necessary and, therefore, also hybrid procedures could more easily be performed: after a subtotal gastrectomy, a small epigastric incision enabled the removal of the specimen as well as the reconstruction by B-I or B-II. As experience grew and each time more surgeons achieved good results, the enthusiasm for this method increased [18] and the low mortality rates, low morbidity, faster recovery, shorter hospital stay and preliminary good long-term survival rates, at least as compared to conventional open surgery have been well received [19-21]. A very large prospective study is going on in Japan and Korea investigating the long-term results for laparoscopic gastric cancer surgery, the preliminary presentations at the actual 10th International Gastric Cancer Congress (IGCC 2013) in Verona, Italy seem very encouraging and more data can be expected at the 11th International Gastric Cancer Congress (IGCC 2015) in Sao Paulo, Brazil.
Minimal Invasive Access Technique
General
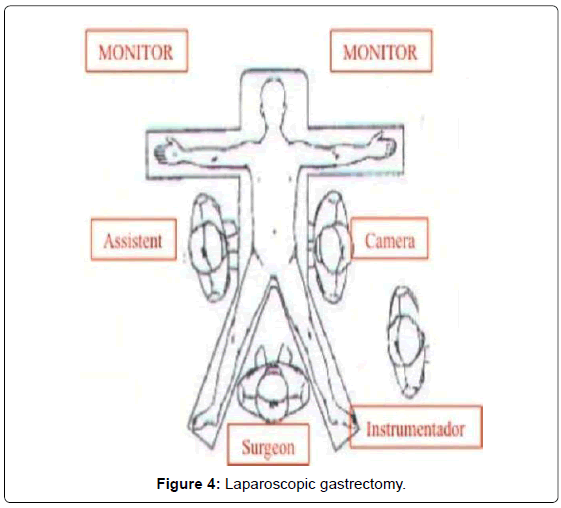
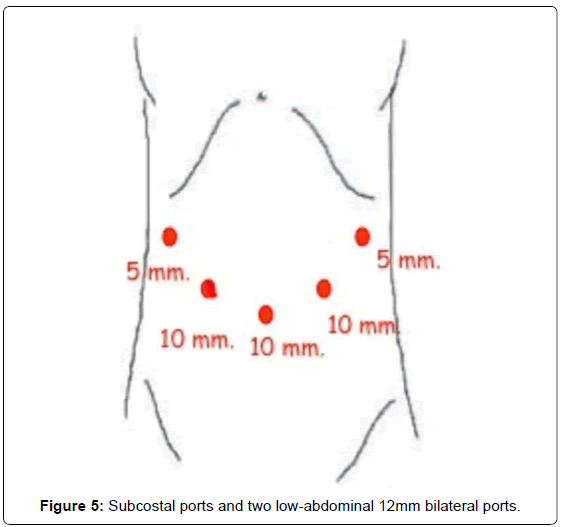
Our method for gastric cancer laparoscopic gastrectomy is performed while the patient is under general anesthesia, in a modified lithotomy position, with the legs apart and flexed slightly; the surgeon is positioned between the legs, the first assistant (camera operator) on the patient’s left and the second assistant to the right (Figure 4). After pneumoperitoneum is established, 6 ports are placed; two 5 mm bilateral subcostal ports and two low-abdominal 12 mm bilateral ports. A 10 mm port is placed 5 cm below and 5 cm to the left of the patient’s umbilicus for the camera. Finally, a 5 mm port is placed in the subxiphoida larea (Figure 5). Intracorporeal pressure is maintained at 12 to 15 mmHg. The gastrectomy begins with the traction of the greater curvature along the transverse colon and the dissection of the gastrocolic ligament, carried out with ultrasonic shears (Laparoscopic coagulating shears [LCS]; Ethicon Endo-Surgery, Cincinnati, OH, USA). The roots of the right gastroepiploic and gastric vessels are exposed by gentle dissection. The infra-pyloric lymph nodes (LN station no. 6) are dissected and the vessels can be afterwards more easily exposed, identified and, then, divided with double clips. During this exposure, lymph nodes of the mesenteric artery and vein (LN station no. 14v and 14a) can be easily removed. Strategically, after this dissection, we move through the dissection of the hepatobiliary ligament, dividing the short vessels irrigating the duodenal first portion and liberating them just under the pylorus to facilitate the introduction of a 45 mm endoscopic linear stapler for the duodenal transection. Routinely, the reinforcement of the linear stapler closure with separated or continuous in absorbable seromuscular suture is performed. The dissection of the right gastric (pyloric) vessels is performed by dividing them with clips and removing the lymph nodes along the hepatic artery in the hepatoduodenal ligament (LN station no. 12a). We continue the preparation with the dissection of the anterior superior lymph node group along the common hepatic artery (LN no. 8a). By pulling up the stomach, the left gastric vessels and their lymph nodes (LN no. 7) are easily identified. Also, during this maneuver, the pancreatic capsule can be removed if necessary. With the harmonic scalpel, the left gastric vessels are gently dissected and the lymph nodes of the left gastric vessels (LN no. 7) and around the celiac artery (LN no. 9) are removed. The dissection continues into the direction of the splenic artery toward the splenic hilum removing the lymph nodes along the proximal splenic artery (LN no. 11p) and along the distal splenic artery (LN no. 11d), when necessary. This procedure is usually performed for D2 dissection in both TG and STG.
Total gastrectomy (TG)
When a TG is necessary, the lymph node dissection continues dividing the peritoneal membrane that covers the esophagogastric junction and after exposing and traction of the esophagus with a Penrose drain, the vagal nerves are easily identified and divided. This is followed by the lymphadenectomy of the right paracardial lymph nodes (LN no. 1) and the lymph nodes along the lesser curvature (LN no. 3). In this manner, the preparation results in liberating the soft tissues on the posterior gastric wall. After this, we move again to the greater curvature finishing and releasing the great omentum from the transverse colon (completion of the dissection of the gastrocolic ligament); this includes the dissection and lymphadenectomy of the left gastroepiploic vessels (LN no. 4sb). The short gastric vessels with its lymph nodes (LN no. 4sa) are divided with the harmonic scalpel and clips, if necessary. After completion of the lymph node dissection of the left paracardial lymph nodes (LN no. 2), the esophagus is transected with a 45 mm linear stapler with blue cartage. By this step, the gastric resection is completed and the specimen can be stored for histopathological examination after marking. As the gastric specimen is large due to the amplified dissection including the greater omentum, we usually perform a small suprapubic Pfannenstiel incision for the removal of the specimen.
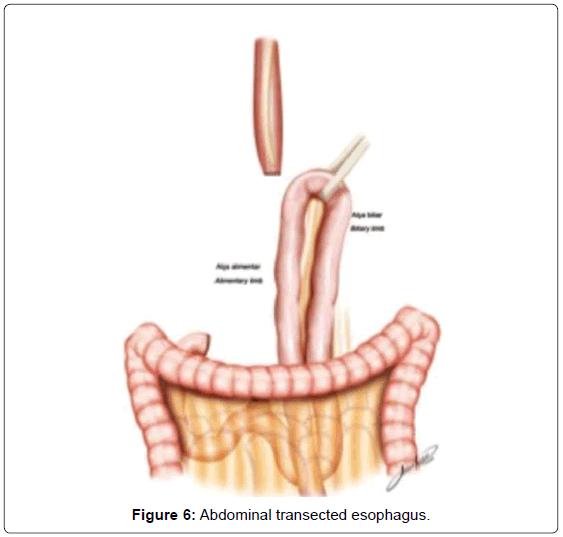
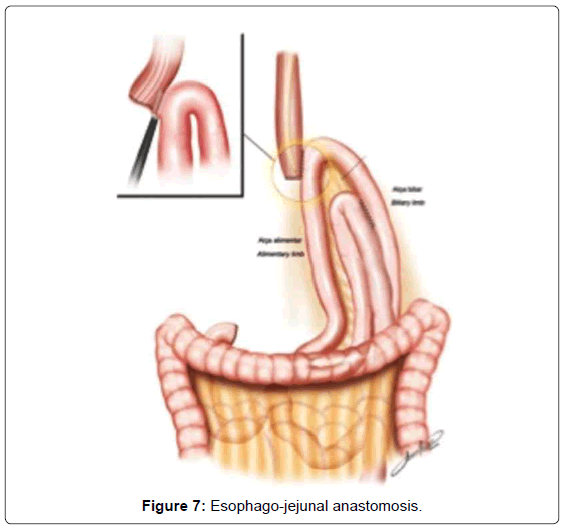
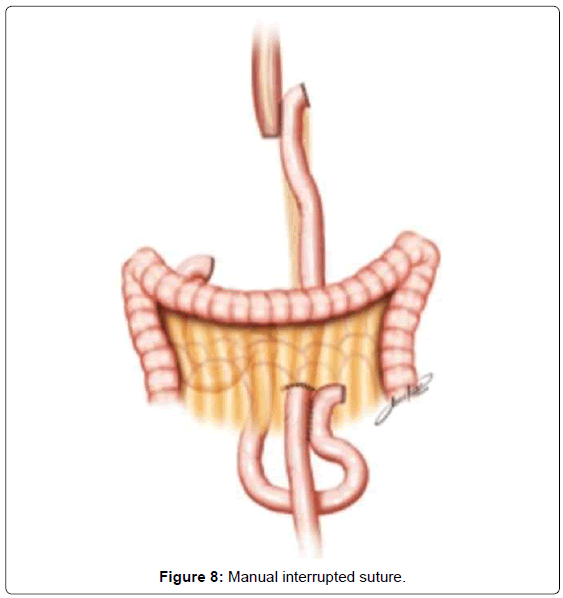
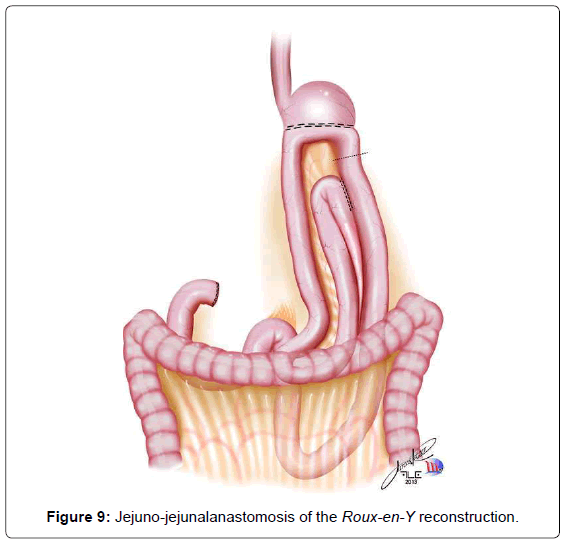
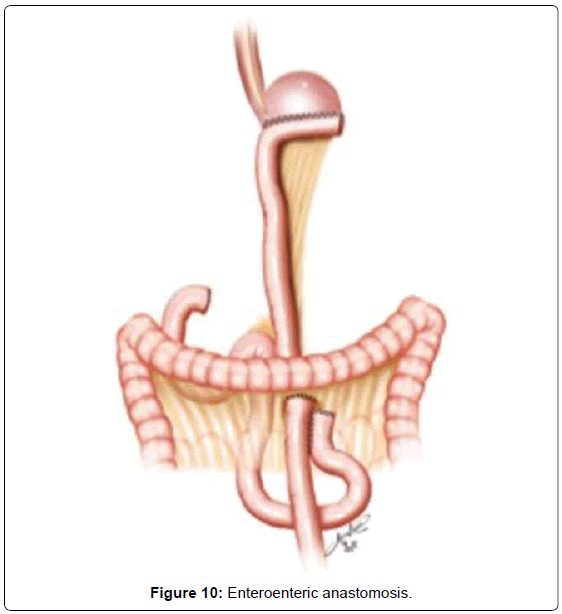
The continuity of digestive tract is performed with a Roux-en-Y diversion totally by laparoscopy. To facilitate this maneuver, the Treitz’ angle is identified and 30-40 cm distal from it, a jejunal loop is identified and transposed through the supramesocolic space using a transmesocolic or precolic route. It is helpful when the jejunal loop is anchored by a stitch to the left lateral wall of the abdominal transected esophagus (Figure 6). A 12 French bougie is orally introduced by the anesthesiologist for an easier exposureof the esophageal stump. A 45mm linear stapler with white cartage is utilized to perform the latero-lateral esophago-jejunal anastomosis (Figure 7). After the completion of the mechanical anastomosis, the bougie already exposing the esophagus is introduced into the jejunal loop to ensure the anastomosis diameter and facilitate the closure of the common entry hole by hand sewn with extramucosal 3-0 PDS® (polydioxanone suture) (Figure 7). Next an alimentary limb is isolated with a length of about 70 cm, brought into the upper abdomen close to the biliary limb in order to perform the jejuno-jejunal anastomosis of the Roux-en-Y reconstruction (Figure 7).We perform this anastomosis with a 45 mm white cartage linear stapler closing the stapler entrance by hand sewing with extramucosal 3-0 PDS®. After the anastomosis had been completed, we test the closures by instilling methylene blue solution through the esophageal bougie to ensure the integrity of the closure and no leaks. Once tested, the biliary limb and the alimentary limb are divided and separated by utilizing linear stapler with a 45 mm white cartage. In the transmesocolic route, the alimentary limb is tractioned and the enteroenteric anastomosis is placed at the inframesocolic space. The mesenteric gap is closed with manual interrupted suture (Figure 8).
Subtotal Gastrectomy (STG)
When a subtotal gastrectomy is indicated, the lymphadenectomy is continued with the removal of the right paracardial lymph nodes (LN no. 1) and along the lesser curvature (LN no. 3). We then move to the greater curvature finishing and releasing the great omentum from the transverse colon, moving through the left gastroepiploic vessels, dividing them with clips (LN no. 4sb). Depending on the gastric margins, the stomach is transected generally at that level, with a 60 mm linear stapler, with one or two blue cartages. The continuity of the digestive tract is performed also by a Roux-en-Y diversion entirely by laparoscopy. The duodenojejunal angle is identified and the jejunal loop about 30-40 cm away from Treitz’is transposed to the supramesocolic space using transmesocolic or precolic route. We again perform a stitch of the jejunal loop to the right lateral wall of the gastric stump. The gastrojejunal anastomosis is performed using a 60 mm linear stapler with blue cartage. The common entry hole is then closed and hand sewn with extramucosal 3-0 PDS®. The alimentary limb is isolated of a length of 70 cm and brought into the upper abdomen, close to the biliary limb in order to perform the jejuno-jejunalanastomosis of the Roux-en-Y reconstruction (Figure 9). This anastomosis is performed with a 45 mm white cartage linear stapler, closing the stapler entrance by hand sewn with extramucosal 3-0 PDS®. The closures are also tested by instillation of diluted methylene blue solutionby a nasogastric tube, to ensure the integrity of the closure. Once tested, the billiary limb and the alimentary limb are separated utilizing linear stapler with a 45 mm white cartage. In the transmesocolic route, the alimentary limb is tractioned and the enteroenteric anastomosis is located in the inframesocolic space. The mesenteric gap is closed with interrupted suture (Figure 10). Until now, we drain the operative situs with two silicon abdominal drains, exteriorized by the lateral 12 mm ports, monitoring the esophago- or gastrojejunal anastomosis at the left and the duodenal stump at the right.
Discussion
Until now, we have performed, during a 7-year period,on more than 70 patients with gastric cancer by laparoscopic gastric resection, 97% because of gastric cancer and 3% with a GIST tumor. In the majority of these cases, 70% underwent subtotal gastrectomy and 30% total gastrectomy. D2-lymphadenectomy was feasible in all cases and at least 37 lymph nodes were removed. There was neither a conversion to an open surgery necessary and the mortality rate was 0%. As for complications, there was one small fistula of the esophagojejunal anastomosis with spontaneous closure and two middle colic vessels lesions.
The international literature has an increasing variety of technical options like hybrid procedures where reconstruction is performed by open access or totally by laparoscopy where the gastrojejunal or esophagus-jejunal reconstruction is made by circular stapler [22,23]. As has been pointed out by the International Gastric Cancer Association (IGAC), the primarily goal for gastric cancer patients is survival and, therefore, until now it is considered in all eastern trials that the survival for early gastric cancer is equal to open surgery and by this we are adding the benefits of this minimally invasive approach to the good oncological results. There is hope that this can be also shown in advanced tumor categories in the future. Until now, large series are retrospective and clear selection criteria have to be elaborated. However, the results until now provide that there might be a shift by adopting a faster learning curve to minimally invasive gastric cancer surgery [24].
Conclusion
The laparoscopic minimally invasive surgery approach is increasingly performed for patients with gastric cancer. The results of ongoing Asian large trials can be expected for the 11th International Gastric Cancer Congress (IGCC 2015) in Sao Paulo, Brazil.
References
- Brookes VS, Waterhouse JA, Powell DJ (1965) Carcinoma of the Stomach: A 10-Year Survey of Results and of factors Affecting Prognosis. Br Med J 1: 1577-1583.
- Ming SC, Hirota T(1998) Malignant epithelial tumors of the stomach. In: MING SC, GOLDMAN H, ed. Pathology of the gastrointestinal tract. Williams & Wilkins, Baltimore 607-650.
- Forman D, Burley VJ (2006) Gastric cancer: global pattern of the disease and an overview of environmental risk factors. Best Pract Res Clin Gastroenterol 20: 633-649.
- Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics, 2010. CA Cancer J Clin 60: 277-300.
- Kubo T (1971) Histologic appearance of gastric carcinoma in high and low mortality countries: comparison between Kyushu, Japan and Minnesota, USA. Cancer 28: 726-734.
- Rios-Castellanos E, Sitas F, Shepherd NA, Jewell DP (1992) Changing pattern of gastric cancer in Oxfordshire. Gut 33: 1312-1317.
- Siewert JR, Sendler A (1999) The current management of gastric cancer. Adv Surg 33: 69-93.
- Bonenkamp JJ, Hermans J, Sasako M, van de Velde CJ, Welvaart K, et al. (1999) Extended lymph-node dissection for gastric cancer. N Engl J Med 340: 908-914.
- Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, et al. (2008) D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med 359: 453-462.
- Wagner AD, Unverzagt S, Grothe W, Kleber G, Grothey A, et al. (2010) Cochrane Database Syst Rev. 17: CD004064.
- Yasuda K, Adachi Y, Shiraishi N, Inomata M, Takeuchi H, et al. (2002) Prognostic effect of lymph node micrometastasis in patients with histologically node-negative gastric cancer. Ann Surg Oncol 9: 771-774.
- Song KY, Park YG, Jeon HM, Park CH (2013)A nomogram for predicting individual survival of patients with gastric cancer who underwent radical surgery with extended lymph node dissection. Gastric Cancer.
- Maruyama K, Sasako M, Kinoshita T, Okajima K (1995) Effectiveness of systematic lymph node dissection in gastric cancer surgery. In: Nishi M, Ichikawa H, Nakajima T, Maruyama K, Tahara E, ed. Gastric cancer. Berlin Heidelberg New York Tokyo 27: 23-27.
- Siewert JR, Böttcher K, Roder JD, Busch R, Hermanek P, et al. (1993) Prognostic relevance of systematic lymph node dissection in gastric carcinoma. German Gastric Carcinoma Study Group. Br J Surg 80: 1015-1018.
- Roviello F, Marrelli D, Morgagni P, de Manzoni G, Di Leo A, et al. (2002) Italian Research Group for Gastric Cancer: Survival benefit of extended D2 lynphadenectomy in gastric cancer with involvement of second level lymph nodes: a longitudinal multicenter study. Ann SurgOncol 9: 894–900.
- Asoglu O, Matlim T, Kurt A, Onder SY, Kunduz E, et al. (2013) Guidelines for extended lymphadenectomy in gastric cancer: a prospective comparative study. Ann Surg Oncol 20: 218-225.
- Goh PM, Alponat A, Mak K, Kum CK (1997) Early international results of laparoscopic gastrectomies. Surg Endosc 11: 650-652.
- Zilberstein B, Jacob CE, Barchi LC, Yagi OK, Ribeiro-Jr U, et al. (2013) Simplified technique for reconstruction of the digestive tract after total and subtotal gastrectomy for gastric cancer. Arq Bras Cir Dig
- Cai J, Wei D, Gao CF, Zhang CS, Zhang H, et al. (2011) A prospective randomized study comparing open versus laparoscopy-assisted D2 radical gastrectomy in advanced gastric cancer. Dig Surg 28: 331-337.
- Martínez-Ramos D, Miralles-Tena JM, Cuesta MA, Escrig-Sos J, Van der Peet D, et al. (2011) Laparoscopy versus open surgery for advanced and resectable gastric cancer: a meta-analysis. Rev Esp Enferm Dig 103: 133-141.
- Zilberstein B, da Costa Martins B, Jacob CE, Bresciani C, Lopasso FP, et al. (2004) Complications of gastrectomy with lymphadenectomy in gastric cancer. Gastric Cancer 7: 254-259.
- Park do J, Han SU, Hyung WJ, Kim MC, Kim W, et al. (2012) Korean Laparoscopic Gastrointestinal Surgery Study (KLASS) Group: Long-term outcomes after laparoscopy-assisted gastrectomy for advanced gastric cancer: a large-scale multicenter retrospective study. SurgEndosc 26: 1548-1553.
- Kim KH, Kim MC, Jung GJ, Kim HH (2012) Long-term outcomes and feasibility with laparoscopy-assisted gastrectomy for gastric cancer. J Gastric Cancer 12: 18-25.
- Woo Y, Hyung WJ, Pak KH, Inaba K, Obama K, et al. (2011) Robotic gastrectomy as an oncologically sound alternative to laparoscopic resections for the treatment of early-stage gastric cancers. Arch Surg 146: 1086-1092.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 14787
- [From(publication date):
December-2013 - Dec 22, 2025] - Breakdown by view type
- HTML page views : 10140
- PDF downloads : 4647