Review Article Open Access
Gastrointestinal Carcinoid Tumours: A Review
Jennifer Ha1,2,3,4*and Weixian Alex Tan3
1Armadale Health Service, 3056 Albany Highway, Armadale, Western Australia 6112, Australia
2Royal Perth Hospital, Wellington Street Campus, Wellington Street, Perth, Western Australia 6000, Australia
3Sir Charles Gairdner Hospital, Hospital Avenue, Nedlands, Perth, Western Australia 6009, Australia
4School of Surgery, University of Western Australia, Australia
- *Corresponding Author:
- Jennifer Ha
Armadale Health Service
3056 Albany Highway
Armadale, Western Australia 6112
Telephone: +61 8 9391 2000
Fax: +61 8 9391
E-mail: jenha81@yahoo.com.au
Received date: February 03, 2012; Accepted date: March 16, 2012; Published date: March 18, 2012
Citation: Ha J, Tan WA (2012) Gastrointestinal Carcinoid Tumours: A Review. J Gastrointest Dig Syst 2:107. doi:10.4172/2161-069X.1000107
Copyright: © 2012 Ha J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Introduction: Carcinoid tumour represents the majority of the neuroendocrine malignancy of the gastrointestinal tract. Diagnosis is difficult as it is often asymptomatic and non-specific, and the presence of symptoms generally implies advanced disease. Whilst surgical resection remains the treatment of choice for primary and respectable disease, there are still significant uncertainties for management of those with metastatic disease.
Methods: We present a review of the literature on the signs and symptoms, classification, diagnosis, prognosis and treatment options of carcinoid tumours of the gastrointestinal tract.
Conclusion: The aim of treatment is to achieve cure and or improve quality of life by palliating symptoms and prolong survival. The recent WHO classification scheme may be helpful with the diagnosis, prognosis as well as consideration of appropriate treatment option.
Keywords
Carcinoid tumour; Gastrointestinal; Management; Diagnosis; Review
Introduction
The first description of carcinoid tumour was made by Lubarsch in 1888 [1]. Gastrointestinal tract [GIT] neuroendocrine tumour was first described by Oberndorfer in 1907 as arising from enterochromaffin cells primarily in the submucosa of the intestine,accounting for 2-5% of all malignancy and carcinoid tumour comprise the majority, 55% [1-14]. It belongs to the apudomas (amine precursor uptake and decarboxylation tumours), as it arises from endocrine amine precursor uptake and decarboxylation cells that can be found throughout the GIT [8,11,13,15,16]. Serotonin (5HT) was first isolated by Rapport et al. from beef serum and Erspamer showed that Kultschitsky cells secreted 5HT [7].
It can occur in any organ derived from the primitive endoderm but 64% originates in the GIT, with the commonest primary sites being the appendix, small intestines and rectum [1,3,5,14-19]. In the small intestine, majority of the cases are found in the ileum, less commonly in the jejunum and rarely involving the duodenum [14, 20-22].
The induction of cellular proliferation by paracrine agents and growth factors is believed to follow mutations in oncogenes and tumour suppressor genes and the amplification of HER-2/neu cellular oncogene expression has been detected in ileal carcinoids [23]. It has been found that stimulation of a number of the 5HT receptor subtypes, of which there are 15, has the potential to induce malignant transformation [18].
The tumour arises in the Kulchitsky cells in the crypts of Liberkuhn and grows as a submucosal nodule [8,11,13]. It is usually small, slowgrowing, and often invades transmurally [5,8,15,20,24,25]. Metastases occur in 30% of patients at the time of diagnosis even though the mucosa is usually intact [6,15]. It spreads characteristically to the lymphatics and adjacent mesentery [6,7,15,19,24,26]. The direct local effect is elastic sclerosis of the mesenteric vessels and due to the marked fibrotic reaction, it results in angulations and fixation of the adjacent bowel loops [1,6,8,13-15,19,24].
The annual incidence is thought to be 1.5-2 cases per 100 000 of the general population [2,4,14,17,18]. Recent studies have reported an increase in incidence to 3.84-8.4 per 100 000 population [14,22,27]. The highest incidence is reported in patients around 50-70 years old with no gender predilection [1,2,6-8,14,21]. Appendiceal carcinoid is an exception as it seems to develop at an earlier age and more frequently in women, which is thought to parallel the mean age at appendectomy [1,2,7,14].
Signs & Symptoms
It is asymptomatic in up to 60% of the patients and autopsy studies showed that up to two thirds of jejunoileal carcinoids remain undetected in life [1,6,11,16,19,26,28,29]. When symptoms occur, they are often non-specific and vary according to the location of the lesion [7,8,15,30]. The average time of onset of symptoms to diagnosis is reported to exceed 9 years, by which it has usually metastasized [16,19]. Up to 50% of patients may present with symptoms of small bowel obstruction, others with abdominal pain, diarrhoea, vomiting, early satiety, weight loss, bleeding, ischemic enteritis or a palpable mass [1,3,5,7-11,13-15,19-21,23,26,28,31-33].
Patients first become symptomatic with symptoms of hormonal hypersecretion rather than symptoms related to tumour bulk [14]. The carcinoid syndrome was first recognised by Thorson in Sweden and Isler in Switzerland [1,7]. This occurs in less than 10% of the cases and the prevalence has been reported to be 0.5 per 100 000 population [2,4,8,9,14,18]. This is more common with tumours of the ileum and jejunum [8,10,11,23]. The classic symptoms are shown in Table 1 [1- 4,7,8,10,13,14,16,17,26,32,34]. As 5HT and amines are cleared rapidly by the liver, therefore the syndrome is uncommon unless the liver is largely replaced by metastases or if the tumour has direct access to the systemic circulation [1,8,13,16,23,26,34]. However, patients can have extensive liver metastases without having carcinoid syndrome [23].
|
Table 1: Main clinical features of carcinoid syndrome.
The symptoms are caused by the tumour’s secretory products, with 5HT being the major one [1-3,5,7,10, 16-18,31]. The urinary 5-hydroxytryptamine metabolite, 5-hydroxyindoleacetic acid [5-HIAA] was found to be virtually always elevated in the carcinoid syndrome by Page et al. [3,7,16,20,35]. 5HT, the precursor of 5-HIAA is one of the mediators of diarrhoea, which is often watery and explosive [1,35]. Kinins appear to cause cutaneous flushing, especially in patients with midgut tumours, in addition to 5HT and prostaglandin [1,7,8,16,35]. This flushing occurs in the face and upper trunk, often provoked by alcohol, tyramine containing food or exertion, lasting a few minutes but occurring several times a day [8,11,16]. Patients with foregut carcinoids may experience a more intense and prolonged flushing accompanied by skin thickening and telangiectasia [8].
Carcinoid heart disease is a late complication associated with carcinoid syndrome leading to right sided heart failure [1,8,27,29,36,37]. This is secondary to the high concentration of circulating amines which cause fibrosis and damage to the tricuspid and pulmonary valves, which has been shown to be the cause of death in a third of the patients [1,8,27,29,36,37]. The aortic and mitral valves are usually spared due to the clearance of 5HT by monoamine oxidase present in the lung [1].
Classification
Up to 85% of the carcinoid tumours in the GIT can be classified according to their anatomical site of origin: Foregut, midgut or hindgut proposed as by Williams and Sandler [2,7,8,10,11,13,23,29]. Whilst these GIT carcinoid tumours have a common cell of origin, they are heterogeneous with a range of morphological appearances and biological behaviours [2,38-40]. It is of clinical value to note that the classical features of carcinoid tumour is associated with development in the foregut and midgut, whereas those in hindgut rarely develop 5-hydroxytryptamine or 5-hydroxytroptophan, and malignant carcinoid syndrome are more likely in metastatic disease from the primary midgut tumours [7,17]. Differentiation of malignant from benign primary carcinoid tumours cannot be made reliably on histology [13]. Furthermore, the tumours have variable biologic behaviour and are all generally considered to have a malignant potential [13].
Diagnosis
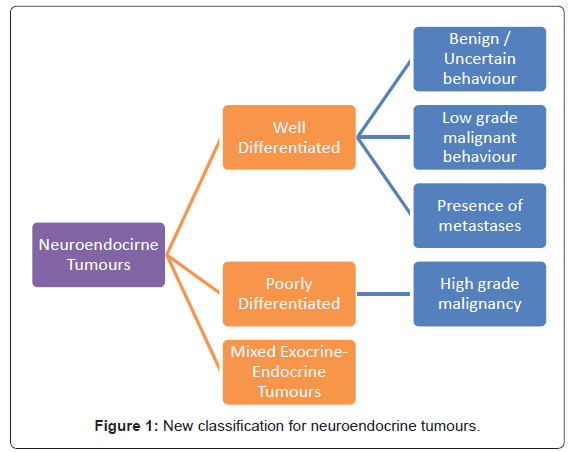
The World Health Organization [WHO] classification scheme is a uniform approach to diagnose the neuroendocrine tumour within the GIT by considering well-defined histopathological and biological features like: cellular grading, primary tumour size and site, lymphovascular invasion, mitotic counts, Ki-67 labelling index, production of biologically active markers, invasion of adjacent organs, presence of metastases and functional status [12,38-40]. Figure 1 depicts the new classification categories that can be applied across all sites within the GIT [12,38,39]. This is helpful with the diagnosis and prognosis, as well as in consideration of the appropriate treatment options [38,39].
The diagnosis is usually based on the clinical features and supported by the presence of high levels of 24-hour urinary 5-HIAA which is the breakdown product of serotonin [2,4,14,23,27,34,41]. This has a sensitivity of 73-75% and a specificity of 90-100% [2,23]. False negatives may occur in patients taking salicylates or L-dopa, in those with malabsorption syndromes, or ingestion of tryptophan or serotonin-rich foods [14,23,27,29,41] Serial measurement has been commonly used for monitoring patients with metastatic tumours [14].
Tumour marker chromogranin A is a 49-kD protein that is contained in neurosecretory vesicles of neuroendocrine tumour cells and is detectable in plasma [14]. Its measurement has been reported to be useful in the detection of carcinoid tumours as it does not rely on serotonin secretion and can be detected in both functioning and non-functioning tumours [14,27,42]. Plasma levels seem to correlate well with tumour burden and treatment response, with a level of >5000 μg/L being associated with a poor prognosis [14,27,29,37,41]. However, levels can be falsely elevated with other neuroendocrine tumours such as that of pancreatic type [29].
Routine laboratory tests and radiological imaging often do not demonstrate the primary lesion [6,16,42]. Standard imaging techniques like computed tomography [CT] and barium contrast series rarely identify the tumour [1,2,14,20,23,41-43]. Detection on CT can be improved by using intraluminal water, instead of barium, or rapid intravenous contrast medium administration and multiplanar reconstructions [13,43]. In the early stages, the tumour is small and confined to the bowel wall therefore small bowel series and enteroclysis may be more sensitive than CT or magnetic resonance imaging [8,13,43].
CT is an excellent technique to show metastatic disease extensions, liver metastases and also useful in monitoring treatment response [1,2,8,13,14,19,37]. The tumour appears as an ill-defined mesenteric mass containing calcification in up to 70% [8,13]. As carcinoid liver metastases are usually hypervascular, administration of intravenous contrast may make it isodense relative to the liver [2].
Transabdominal ultrasound is only useful in identifying a small proportion of small bowel carcinoids and up to 2/3 of liver metastases [37,43]. It is more commonly used to guide needle biopsy to establish diagnosis in equivocal cases [19,37,43]. Endoscopic ultrasound is reported to be highly sensitive in detecting primary tumours of the stomach, duodenum, pancreas and rectum, as well as local nodal involvement [37,41].
A few other investigatory tools used are endoscopy, which is valuable in diagnosing rectal as well as gastric carcinoids [1,37,41,43]. Angiography may be helpful in showing irregularity of the mesenteric and intestinal arcade branches, with stellate arrangements of the arteries, lack of identifiable venous drainage, and minimal tumour stain [8,13,19,20,23].
The unique metabolic features of the neuroendocrine allows for nuclear medicine molecular imaging techniques to be used with great sensitivity and specificity [42]. These include single photon emission computed tomography (SPECT) and positron emission tomography (PET) [42]. PET scan is able to produce better image quality, therefore is more accurate in detecting small tumour foci [42]. The conventional use of 18F-fluorodeoxyglucose is less useful due to the low glycolytic rate of neuroendocrine tumour cells [27,29,37,41,43]. Hence other techniques using tracers such as 5-hydroxytryptophan or iodine-123- labeled octreotide are helpful in diagnosing and locating carcinoid tumours and identifying metastases [4,8,13,14,23,27,29,37,41,43].
Scintigraphy with radio-labelled octreotide has also been successfully used to localise previously undetected primary or metastatic lesions, with a sensitivity of 80-90% [2,23,27,29,43,44]. It is more sensitive for extrahepatic than hepatic lesions due to the heterogeneous octreotide uptake in normal liver [14]. Furthermore, octreotide scans may have a role in predicting the clinical response to somatostatin analogues treatment [14,27,41,43]. Lastly, 123I-labelled MIBG scan has a reported sensitivity of 55-70% and maybe useful in negative octreotide scan cases or patients on long acting octreotide [29,43].
Prognosis
The prognosis varies depending on multiple factors including location, histological subtype, residual disease post resection and metastasis [2,4,6,10,11,17,21,23,28,43,45]. In addition, some authors have reported the levels of 5-HIAA, plasma chromagranin A, weight loss and tumour size to be important factors [2,5,6,17,45]. A table detailing the location, size, incidence, metastatic rates, 5-year survival, rate of carcinoid syndrome and metastatic sites is shown in Table 2 [1-3,8,13,21].
| Location | Tumours size | Incidence | Metastases | 5-Year Survival | Rate of Carcinoid Syndrome | Metastatic sites |
|---|---|---|---|---|---|---|
| Duodenum | Unavailable | 1-3% | 20% | Unavailable | Unavailable | Local lymph nodes & liver |
| Ileum | Unavailable | 11-43% | 35% | 47-65% | 5-7% | Local lymph nodes & liver |
| Jejunum | Unavailable | 1-6% | 35% | 47-65% | 5-7% | Local lymph nodes & liver |
| Appendix | >95% <2cm | 5-44% | 2% | 76% | Unknown | Local lymph nodes & liver |
| Meckel’s Diverticulum | Unavailable | 1% | 18% | Unavailable | Unknown | Unavailable |
| Colon | Avg size at presentation, 5cm | 2% | 60% | 52% | <5% | Unavailable |
| Rectum | 2/3 <1cm | 13-30% | 3% | 18-81% | Unknown | Local lymph nodes & liver. Uncommonly to lungs, bone |
Table 2: Location of primary tumour, presumed cell of origin, metastases and 5-year survival.
Complete removal of a small tumour is associated with a 90% 5-year survival [45]. The 5-year survival rate of appendiceal carcinoid tumour is 76%, whereas that of ileal carcinoid is 47-65% and 70% for carcinoid tumours of other GIT sites [1,4,21]. This decreases to 30% or less in those with distant metastases, which is more likely to occur in small intestinal carcinoids [4]. Larger tumours have a poor prognosis, with a reported 5-year survival of 20% [3].
In small intestinal carcinoids, it is reported that only 15-20% of the smaller primary carcinoid tumours have metastases: 2-18% of tumours < 1cm, 50% of those between 1- 2cm, and 80-100% in > 2cm [3,4,17,20,30]. Up to 69% of cases have lymph node metastases, and up to 50% of the small primary midgut carcinoid tumours may metastasize distally to the liver [3,5]. In general the likelihood of metastasis is related to tumour size, however, size of does not influence the prognosis and is an unreliable predictor of metastatic diseasfe [1-4,8,10,13,14,21,23,45].
Appendiceal tumours are usually < 2cm in > 95% of cases [2]. About 30% of tumours > 2cm have metastasised at the time of diagnosis [2]. The overall 5-year survival rate is 76%, 94% for those with local disease, 85% for those with regional metastases and 34% for those with distant metastases [1,2]. Some authors postulated that appendiceal carcinoids may regress with age, paralleling the behaviour of appendiceal subepithelial argentaffin cells, which are most numerous in young people and decrease in number with time after a peak in early adulthood [1,2,4]. The average survival from time of diagnosis of ileal carcinoid is 8.1 years [35]. The 5-year survival rate is 60% for localised disease, 73% for those with regional metastases [14].
The average colonic carcinoid is 5 cm at presentation and over 60% have either nodal or distant metastases at diagnosis [2]. The size of the primary lesions of rectal carcinoids also closely correlates to the metastatic probability, which occurs in < 5% in tumours < 1cm [2]. The overall 5-year survival is 52%, 70% for those with local disease, 44% in those with regional metastases, and 20% in those with distant metastases [1,2,46].
Rectal carcinoids have an overall five-year survival of 81% in patients with local disease, 47% for regional metastases and 18% for distant metastases, with metastasis commonly to local lymph nodes and the liver [2].
Treatment
Treatment can either be surgical, medical or radiological and this section shall illustrate these various options. The presence of intraabdominal or hepatic metastases warrants a combined medical, surgical and radiotherapeutic approach to control the symptom and the size of the tumour [4]. There are still significant uncertainties regarding the management options for patients with metastatic disease. In general, the more aggressive and poorly differentiated tumours are treated with chemotherapy, whilst the well differentiated tumours may be treated with a variety of biotherapeutic regimens [40].
A treatment summary from the available literature is presented in Table 3 [2-6,16,18,20,28,40].
| Type of Disease | Treatment | |
|---|---|---|
|
|
Well differentiated Tumours | Variety of biotherapeutic regimens (eg. Octreotide, lanreotide± IFN α) |
|
|
Poorly Differentiated Tumours | Chemotherapy |
|
|
Primary Tumours
|
Definitive treatment with surgical resection
|
|
|
Presence of Distant Metastasis
|
|
Table 3: Treatment summary of the available literature.
Surgical Treatment
Surgical removal of the primary tumour is the mainstay treatment [1-5,7,8,11,14,19,20,23,26,28-32,35,36, 40,44,46]. The patients will have either localized surgically curable disease or metastatic incurable disease [3]. The goal is to obtain tissue for diagnosis, document the extent of disease, remove tumour for potential cure, palliate symptoms and to prolong survival [3,32]. The success depends on the site, size and presence of metastases [3,4,6,7].
Whilst generally the success of surgery is related to the size of the primary tumour, this does not pertain to the small intestine [3]. Carcinoid tumours of the small intestine often presents as small bowel obstruction and is treated with resection of the involved segments and mesentery, wide enough to ensure negative margins and removal of the involved lymph nodes [1-3,5,10,16,28,44]. This may benefit even patients with extensive liver or lymph node metastases [3].
Duodenal lesions ≤ 1cm can be excised via endoscopic approach, however, as the lesions are generally submucosal, it is usually not possible to completely excise them [1,3,29,44]. Hence lesions ≥ 2cm generally requires a pancreaticoduodenectomy or segmental resection [44]. Furthermore, there could be synchronous or metachronous tumour, therefore, the entire bowel must be examined carefully [1,5,7,8,16,19,20].
About two thirds of the appendiceal carcinoids arise in the tip, whereas less than 10% arise in the base [14]. Simple appendectomy of a tumour < 1cm in diameter, which is usually 99% of the occurrences is curative [2,3,14,44]. For tumours 1-2 cm, appendectomy is adequate unless there is evidence of local mesenteric invasion or presence of tumour at the base of the appendix [1-3,11,14,16,23]. In the latter and those ≥ 2cm standard right hemicolectomy is recommended [1,3,5,11,14,16,23,44].
In the colon, the tumours are usually seen in the caecum due to the greater concentration of Kulchitsky cells there and are treated with a right hemicolectomy [5]. Whereas sigmoid carcinoids are treated by anterior resection and unresectable lesions by colostomy [5]. Rectal lesions between 1-2 cm without evidence of invasion can be locally excised and regular examination will be sufficient treatment as less than 5% of tumours < 1cm metastasize [1,2,5,7,16]. Rectal lesions > 2cm require low anterior resection or abdominoperineal resection as metastasis occurs in 18-20% of the cases [1,2,5,7,16]. However, in the latter group, retrospective studies have shown that these procedures do not appear to extend the survival beyond that observed with local excision [2]. Therefore one should take into account the patient’s age and co-morbidities as the approach for larger rectal carcinoids [2,16].
Isolated distant metastatic resection may be curative in selected patients [10-25%] with metastatic carcinoid tumours but it is often multifocal, and may be followed by arterial embolization and/or interferon treatment [2-4,6, 14,16,19,20,23,28,32,44]. Attempted treatment of liver metastases can improve quality of life and survival. [1-3,19,26,32,44]There are reported cases of resection of solitary liver metastases resulting in symptomatic relief of carcinoid syndrome and urinary levels of 5-HIAA were reduced to normal and has been shown to prolong median survival from 5 to 11 years for patients [3,6,20,26]. The mean survival following surgery has been reported to be 60 to 216 months, while untreated patients is 65 months [3].
There is limited data on liver transplantation in the treatment of metastatic carcinoid tumours [14,23] It has been reported to have high rates of peri-operative mortality and tumour recurrence [2,14]. A recent study by Le Treut YP et al. reported a 5-year survival rate of 69% among highly selected group of patients [2,29].
Carcinoid crisis can occur during the induction of anaesthesia at the time of surgery or during radiologic interventions that reduce or interrupt blood flow to the tumours in patients with carcinoid syndrome [3,44]. This is due to the bradykinergic effects associated with bronchospasm, hypotension and electrolyte disturbances [5]. It may also result in prolonged recovery from anaesthesia in some patients, but premedication with anti-5HT agents and a smooth nontraumatic induction may be reduce this risk [5]. The administration of intravenous somatostatin analogue such as octreotide has been reported to reverse the life threatening hypotension and reduce intraoperative complications in 11% of patients [3,6,44].
Initial adjuvant therapy is withheld post-operatively until there is definite evidence that tumour progression is causing disability [35]. This reduces the risk of exacerbation of carcinoid syndrome due to chemotherapy-induced tumour cells lysis causing increased release of carcinoid mediators [35]. Some authors found that the increased frequency and intensity of flushing and changes in mentation before chemotherapy appear to indicate the increased risk of chemotherapy induced crisis [35]. Halving the usual dose of chemotherapy is thought to be beneficial in these cases [35].
Hepatic Artery Embolization
Hepatic artery embolization was first reported in 1977 and is now achieved by arterial embolization with gelfoam, steel coils, or surgical ligation [2,4,8,16,18]. It takes away approximately 90% of oxygenation from the tumour causing cell death whereas normal liver will lose only 50% as it is sustained by portal vein flow [14,16]. It is not curative and duration of response is often short-lived, 4-24 months as collateral blood supplies develop [1,2,14,18,29]. It is effective for symptomatic control, to achieve tumour shrinkage and reduce 5-HIAA excretion [1,2,8,14,23,32,41]. However, there is no reported survival advantage with this technique [17]. Side effects commonly reported include pain, pyrexia, nausea, liver function test derangement, hepatic necrosis, carcinoid crisis or acute cardiovascular collapse [2,4,16-18,29]. This may also result in an overwhelming release of vasoactive substances leading to circulatory instability and precipitation an acute carcinoid crisis [17,41].Thus this procedure with a reported mortality of 4% is seldom performed these days [4,17,29].
Chemoembolization may play a role in symptomatic relief and providing sustained tumour control [8,27]. Chemoembolization with doxorubicin and dacarbazine alternating with streptozocin and 5FU may be useful, with an objective response rate of 56% and a median time to progression of 9.9 months [2,18]. Symptomatic palliation has been reported to last for 6-12 months [17,18]. Hepatic artery ligation and infusion with 5-FU are useful if the liver disease is extensive and cannot be resected [5]. Also, combination of embolization with interferon-α has been shown to reduce urinary excretion of 5-HIAA in more than 70% of all patients [4].
Radiofrequency Ablation
This relatively new technique has also been reported in the literature with good symptom control in metastatic disease [2,8,14,27]. It is useful in treatment of unresectable primary and secondary hepatic nodules up to 4 cm in diameter with relatively low complication rate and low mortality [27]. The use of this technique has resulted in local tumour control in majority of patients, as well as biochemical and symptomatic response in approximately 60-80% of patients [27]. Unfortunately, the development of new metastases and local recurrence have been reported [27].
Radionucleide Therapy
Iodine-131-labelled metaiodobenzylguanidine [131I-MIBG] requires careful selection of patients with good uptake of the radioactive tracer and it has been found to be useful in controlling symptoms and may reduce the tumour size in a small number of patients [4,14]. In a prospective study by Taal et al. [27,47] significant symptomatic response was reported in 60% of patients treated with 131I-MIBG, for a period of 8 months and the treatment with unlabelled MIBG resulted in a 60% symptomatic response rate for a shorter median duration of 4.5 months. Side effects were reportedly mild and short-lived, with the main disadvantage being the requirement for isolation [27].
Medical Treatment
Patients with metastatic carcinoid tumour should be treated medically unless they have intestinal obstruction, ischemia, or are refractory to medical therapy [30]. The aim of treatment is for symptomatic relief by reducing hormone levels and tumour growth, and to improve quality of life as tumour load may not be significantly altered [17,36,46]. There is no clear evidence that the particular treatment is able to reduce tumour size in the majority of patients, therefore, it should be chosen as a function of adverse effects, long term efficacy and possibility of repetition of treatment [4,7].
Chemotherapy
The objective response rate of conventional chemotherapy has been reported to be 10-30% [1,2,36,46]. Clear therapeutic advantage has not been demonstrated and due to this questionable efficacy and associated toxicity, chemotherapy should be reserved for those who have not responded to other therapies [2,4,7,14,16,23,27,36].
When used individually, symptomatic remission is only transient, lasting a few months and no positive result is found in relation to the tumour size [4,17,23]. The response rate has been reported to be 10-33% and this is increased with combination therapy [4,17,18,41,44]. Combination therapy, especially the four drug regimen of 5-fluorouracil, streptozotocin, doxorubicin and cyclophosphamide has been reported to be superior to single therapy [4,40]. Combination of streptozocin and 5-fluorouracil has been shown to have an overall response rate of 29-33%, with median survival of 28 months in patients with primary small bowel carcinoid tumours [18,44,48].
Serotonin antagonists
There are multiple agents which inhibit 5HT synthesis and peripheral 5HT antagonists many of which are poorly tolerated due to their side effects and have fallen out of favour [4,17,18]. In addition they are rarely associated with tumour regression [14].
Parachlorphenylalanine is an inhibitor of tryptophan hydroxylase which prevents the conversion of tryptophan to 5HT [17]. It relieves flushing and diarrhoea in 50-60-% of patients and reduces urinary 5-HIAA excretion in up to 80% of patients [16,17]. Unfortunately, there is significant incidence of psychiatric symptoms like confusion and depression, and may cause significant sedation and hypersensitivity reaction [16,17].
P-chlorophenylalanine reduces 5HT synthesis, and is reported to reduce diarrhoea, flushing and 5-HIAA excretion by 50-90% [4,18]. However, it causes hypersensitivity reactions and psychiatric disturbances [4,18].
Cyproheptadineis a serotonin and histamine antagonist [16]. It has only been shown to be useful in reducing diarrhoea in 60% of patients and flushing in 47% of patients, but has a mean duration of response of 8 months and has no effect on the tumour size and may exacerbate other complications of carcinoid syndrome like heart disease [4,16,17,20].
Methysergide has been found to provide some symptomatic relieve [18,20]. Ketanserinis shown to reduce the frequency and severity of flushing by 70% and diarrhoea by 30% [18]. However due to their significant side effects like dizziness, sedation, nausea and hypotension, they are not used routinely [18].
Ondansetron has been reported to provide immediate and sustained symptomatic relief [18,36]. However, it has no advantage in terms of reducing tumour growth and tachyphylaxis is often encountered [36].
Interferon α
The recombinant form, or the one derived from human leukocytes is thought to be a cytotoxic agent, acting selectively on tumour cells to inhibit cell division by prolonging the cell cycle [4,27,29]. Interferon α is found to significantly decrease the tumour markers in 40-55% of patients and reduce tumour size in 10-20% of the patients [4,14,18,27,36]. Stable disease is reported in 78% of patients and this is thought to be due to its cytostatic effect [17].
However, the objective tumour response rate is reported to range from less than 10% to 48% [2,17,18,29,44]. Symptomatic response rate has been reported to be 70% and the duration of response is only seven weeks [18,36]. The adverse effects include flu-like symptoms and chronic fatigue, development of interferon antibodies that affected the therapy’s effectiveness and autoimmune hypothyroidism in 20% of the patients [2,4,18,23,36]. Some of the adverse reaction are only common at the initiation of treatment, often transient and can be managed by dose reduction [36]. Higher doses are associated with slightly increased rate of tumour regression but are poorly tolerated [18].
Somatostatin analogue
This is considered the first line treatment for patients with low grade tumours and carcinoid syndrome, acting to inhibit the neuropeptide release and therefore the gut endocrine and exocrine function [14,17,37,39]. This is effective in controlling symptoms like diarrhoea, flushing and halves urinary 5-HIAA in more than 70% of patients [2-4,17,18,29,37,43]. However it has a very low rate of objective response of 10%, with commonly reported side effects of cholelithiasis in up to 50% of patients and decreased sensitivity to somatostatin due to tachyphylaxis [2,4,17,37,39,43]. Rarely, cardiac conduction abnormalities and arrhythmias, as well as endocrine abnormalities including hypothyroidism and hyperglycaemia have been reported [29].
Octreotide is particularly effective in abolishing flushing and has been reported to improve carcinoid syndrome in up to 88% of the patients [14,23,29]. It needs to be administered three times daily subcutaneously due to its short half-life of 90-120 minutes, but new slow release analogues and depot preparations have been developed that can be given every 2-4 weeks [14,18,23,27,29,39]. The median duration of response is 12 months, and the mean remission time has been reported to be 9 months [4,17,18,44]. Whilst the tumour growth is reported to be lower during treatment, there is no advantage in terms of tumour size reduction [4,18,44]. Also, it is effective in preventing carcinoid crisis associated with surgical removal of the tumour [4].
Combination therapy of interferon α and octreotide have been shown to be effective and better tolerated than monotherapy [14,18]. Up to 77% of patients had a biochemical response, but no significant tumour regression was reported [18,29]. The combined approach with surgical resection of the primary tumour, followed by hepatic artery embolization and 12 months of interferon has been reported to produce an objective tumour response rate of 85% at 12 months compared with 40% with interferon alone [17].
The development of several radioactive-labelled somatostatin analogues for use in neuroendocrine tumours with positive 111In-pentetreotide scintigraphy scans has brought symptomatic improvement frequently and tumour reduction in a minority of patients [27]. Partial remission was reported in 40% of patients treated with 90Y-labelled octreotide at 9 months [43]. The objective response rate has been reported to below with indium-111-labeled octreotide [14]. Whilst there were some radiologic response reported (27%), this did not correlate with improved survival [14]. However, with the development of higher tumour radiation particles, a greater tumour reduction response of 15-30% has been reported, with a clinical benefit of up to 60% [27]. However, side effects including nausea, vomiting, renal impairment and haematological toxicities have been reported [27].
Others
Adrenergic blocking agents like clonidine presumably exert their effect by inhibiting catecholamine triggering of the kallikreinbradykinin sequence [16]. Chlorpromazine act as a kinin antagonist but may exert its effect by blocking the emotional stimulus to flushing [16]. Corticosteroids have been effective by blocking kallikrein release [20]. Aprotinin and epsilon-tachostyptan have been used in isolated cases of refractory hypotension in carcinoid crisis [20].
Novel therapeutic approaches with regimen incorporating angiogenesis inhibitors and small molecule tyrosine kinase inhibitors have been reported [14]. The abundant vasculature and high levels of plasma vascular endothelial growth factors (VEGF) has led to the development of thalidomide which is postulated to have antiangiogenic activity, bevacizumab which is a humanised monoclonal antibody targeting VEGF [14]. Sunitinib is an orally active, multi-targeted tyrosine kinase inhibitor that specifically inhibits the VEGF receptor, platelet derived growth factor receptor and c-kit [14]. The preliminary report of a phase II study has suggested antitumor efficacy [14].
Radiotherapy
This form of treatment has not been extensively studied [2]. It may be effective in palliation of bone or central nervous system metastases [2,29].
Conclusion
Gastrointestinal tumours are generally slow-growing and malignant tumours and patients may live for years with the indolent disease. Diagnosis is difficult as it is often asymptomatic and nonspecific, and the presence of symptoms generally implies advanced disease. Whilst surgical resection remains the treatment of choice for primary and respectable disease, there are still significant uncertainties for management of those with metastatic disease. The aim of treatment is to achieve cure and or improve quality of life by palliating symptoms and prolong survival.
References
- Thompson GB, van Heerden JA, Martin JK, Schutt AJ, Ilstrup DM, et al. (1985) Carcinoid tumors of the gastrointestinal tract: presentation, management, and prognosis. Surgery 98: 1054-1063.
- Kulke MH, Mayer RJ (1999) Carcinoid Tumors. N Engl J Med 340: 858-868.
- Norton JA (1994) Surgical management of carcinoid tumors: role of debulking and surgery for patients with advanced disease. Digestion 55: 98-103.
- Napolitano G, Bucci I, Lio S, Giuliani C, Minnucci A, et al. (1991) An overview on the management of carcinoid tumors. J Nucl Biol medicine 35: 337-340.
- Aranha GV, Greenlee HB (1980) Surgical management of carcinoid tumors of the gastrointestinal tract. Am Surg 46: 429-435.
- Zarrilli L, Marzano LA, Porcelli A, D'Avanzo A, Misso C (1991) The surgical management of carcinoid tumors. J Nucl Biol Med 35: 341-342.
- Martin RG (1970) Management of carcinoid tumors. Cancer 26: 547-551.
- Horton KM, Kamel I, Hofmann L, Fishman EK (2004) Carcinoid tumors of the small bowel: a multitechnique imaging approach. AJR Am J Roentgenol 182: 559-567.
- Mrevlje Z, Stabuc B (2006) Pitfalls in diagnosing small bowel carcinoid tumors. J BUON 11: 83-86.
- Onaitis MW, Kirshbom PM, Hayward TZ, Quayle FJ, Feldman JM, et al. (2000) Gastrointestinal carcinoids: characterization by site of origin and hormone production. Ann Surg 232: 549-556.
- Shebani KO, Souba WW, Finkelstein DM, Stark PC, Elgadi KM, et al. (1999) Prognosis and survival in patients with gastrointestinal tract carcinoid tumors. Ann Surg 229: 815-823.
- Moran CA, Suster S (2007) Neuroendocrine carcinomas (carcinoid, atypical carcinoid, small cell carcinoma, and large cell neuroendocrine carcinoma): current concepts. Hematol Oncol Clin North Am 21: 395-407.
- Tamm EP, Kim EE, Ng CS (2007) Imaging of neuroendocrine tumors. Hematol Oncol Clin North Am 21: 409-432.
- Kulke MH (2007) Clinical presentation and management of carcinoid tumors. Hematol Oncol Clin North Am 21: 433-455.
- Zander DR, Rosenbloom M, Bégin LR (1998) Residents' corner. Answer to case of the month #52. Carcinoid tumour of the small bowel. Can Assoc Radiol J 49: 49-51.
- Moertel CG (1983) Treatment of the carcinoid tumor and the malignant carcinoid syndrome. J Clin Oncol 1: 727-740.
- Saini A, Waxman J (1991) Management of carcinoid syndrome. Postgrad Med J 67: 506-508.
- Halford S, Waxman J (1998) The management of carcinoid tumours. QJM 91: 795-798.
- Makridis C, Oberg K, Juhlin C, Rastad J, Johansson H, et al. (1990) Surgical treatment of mid-gut carcinoid tumors. World J Surg 14: 377-383.
- Strodel WE, Talpos G, Eckhauser F, Thompson N (1983) Surgical therapy for small-bowel carcinoid tumors. Arch Surg 118: 391-397.
- Burke AP, Thomas RM, Elsayed AM, Sobin LH (1997) Carcinoids of the jejunum and ileum: an immunohistochemical and clinicopathologic sutdy of 167 cases. Cancer 79: 1086-1093.
- Maggard MA, O'Connell JB, Ko CY (2004) Updated Population-Based Review of Carcinoid Tumors. Ann Surg 240: 117-122.
- Janmohamed S, Bloom SR (1997) Carcinoid tumours. Postgrad Med J 73: 207-214.
- Tsoukas E (1965) Radiological notes. Case No. 257. Carcinoid tumor of the small bowel (2 cases). J Mount Sinai Hosp N Y 32: 689-693.
- Miller GA, Borten MM (1991) Primary carcinoid tumour of the ileum associated with massive gastrointestinal haemorrhage. Aust N Z J Surg 61: 645-646.
- Makridis C, Rastad J, Oberg K, Akerström G (1996) Progression of metastases and symptom improvement from laparotomy in midgut carcinoid tumors. World J Surg 20: 900-906 .
- Zuetenhorst JM, Taal BG (2005) Metastatic Carcinoid Tumors: A Clinical Review. The Oncologist 10: 123-131.
- Ormandy SJ, Parks RW, Madhavan KK (2000) Small bowel carcinoid tumour presenting with intestinal ischaemia. Int J clin Practice 54: 42-43.
- Schnirer II, Yao JC, Ajani JA (2003) Carcinoid-- a comprehensive review. Acta Oncol 42: 672-692.
- Tessier DJ, Harris E, Johnson DJ (2002) Synchronous carcinoid tumor of the small bowel. J Am Coll Surg 195: 890-891.
- von Knorring J, Höckerstedt K, Holmström T, Salaspuro M, Scheinin TM (1981) Severe gastrointestinal bleeding due to carcinoid tumour of the ileum. Ann Chir Gynaecol 70: 18-21.
- Søreide O, Berstad T, Bakka A, Schrumpf E, Hanssen LE, et al. (1992) Surgical treatment as a principle in patients with advanced abdominal carcinoid tumors. Surgery 111: 48-54.
- Payne-James JJ, de Gara CJ, Lovell D, Misiewicz JJ, Gow NM (1990) Metastatic carcinoid tumour in association with small bowel ischaemia and infarction. J R Soc Med 83: 54.
- Costello C (1965) Management of carcinoid syndrome. Arch Surg 90: 787-792.
- Bonomi P, Hovey C, Dainauskas JR, Slayton R, Wolter J (1979) Management of carcinoid syndrome. Med Pediatr Oncol 6: 77-83.
- Oberg K, Eriksson B (1991) The role of interferons in the management of carcinoid tumours. Br J Haematol 79: 74-77.
- Ganim RB, Norton JA (2000) Recent advances in carcinoid pathogenesis, diagnosis and management. Surg Oncol 9: 173-179.
- Chetty R (2008) Requiem for the term 'carcinoid tumour' in the gastrointestinal tract? Can J Gastroenterol 22: 357-358.
- Bajetta E, Catena L, Procopio G, Bichisao E, Ferrari L, et al. (2005) Is the new WHO classification of neuroendocrine tumours useful for selecting an appropriate treatment? Anna Oncol 16: 1374.
- Artale S, Giannetta L, Cerea G, Maggioni D, Pedrazzoli P, et al. (2005) Treatment of metastatic neuroendocrine carcinomas based on WHO classification. Anticancer Res 25: 4463-4469.
- Modlin IM, Moss SF, Oberg K, Padbury R, Hicks RJ, et al. Gastrointestinal neuroendocrine (carcinoid) tumours: current diagnosis and management. Med J Aust 193: 46-53.
- Goldsmith SJ (2009) Update on nuclear medicine imaging of neuroendocrine tumors. Future Oncol 5: 75-84.
- Wong M, Kong A, Constantine S, Pathi R, Parrish F, et al. (2009) Radiopathological review of small bowel carcinoid tumours. J Med Imaging Radiat Oncol 53: 1-12.
- Woodside KJ, Townsend CM, Mark Evers B (2004) Current Management of Gastrointestinal Carcinoid Tumors. J Gastrointest Surg 8: 742-753.
- Agranovich AL, Anderson GH, Manji M, Acker BD, Macdonald WC, et al. (1991) Carcinoid tumour of the gastrointestinal tract: prognostic factors and disease outcome. J Surg Oncol 47: 45-52.
- Oberg K, Eriksson B (1991) The role of interferons in the management of carcinoid tumors. Acta Oncol 30: 519-522.
- Taal BG, Hoefnagel CA, Valdes Olmos RA, Boot H, Beijnen JH (1996) Palliative effect of metaiodobenzylguanidine in metastatic carcinoid tumors. J Clin Oncol 14: 1829-1838.
- Moertel CG, Hanley JA (1979) Combination chemotherapy trials in metastatic carcinoid tumor and the malignant carcinoid syndrome. Cancer Clin Trials 2: 327-334.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 16297
- [From(publication date):
June-2012 - Nov 15, 2025] - Breakdown by view type
- HTML page views : 11499
- PDF downloads : 4798