Global Climate Change and Infectious Diseases: Invasive Mycoses
Received: 21-Sep-2011 / Accepted Date: 14-Oct-2011 / Published Date: 17-Oct-2011 DOI: 10.4172/2157-7617.1000108
Abstract
Global climate change through rising temperatures and changing rainfall patterns is leading to disparate patterns of infectious diseases across space and time. Although the seasonality of respiratory viral illnesses, gastrointestinal infections, and vector-borne-diseases has been well described, the geoclimatic influences on invasive mycoses are less elucidated. Herein is presented a narrative review of the impact of world-wide climate change on the development of endemic and non-endemic invasive fungal infections (IFI) in humans framed within the classic “disease triangle” paradigm.
Keywords: Invasive Mycoses; Seasonality; Biogeoclimatic; Endemic Fungi
5427Introduction
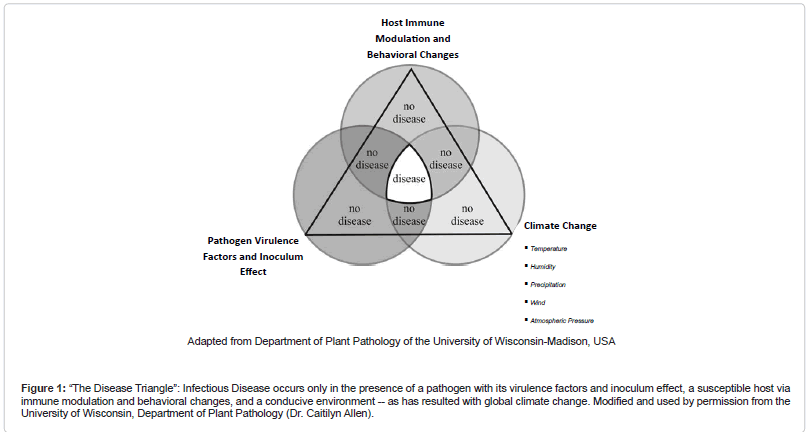
The impact of global climate change on the development of infectious diseases is depicted well by the classic “disease triangle.” In this paradigm, the pathogen, with its virulence factors and inoculum effect, serves as a component cause for disease only in the presence of a susceptible host via immune modulation and behavioral changes, and a favorable environment (Figure 1 (The Disease Triangle”: Infectious Disease occurs only in the presence of a pathogen with its virulence factors and inoculum effect, a susceptible host via immune modulation and behavioral changes, and a conducive environment -- as has resulted with global climate change. Modified and used by permission from the University of Wisconsin, Department of Plant Pathology (Dr. Caitilyn Allen))) [1].
Figure 1: “The Disease Triangle”: Infectious Disease occurs only in the presence of a pathogen with its virulence factors and inoculum effect, a susceptible host via immune modulation and behavioral changes, and a conducive environment -- as has resulted with global climate change. Modified and used by permission from the University of Wisconsin, Department of Plant Pathology (Dr. Caitilyn Allen).
Variations in host susceptibility and other physiological changes due to light-dark cycles (photoperiodism) and consequent alteration in circadian rhythms and changes in the daily secretion duration of melatonin – the byproduct, melanin, being a known immunomodulator – have been reported [2]. Malnutrition due to climate-induced damage to agricultural produce can suppress the body’s immunity to infection. Ultraviolet radiation influx may further alter human cell mediated immunity and cause damage to skin and genes [3,4].
Psychoneuroimmunology is a field, the premise of which is that human psychological stress from life situations that may be elicited by the environment can depress the immune system via a variety of mechanisms [5]. To the extent that such stressors affect humans also psychologically, immune function weakening can occur. Moreover, in response to such stressors, humans may change behavior to cluster in enclosed areas during the cold season, making them more likely to be exposed to communicable diseases. Global migratory patterns may change in response to climate leading to novel exposures for immune naïve individuals.
The net emission of the “greenhouse gases” (CO2, NO, CH4), resulting from fossil fuel consumption and deforestation/land use, has induced a conducive environment for the emergence of several infectious diseases beyond their previously defined niches [6]. For example, mosquito-borne diseases, such as malaria and dengue, have shifted their geographic ranges in relation to warming effects that increase vector biting and reproductive rates, and shorten pathogen incubation period. In central Ethiopia, after controlling for drug resistance, population migration, and vector control efforts, an association between warming trends and malaria incidence has been noted in the highlands [7]. Food and waterborne illness, such as cholera and algal toxins, have occurred after rising sea levels and frank flooding due to the El Niño Southern Oscillation (ENSO) and tropical storms/hurricanes, resulting from rising sea surface temperature and air pressure anomalies. For instance, cholera outbreaks in Bangladesh have coincided with an increasing Southern Oscillation Index (SOI) [8,9]. The SOI measures fluctuations in sea surface temperature across the Pacific linked to atmospheric pressure and wind anomalies. “Red tide” algal blooms (e.g. Noctiluca scintillans and Karenia brevis) have affected the shellfish industry through water current shifts in the South China Sea [10]. Viruses, such as influenza and poliomyelitis, exhibit seasonality at various times of the year dependent upon distance from the equator and latitude [2].
But what about fungal infections? The relationship between climate change and allergic phenomenon, such as “thunderstorm asthma” triggered by inciters such as mould spores, is well documented [11]. Further, superficial mycoses such as oncychomycosis occur more commonly in humid, tropical ecologic zones [12]. However, the patterns of invasive mycoses with respect to environmental acquisition of mould spores and climatic variables are less often described. We have previously reviewed fungal infections in returning travelers and the consequent migratory effects [13]. Herein, we report the first review of the impact of climate change on invasive human mycoses, beyond merely those with known geographic restrictions.
Endemic mycoses
Coccidioidomycosis (Valley fever) is caused by the dimorphic fungus Coccidioides immitis in central and southern California and by C. posadasii outside California -- being found in the soil of certain arid, semidesert regions of the southwestern United States (Arizona, New Mexico, western Texas, and parts of Utah). The disease is also endemic in parts of Central and South America extending from Mexico to Argentina. Human infection occurs after inhalation and has been associated with ground disturbing activities, such as earthquakes [13]. Its seasonality has been documented since the 1930’s [14]. Comrie has demonstrated a bimodal seasonality for exposure to spores in Tuscon, Pima County, Arizona. Peaks occurred during the summer months of June-July and fall months of October-November. He used cross-validated time series and regression techniques. Previous significant climatologic variables included: drought indices; lagged precipitation; temperature; wind speed; and dust during the preceding one or more years. However, precipitation -- during the normally arid foresummer 1.5-2 years before the presumed season of exposure (known because the incubation period is approximately 14 days) -- was found to be the dominant predictor of disease in all seasons [15,16]. Furthermore, Park et al. [17] found the highest incidence of disease in Phoenix, Maricopa County, Arizona to occur in the winter (from November-February) using Poisson regression, after controlling for lagged variables including average wind velocity during the previous 3 months, cumulative rainfall during the previous 2 and 7 months, and average dust.
Acute and subacute paracoccidioidomycosis, endemic to many parts of Latin America, especially southeastern Brazil, is also influenced by climate. Caused by Paracoccidioides brasiliensis, disease may take an acute form, in which rapid dissemination to the reticulo endothelial system (RES) occurs in the mostly young, or a chronic form, which has an incubation period that spans years and primarily affects the lungs and oropharynx. In studies by Barrozo et al. [18] space-time clusters of disease were detected retrospectively in the central and western regions of Sao Paulo State, Brazil with a scan test and found to occur in areas where soil water storage and absolute air humidity were atypically high in the year prior to illness identification and independent of land use [18]. In another study spanning 31 years, these same investigators, found that 49% of the disease variability could be explained by air humidity in the year of exposure, soil water storage, and SOI – a measure of the El Niño effect – lagged 3 years before disease identification [19].
Penicilliosis is endemic to parts of Southeast Asia (Thailand, Vietnam, Myanmar, parts of Hong Kong, Taiwan, Indonesia, Laos, Malaysia, Singapore, and the Guang Xi province in southern China) and northeast India. It is caused by the dimorphic fungus, Penicillium marneffei, which is thought to be acquired from the soil. It affects mostly the immuncompromised such as patients with HIV and cancer. The most common symptoms are fever, weight loss, and anemia and the disease’s extent depends on the level of immune suppression, which can include skin lesions, fungemia, and RES involvement [13]. Recently, Le et al. [20] used cosinor models to find that there was a 27% (95% CI 14%-41%, p<0.001) increased incidence of penicilliosis among AIDS patients in Vietnam from 1996-2009 during the rainy months of May- November with peak cases in August – the amplitude corresponding to a 32% higher odds (95% CI: 17%-48%) for penicilliosis admission compared with the mean [20].
Blastomycosis, caused by Blastomyces dermatitidis, may become acquired after inhalation. After an incubation period of approximately one month, the lung, skin, and bones may become affected. It is endemic to the river estuaries in midwestern, southeast, and south central U.S. and Canadian provinces bordering the Great Lakes and St. Lawrence Seaway and scattered foci in Europe, Asia, Central and northern South America, and Africa (especially South Africa) and demonstrates an interesting seasonal pattern in disease extent. In Manitoba and northwestern Ontario, 63% of 324 patients with blastomycosis were identified in the autumn and winter (September-February). However, localized disease peaked during the autumn whereas the preponderance of diffuse lung and disseminated disease was in the spring (p<0.0001). The authors concluded that summer spore acquisition resulted in early, localized disease in the 1-6 months post-exposure, but gave rise to later reactivation or slower progression 4-9 months post-exposure as manifest by diffuse and extrapulmonary disease [21].
Nonendemic mycoses
Invasive aspergillosis (IA) leads to high morbidity and mortality (67%-80%) in the immunocompromised host, many of whom are spending more time outside the healthcare setting shortly after hospitalization with improved care. Unfortunately, the lack of a known incubation period and minimum infectious inoculum, and the high genetic diversity and likely nonclonality of infection make the source of IA and climatic effects difficult to identify. We have previously described a retrospective cohort study of hematopoietic stem cell transplant recipients (HSCT) from two geographically and climatologically disparate cities: Seattle, Washington, USA in the temperate Pacific Northwest and Houston, Texas, USA in the humid South. Though both cities are near a coast, Houston weather is much warmer in the spring and summer due to the warming effects from the Gulf of Mexico, but unlike Seattle, precipitation is increased during the warmer weather. The study encompassed the period of 1992-2003 and involved over 4000 HSCT from each site. After dividing seasons into the ‘warm’ periods (April-September) and ‘cool’ periods – the remainder of the year and collecting mould spore count information from allergy clinics, IA incidence 3 month post-transplant exhibited a significant seasonality during the ‘warm’ season in Seattle (adjusted HR=1.59, 95% [1.21,1.99], p=0.0006) but not in Houston (adjusted HR=0.68, 95%CI [0.36-1.24], p=0.20). In Seattle, the monthly spore count increased with high temperature (Spearman ρ=0.6, p<0.00001) and low precipitation (Spearman ρ = -0.3, p <0.00001). Similarly, in Houston, the monthly spore count also increased with rising temperature (Spearman ρ=0.5658, p<0.00001), but was only marginally correlated with precipitation, and decreased with lower average wind velocity (Spearman ρ = -0.4, p = 0.0022). In addition, the barometric pressure was directly proportional to spore count (Spearman ρ=0.3735, p=0.0001). No lag effect was identified and, thus, the finding suggests spore acquisition at the time of transplant and induction immunosuppression is a major environmental exposure in developing IA. To further this assertion, recrudescence of latent disease was excluded since most IA cases are believed to develop de novo. In Houston, there was a significant difference in time to diagnosis of IA by species with A. flavus being diagnosed sooner than A. terreus and A. fumigatus (log rank test p=0.0224) [22]. Of note, in Spain, Guinea et al found a similar pattern to Seattle in that environmental air levels of Aspergillus conidia increased when temperature increased but decreased with rainfall, but like Houston, species (the most common being A. fumigatus followed by A. niger and A. flavus) did not vary significantly by season, but, in contrast, most isolates were identified during the Autumn – albeit just environmental samples and not human samples [23].
Invasive candidiasis (IC) may also exhibit seasonality. In the UK, premature neonates < 32 weeks gestation were more than three times at risk of being diagnosed with IC during the autumn and winter months of September-February. The result was statistically significant after controlling for other factors and may be explained by seasonal variation in the antenatal environment, though acquisition via the birth canal is unlikely since the seasonality of vulvovaginal candidiasis has never been documented [24]. In another study, we analyzed the National Hospital Discharge Survey (NHDS) for invasive fungal infections (IFI) from 1996-2006 in the U.S. The majority were due to Candida (63%). Although the adjusted relative risk for IFI hospitalization by region was not significant and by season appeared to decrease significantly by 81% (p=0.0082) in the autumn, the seasonal effect was modified by geographic region. For example, being discharged from a hospital in the northeast and midwest in the autumn actually increased the risk of IFI hospitalization and hospital days of care rate dramatically (RR=8.14 [95%CI: 2.03, 32.6], p=0.0030, IRR=5.31 [95%CI: 1.02, 25.3], p=0.05; and RR=6.25 [95%CI: 1.57, 24.9], p=0.009, IRR=5.09 [95%CI:1.02, 27.7], p=0.05, respectively). Though the IFI-related mortality rate was least for the youngest age group, regardless of season (IRR=0.155[95%CI: 0.044, 0.550]), there was a reduced rate of such mortality in the spring of Midwest among all age groups (IRR=0.44 [0.20, 0.95], p=0.03) [25].
Cryptocococcus gattii, formerly C. neoformans var. gattii, has adapted from the historically desert-like and tropical climates of Australia and South Africa, respectively to a new microniche in the temperate climate of the U.S. Pacific Northwest that has been attributed to the effects of global warming – with increasingly warm, dry summers, and mild, wet winters [26,27]. It is a basidiomycetous yeast that is able to survive wide temperature ranges through production of a manganese superoxide dismutase and resists UV irradiation through its production of melanin from soil nutrients [27], but cannot make creatinine deiminase like C. neoformans and so is unable to grow in alkaline pigeon droppings appreciably. C. gattii infections cause granulomatous, solitary cryptococommas in the lung and brain with neurological sequelae more commonly than its cogeners. Though seasonal effects in environmental sampling have been found, no seasonality in disease incidence has been identified.
Pneumocystis jirovecii was formerly called P. carinii and was classified as a sporozoan. However, through molecular techniques, it is now classified as a fungus. It causes Pneumocystis pneumonia (PCP) in immunocompromised patients with AIDS and malignancy, and transplant recipients. It may exhibit seasonal patterns dependent upon geographic locale, but controversy exists. In Seville, Spain, Varela et al. [28] showed that there was an inverse relationship between PCP incidence and minimum mean ambient temperature (Spearman ρ = -0.30 (p=0.02); ARIMA model: r= -0.250, p=0.07) with most cases occurring during the winter, December-February (ANOVA, p<0.05) [28]. In contrast, in Germany, Sing et al. [29] showed the opposite wherein PCP incidence was positively correlated with mean temperature, after controlling for total precipitation, mean and maximum wind force, and highly active antiretroviral (HAART) use. A higher risk for disease occurred in the summer (May-August) in the absence of a lag effect (4-8 weeks incubation period) [29]. Also controversial, in the UK, Miller and colleagues have noted not only a similar summer pattern, but genotypic differences. Mixed genotypes and genotype 2 showed a significant association with calendar month (May-July) (p=0.018 and p=0.029, respectively) but no significant association with temperature or rainfall [30]. Drawing from the findings, of the Multicenter AIDS Cohort Study during the pre-HAART era in the U.S. in which an increase PCP incidence was found among HIV+ men who have sex with men from colder cities than warmer ones, but peak incidences were in May-June, suggesting the seasonal incidence amplitude may be inversely related to the overall average yearly temperature for a locale [31]. These observations support the contrasting findings from the afore-mentioned studies. The timing of peak PCP incidence in the overall warm, Mediterranean climate of southern Spain -- in which there are hot, dry summers and mild winters, and the overall cooler temperate climate of Germany and the UK implies a moderate temperature ideal for PCP occurrence.
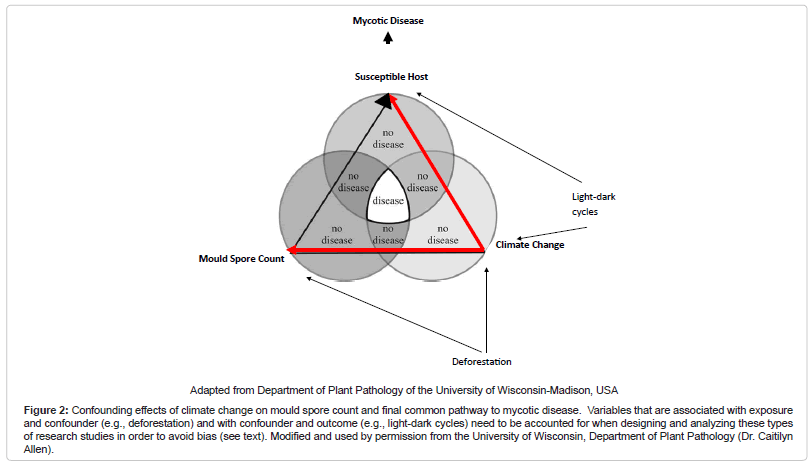
In conclusion, one can posit from these studies that a “biogeoclimatic hypothesis” for mycoses exists. A common pattern appears to be that the growth of fungal propagules is facilitated by soil moisture from precipitation and humidity followed by a relatively dry period when fungal hyphae dessicate and form spores, and then a dispersion event when wind or other disturbances such as seismic activity permits acquisition by a host and disease. Of note, others have coined this phenomenon the “grow and blow” hypothesis [16]. The exact time period between such events and patterns is variable, dependent on subcomponents of the “disease triangle.” In particular, weather variables may affect one another differentially under varied circumstances, such as in different ecological zones. Therefore, development of a composite climatic index based on a score may become desirable given the intricacies of these analyses. For instance, such a score would be favorable over stratification which may lead to a sparse data problem. Moreover, understanding the relationship of variables within the “disease triangle” is necessary to appropriately control for confounding. For example, if we control for climate change, which is a confounder between the relationship of mould spore count and the host – the final common pathway to the disease state – but fail to control for variables such as deforestation (that is associated with mould spore count and climate change) and light-dark cycles (that is associated with climate change and host immune modulation), there may be confounding within the climate change strata that may lead to an upward or downward estimate of risk between mould spore exposure and disease, depending upon the magnitude and directionality of the influence (Figure 2 (Confounding effects of climate change on mould spore count and final common pathway to mycotic disease. Variables that are associated with exposure and confounder (e.g., deforestation) and with confounder and outcome (e.g., light-dark cycles) need to be accounted for when designing and analyzing these types of research studies in order to avoid bias (see text). Modified and used by permission from the University of Wisconsin, Department of Plant Pathology (Dr. Caitilyn Allen))) [32]. Furthermore, preventative strategies for IFI need to focus on non-healthcare associated sources given the likelihood of environmental exposure outside the healthcare setting. Increase antifungal prophylaxis among high risk persons during predicted high incidence time periods may be warranted. For those mycoses that are highly dependent on an immunocompromised host to effect invasive disease, a lag period for exposure when modeling may not be necessary, as illustrated by IA and PCP, unless reactivation disease is considered. Finally, it will be useful to determine if the known seasonality of other pathogens such as respiratory viruses that co-infect with fungi induce an “apparent seasonality” among mycoses or are the effects independent of one another.
Figure 2: Confounding effects of climate change on mould spore count and final common pathway to mycotic disease. Variables that are associated with exposure and confounder (e.g., deforestation) and with confounder and outcome (e.g., light-dark cycles) need to be accounted for when designing and analyzing these types of research studies in order to avoid bias (see text). Modified and used by permission from the University of Wisconsin, Department of Plant Pathology (Dr. Caitilyn Allen).
References
- Francl LJ (2001) The Disease Triangle: a plant pathological paradigm revisited. The Plant Health Instructor.
- Dowell SF (2001) Seasonal variation in host susceptibility and cycles of certain infectious diseases. Emerg Infect Dis 7: 369-374.
- Patz JA, Epstein PR, Burke TA, Balbus JM (1996) Global climate change and emerging infectious diseases. Jama 275: 217-223.
- Norval M, Lucas RM, Cullen AP, De Gruijl FR, Longstreth J, et al. (2011) The human health effects of ozone depletion and interactions with climate change. Photochem Photobiol Sci 10: 199-225.
- Irwin MR (2008) Human psychoneuroimmunology: 20 years of discovery. Brain Behav Immun 22: 129-139.
- Shuman EK (2010) Global climate change and infectious diseases. N Engl J Med 362: 1061-1063.
- Patz JA, Campbell-Lendrum D, Holloway T, Foley JA (2005) Impact of regional climate change on human health. Nature 438: 310-317.
- Pascual M, Rodo X, Ellner SP, Colwell R, Bouma MJ (2000) Cholera dynamics and El Nino-Southern Oscillation. Science 289: 1766-1769.
- Rodo X, Pascual M, Fuchs G, Faruque AS (2002) ENSO and cholera: a nonstationary link related to climate change? Proc Natl Acad Sci USA 99: 12901-12906.
- James KJ, Carey B, O'Halloran J, Van Pelt FN, Skrabakova Z (2010) Shellfish toxicity: human health implications of marine algal toxins. Epidemiol Infect 138: 927-940.
- Nasser SM, Pulimood TB (2009) Allergens and thunderstorm asthma. Curr Allergy Asthma Rep 9: 384-390.
- Kamalam A, Thambiah AS (1976) A study of 3891 cases of mycoses in the tropics. Sabouraudia 14: 129-148.
- Panackal AA, Hajjeh RA, Cetron MS, Warnock DW (2002) Fungal infections among returning travelers. Clin Infect Dis 35: 1088-1095.
- Smith CE (1940) Epidemiology of Acute Coccidioidomycosis with Erythema Nodosum ("San Joaquin" or "Valley Fever"). Am J Public Health Nations Health 30: 600-611.
- Comrie AC (2005) Climate factors influencing coccidioidomycosis seasonality and outbreaks. Environ Health Perspect 113: 688-692.
- Tamerius JD, Comrie AC (2011) Coccidioidomycosis incidence in Arizona predicted by seasonal precipitation. PLoS One 6: e21009.
- Park BJ, Sigel K, Vaz V, Komatsu K, McRill C, et al. (2005) An epidemic of coccidioidomycosis in Arizona associated with climatic changes, 1998-2001. J Infect Dis 191: 1981-1987.
- Barrozo LV, Benard G, Silva ME, Bagagli E, Marques SA, et al. (2010) First description of a cluster of acute/subacute paracoccidioidomycosis cases and its association with a climatic anomaly. PLoS Negl Trop Dis 4: e643.
- Barrozo LV, Mendes RP, Marques SA, Benard G, Silva ME, et al. (2009) Climate and acute/subacute paracoccidioidomycosis in a hyper-endemic area in Brazil. Int J Epidemiol 38: 1642-1649.
- Le T, Wolbers M, Chi NH, Quang VM, Chinh NT, et al. (2011) Epidemiology, seasonality, and predictors of outcome of AIDS-associated Penicillium marneffei infection in Ho Chi Minh City, Viet Nam. Clin Infect Dis 52: 945-952.
- Bruce Light R, Kralt D, Embil JM, Trepman E, Wiebe L, et al. (2008) Seasonal variations in the clinical presentation of pulmonary and extrapulmonary blastomycosis. Med Mycol 46: 835-841.
- Panackal AA, Li H, Kontoyiannis DP, Mori M, Perego CA, et al. (2010) Geoclimatic influences on invasive aspergillosis after hematopoietic stem cell transplantation. Clin Infect Dis 50: 1588-1597.
- Guinea J, Pelaez T, Alcala L, Bouza E (2006) Outdoor environmental levels of Aspergillus spp. conidia over a wide geographical area. Med Mycol 44: 349- 356.
- Edi-Osagie NE, Emmerson AJ (2005) Seasonality of invasive Candida infection in neonates. Acta Paediatr 94: 72-74.
- Panackal AA (2010) Spatio-temporal and healthcare trends of non-endemic, invasive fungal infections in the United States, National Hospital Discharge Survey--1996 to 2006. Med Mycol 48: 449-457.
- Datta K, Bartlett KH, Baer R, Byrnes E, Galanis E, et al. (2009) Spread of Cryptococcus gattii into Pacific Northwest region of the United States. Emerg Infect Dis 15: 1185-1191.
- Datta K, Bartlett KH, Marr KA (2009) Cryptococcus gattii: Emergence in Western North America: Exploitation of a Novel Ecological Niche. Interdiscip Perspect Infect Dis 2009: 176532.
- Varela JM, Regordan C, Medrano FJ, Respaldiza N, de La Horra C, et al. (2004) Climatic factors and Pneumocystis jiroveci infection in southern Spain. Clin Microbiol Infect 10: 770-772.
- Sing A, Schmoldt S, Laubender RP, Heesemann J, Sing D, et al. (2009) Seasonal variation of Pneumocystis jirovecii infection: analysis of underlying climatic factors. Clin Microbiol Infect 15: 957-960.
- Miller RF, Evans HE, Copas AJ, Cassell JA (2007) Climate and genotypes of Pneumocystis jirovecii. Clin Microbiol Infect 13: 445-448.
- Hoover DR, Graham NM, Bacellar H, Schrager LK, Kaslow R, et al. (1991) Epidemiologic patterns of upper respiratory illness and Pneumocystis carinii pneumonia in homosexual men. Am Rev Respir Dis 144: 756-759.
- Greenland S, Pearl J, Robins JM (1999) Causal diagrams for epidemiologic research. Epidemiology 10: 37-48.
Citation: Panackal AA (2011) Global Climate Change and Infectious Diseases: Invasive Mycoses. J Earth Sci Climat Change 1: 108. DOI: 10.4172/2157-7617.1000108
Copyright: ©2011 Panackal AA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 18575
- [From(publication date): 10-2011 - Nov 04, 2025]
- Breakdown by view type
- HTML page views: 13556
- PDF downloads: 5019