Review Article Open Access
Metabolic Bone Disease and Bariatric Surgery
| Tanjinder S Sanghera1*, Sertaz-Niel Kang2 and Khaled Hamdan3 | |
| 1Digestive Diseases Unit, Brighton and Sussex University Hospitals, Brighton, UK | |
| 2Royal National Orthopaedic Hospital, Stanmore, Middlesex, UK | |
| 3Digestive Diseases Unit, Brighton and Sussex University Hospitals, Brighton, UK | |
| Corresponding Author : | Tanjinder S Sanghera Digestive Diseases Unit Brighton and Sussex University Hospitals Brighton, UK E-mail: Tanjinder.sanghera@bsuh.nhs.uk |
| Received November 16, 2011; Accepted April 21, 2012; Published April 23, 2012 | |
| Citation: Sanghera TS, Kang SN, Hamdan K (2012) Metabolic Bone Disease and Bariatric Surgery. J Gastroint Dig Syst S10:001. doi:10.4172/2161-069X.S10-001 | |
| Copyright: © 2012 Sanghera TS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
The prevalence of obesity is increasing worldwide and the past decade has witnessed an exponential rise in the number of bariatric operations performed. As a consequence, it is expected that an increasing number of patients are likely to be at risk of long term complications that may not manifest until years or decades later. Several studies have investigated the short and long-term complications of bariatric surgery but there is little available data on the longterm consequences on bone metabolism and the consequences of this after bariatric surgery. This paper reviews the current literature for further information on this clinically relevant issue.
| Keywords |
| Bariatric surgery; Bone disease; Osteoporosis; Osteomalacia |
| Abbreviations |
| LAGB: Laproscopic Adjustable Gastric Banding; VGS: Vertical Sleeve Gastrectomy; RYGB: Roux-en-Y Gastric Bypass; BPD-DS: Biliopancreatic Diversion with a Duodenal Switch; PTH: Parathyroid Hormone; DEXA: Dual-Energy X-Ray Absorptiometry; ALP: Alkaline Phosphatise; BMD: Bone Mineral Density; FBC: Full/ Complete Blood Count; LFT: Liver Function Tests; TSH: Thyroid Stimulating Hormone |
| Introduction |
| The prevalence of obesity is increasing worldwide with more than 500 million people estimated to be clinically obese worldwide [1]. Obesity is an established risk factor for several cardiovascular, metabolic and respiratory conditions and is considered to be a major cause of increased mortality. It is estimated that more than 30,000 deaths each year in England are attributed to obesity alone, taking up to 9 years off a normal lifespan [2]. |
| The dramatic increase in the prevalence of obesity, coupled with the poor long-term outcomes of current nonsurgical treatment, has led to a rapid growth in the number of bariatric procedures performed worldwide. Data from the USA and Europe indicate that the number of bariatric procedures performed has increased exponentially. For example, the number of bariatric procedures performed in England has more than doubled in 2010 (10,000 procedures) compared to 2009 (~5000) [3]. In the USA the number rose by 50% to 1, 20, 000 compared with 2002 [4]. |
| Whilst the beneficial effects of bariatric surgery have been clearly demonstrated and some of the short and long term complications have been extensively investigated [2,5,6] the data on the long-term effects on bone metabolism are scarce. |
| It is widely accepted that bariatric surgery is likely to place the patient at risk of developing metabolic bone disease, whether that is by reducing the intake of calcium or vitamin D or by impairing its absorption. This paper will review the current literature for further information on this clinically relevant issue. |
| Methods |
| We conducted a search of the following electronic databases: MEDLINE, EMBASE, CINAHL and the Cochrane Library with no date restrictions through October 06, 2011. The following keywords and Medical subject headings (MeSH) terms used were: |
| • “Bariatric surgery” and “Bone diseases” |
| • “Bariatric surgery” and “Metabolic bone diseases” |
| • “Bariatric surgery” and “Osteoporosis” |
| We screened the reference lists of included articles to identify any further relevant studies, then reviewed the titles, abstracts and full text of apparently relevant articles to determine their eligibility. |
| Eligibility criteria |
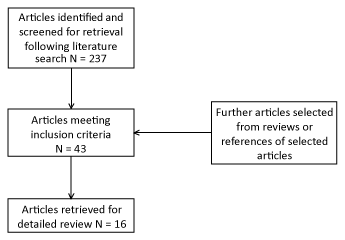
| Only comparative studies, analytical studies and case series written in English were included. We excluded studies that were not human, reviews and posters and articles not related to the aims of this study. All relevant articles were cross-referenced between two of the present authors, and the selected studies were assessed further for quality and validity (Figure 1). |
| Types of Bariatric Procedure |
| To fully appreciate the potential long-term consequences of bariatric surgery on bone metabolism it is essential to have a basic understanding of the different bariatric procedures that are currently in common use. It is also important to be aware of normal vitamin D and calcium homeostasis and physiology. |
| Bariatric procedures are generally classified as restrictive procedures that include Laparoscopic Adjustable Gastric Banding (LAGB) and Vertical Sleeve Gastrectomy (VGS) or restrictive with malabsorption procedures that include Roux-en-Y Gastric Bypass (RYGB) and Biliopancreatic Diversion with a Duodenal Switch (BPD-DS) [2,7]. LAGB involves the insertion of an adjustable silicon band around the upper part of the stomach creating a small gastric pouch with a narrow stomal size, thereby restricting the overall caloric intake and slowing the passage of food. The degree of intake restriction can be controlled by injecting normal saline into a subcutaneous port, which is connected to the band via a silicon catheter. VGS involves the creation of a narrow gastric tube through the excision of most of the stomach. In addition to the restrictive nature of the procedure, weight loss is aided by removing most of the endocrine and paracrine function of the gastric mucosa, including ghrelin-producing cells. RYGB involves the creation of a small gastric pouch from the cardia of approximately 20 ml in size. The distal stomach and proximal small bowel are bypassed by anastomosing the gastric pouch to the divided jejunum (Roux alimentary limb). The biliary pancreatic limb is then anastomosed to the small bowel at about 80–150 cm distal to the point of division of the jejunum. The ‘common channel’ for food digestion and absorption following RYGB is usually in excess of 2 m. BPD-DS involves excising most of the stomach, leaving only a gastric sleeve. The first part of the duodenum is divided, and then reconstituted by a long Roux-en-Y anastomosis to the distal jejunum, leaving a common digestive channel of 75–100 cm. |
| Bone metabolism |
| Ninety-nine per cent of the body’s calcium is contained within bone. These stores are not static and through formation and resorption, bone plays a vital role in calcium homeostasis. Calcium has a number of important roles beyond bone formation. It is involved in signal transduction during synapse function, muscle contractility and cell division; it also maintains excitable cell membranes. It is a co-factor for many enzymes, particularly those involved in cell death and coagulation. |
| Calcium homeostasis is maintained, principally, by the actions of three hormones, the active metabolite of vitamin D3, 1,25-dihydroxycholecalciferol, Parathyroid Hormone (PTH) and calcitonin. |
| The complex control of calcium homeostasis is illustrated in (Figure 2) and highlights the importance of the alimentary canal in maintaining homeostasis through endocrine and absorptive mechanisms. |
| Obesity, metabolic disease and the metabolic complications of bariatric surgery |
| Weight loss induces bone loss that correlates with the amount and speed at which it is lost. However the degree of this loss following bariatric surgery is significantly higher [8]. Further, if bone loss continues after three years then it cannot be explained by weight loss alone, as weight loss should have plateaued and patients may even be regaining weight [9]. This strongly suggests that bone mass loss is not due to weight loss alone and may be explained by the interplay of at least three distinct factors. |
| Inadequate intake of vitamin D and calcium |
| Hypocalcaemia has been observed in some obese individuals, however this may be due to poor diet [10]. Up to 60-90% of the obese are known to have vitamin D deficiency, even without surgery [11,12] and an inverse correlation between serum vitamin D levels and obesity has been documented[13,14]. Despite this, the deficiency does not seem to translate to clinically relevant consequences such as osteoporosis. |
| Bone health and obesity have a complicated relationship and obesity has been shown to exert a protective effect against osteoporosis. This is likely a result of Wolff ’s law that states bone is laid down in response to the stresses placed upon it, in this case the skeletal loading that obesity generates. Meanwhile the interplay of osteokines and adipokines has shown homeostatic feedback between bone and the adipose tissue [15]. Leptin, produced by adipose tissue, increases trabecular bone though reduces overall bone mass [16]. Hyper-insulinaemia also seems to plays a role, as mice osteoblasts respond by increasing osteocalcin secretion and subsequently bone turnover and mass [17]. In humans, although weight loss has been shown to reduce insulin, it is unclear whether it is an additional factor [18]. |
| Altered vitamin D metabolism and absorption |
| Reduced bioavailability of vitamin D in the obese [19], poor diet, reduced sun exposure and reduced production of vitamin D precursors in the liver are all thought to contribute [20] to reduced levels of vitamin D in the obese. A degree of hyperparathyroidism has also been observed, though it is thought that low vitamin D is only partially to be the cause of this. |
| Bariatric surgery can exacerbate this as it involves significant changes to the foregut and normal digestion causing poor gut absorption by design. This poor gut absorption leads to bypass of the duodenum and proximal jejunum, the main site of vitamin D3 absorption [21]. Poor fat absorption also leads to insoluble soap formation and further decreases calcium absorption. |
| Together this can cause a vitamin D3 deficiency that leads to inadequate mineralisation of bone matrix (osteomalacia) and defective endochondral bone formation (rickets). This differs from osteoporosis which features reduced trabecularisation of bone and subsequent loss of strength and increased fracture risk. |
| Compromised calcium absorption |
| Calcium is absorbed from the gut in two ways. Active transcellular transport in the duodenum involves entry into intestinal epithelial cells, transport across and exit out. Vitamin D plays a large role in achieving this; the protein required for intracellular transport (calbindin) and basolateral transport are upregulated by the active metabolite 1,25-dihydroxycholecalciferol. |
| Passive paracellular absorption of ionized calcium occurs elsewhere in the small intestine and in the colon. Unlike the duodenum, however, the calcium needs to be ionized and this requires an acidic environment. In bariatric surgery there may be significant changes not only to the site of active calcium absorption but also to the site of calcium ionization, namely the stomach [22]. |
| The clinical presentation of osteomalacia |
| Osteomalacia, literally “soft-bones”, is characterised by the poor mineralisation of bone and thickening of the osteoid. It is most commonly caused by vitamin D deficiency or abnormal metabolism but can also be caused by phosphate deficiency. The symptoms are normally non-specific and onset is insidious. This makes diagnosis difficult, especially by clinicians unfamiliar or unsuspecting of the condition. Usually there is deep vague bony pain, proximal muscle weakness/pain and gradual bony deformity. Pathological fractures can be common but for many years the only complaint maybe chronic fatigue. Eventually the patient may develop clinically evident hypocalcaemia, a waddling gait or loss of mobility [23]. |
| Methods for assessing metabolic bone changes |
| Several methods/techniques have been used to quantify bone changes particularly in the context of osteomalacia. Bone biopsy is considered as the gold standard for confirming a suspected diagnosis of osteomalacia [24] but can be unpractical for routine follow up and is not widely practiced. Plain radiographs are useful in identifying osteomalacia as it can cause some typical features. These relate to the softening of bone and include kyphosis, Looser’s zones (pseudofractures), amyloid deposition and osteopaenia. Dual-Energy X-ray Absorptiometry (DEXA) is also useful and frequently shows reduced Bone Mineral Density (BMD) throughout the skeleton, reflecting the osteopaenia seen in plain radiographs. Radionuclide uptake displays more widespread patterns; these can be diffuse (a super scan) or be discrete (hotspots) [22,25]. However, these changes can frequently be mistaken for metastatic disease, limiting its use in isolation [24,26]. |
| Biochemical studies are routinely used in the identification of metabolic bone disease and whilst hypocalcaemia and hypophosphatemia can be characteristic of osteomalacia, an increase in the levels of serum alkaline phosphatase (ALP) is the most common biochemical sign. Levels can rise to more than eight times the normal value [27]. Secondary hyperparathyroidism is also characteristically seen; as vitamin D levels fall, its ability to suppress PTH levels fall too. This rise in PTH increases osteoclastic activity and bone turn over, resorbing calcium from bone. Serum calcium, however, is an unreliable marker, as although total body calcium maybe reduced, serum calcium is often maintained by the action of PTH. Bone turnover can also be measured using markers such as osteocalcin, which in surgically induced weight loss is significantly raised [27,28]. |
| Changes associated with bariatric surgery |
| The signs and symptoms of metabolic bone disease in the bariatric surgical patient are infrequently mentioned outside case reports. Progressive and persistent bone and joint pain, muscle weakness and fatigue were commonly described in the literature and in one study occurred in 75% of the patients. These symptoms, once established, require aggressive therapy with calcium, phosphate and vitamin D supplementation [24,29,30]. Localised bone pain may be due to underlying lesions such as lytic lesions like osteitis fibrosa cystica or pseudofractures [26] and an increased reporting of fractures and height reduction has been seen in patients following RYGB [31]. |
| Low levels of both serum calcium and urinary calcium excretion and elevated levels of serum ALP and PTH are commonly reported amongst the articles reviewed. In some an increase in 1,25-dihydroxycholecalciferol was noted, this was thought to be due to a drive in vitamin D 1-α-hydroxylase activity by PTH [12,24,26,29,32]. |
| Three studies stood in contrast though to the common biochemical findings; Bano G et al. [33] investigated five groups of people all of whom had had surgical treatment for obesity, pre-menopausal women, post-menopausal women, post-menopausal women with oestrogen, men and women who had undergone a reversal of juunoileal or pancreaticobiliary bypass surgery. No difference was found in serum calcium, ALP or 25-hydroxycholecalciferol. |
| Valderas JP et al. [9] noted that although hyperparathyroidism was seen post RYGB there was no statistical difference in vitamin D, ALP or BMD between their cohort and a matched control. However, both groups did have a vitamin D deficiency, supporting the evidence that the obese continue to have poor diet and vitamin deficiencies are endemic. When serum carboxy telopeptide (a marker of bone turnover) was measured the study concluded that bone resorption was associated with hyperparathyroidism. But the cause of the hyperparathyroidism was undeterminable; the PTH could be raised due to calcium malabsorption or perhaps, due to this population having long standing vitamin D deficiency, they already had a degree of hyperparathyroidism and the surgery had just exacerbated it. |
| Hyperparathyroidism was commonly reported in the long-term follow up of patients. Kumar et al. [21] had a RYGB patient that preoperatively had normal serum calcium and ALP, two years postoperatively those values remained normal but vitamin D levels were on the low end of normal. At seven years she was seen to have low levels of calcium and vitamin D with elevated levels of PTH and ALP consistent with osteomalacia. Vilarrasa et al. [34] looked at post-RYGB patients at 12 months and 36 months. They noted that whilst at 12 months plasma PTH and 25-hydroxycholecalciferol were unchanged, at 36 months PTH rose and 25-hydroxycholecalciferol significantly fell. Despite these findings a multiple regression analysis suggested they were not associated with the bone loss seen but rather menopause was a more significant factor [34]. One study found that 8 years after RYGB although the vast majority showed a strong inverse correlation between vitamin D and PTH, 10% of patients had a vitamin D deficiency with normal PTH. They posed that whilst the vitamin D deficiency was due to malabsorption, the normalisation of PTH was “more intense in the first years” and would eventually resolve [14]. |
| Histological changes consistent with osteomalacia including reduced mineralisation and increased osteoid surface, width and volume [24,32] were a common finding however one study found that 6 years after the bariatric surgery the amount of osteoid eventually decreased and trabecular bone increased, with no evidence of osteomalacia at the end of the study [35]. |
| The management of metabolic disease after bariatric surgery |
| Several articles have been published outlining management for metabolic disease following bariatric surgery. Although they differ in specifics they share common principles. Each recommends pre- and post-operative nutritional screening, investigation and evaluation and appropriate calcium and vitamin D supplementation [36-38] (Table 1). |
| The American Association of Clinical Endocrinologists, the Obesity Society and the American Society for Metabolic and Bariatric Surgery guidelines discuss a range of metabolic disturbances and their corresponding clinical manifestations. Electrolyte abnormalities, fat-soluble vitamin deficiencies, osteoporosis and secondary hyperparathyroidism were explored and their management suggested. |
| Bisphosphonate therapy has been suggested to treat the low bone mass density, but in studies has not shown to resolve symptoms. Additionally, it has been pointed out that bisphosphonate therapy in the hypocalcaemic can exacerbate the hypocalcaemia and be life threatening [8,30]. |
| Ensuring patients have adequate follow up and management to prevent or control deficiencies may be just one way of preventing bone disease. Srikanth et al. [39] argued that in long-term follow-up patients who display signs and symptoms of malabsorption are typically those who are non-compliant with supplement therapy and, importantly, had shorter common digestive channels. They suggested that a RYGB with a common digestive channel of >150 cm together with a small gastric pouch provided a balance between weight loss and malnutrition [39]. |
| Discussion |
| Evidence for metabolic bone disease after bariatric surgery is far from clear, whilst shorter studies do show evidence of bone loss these may just be reflecting Wolff ’s law and early changes rather than the effect of the surgery itself (Table 2). For this reason, a follow up of three years or more was selected. |
| Previously in this article we discussed three factors that may contribute to the findings. The inadequate dietary intake of the patients who typically present for bariatric surgery is well known. The fact that they have increased weight and thus a higher BMI does not necessarily reflect a positive nutritional status. The obese patient may often have significant vitamin, mineral and nutritional deficiencies that, if not already causing undiagnosed bone changes prior to surgery, may leave them increasingly exposed to the changes introduced by surgery. These changes reduce not only the ability of the gut to absorb vitamin D but also fundamentally alter calcium homeostasis. In the absence of vitamin D, an important feedback mechanism in controlling PTH is lost and in the absence of exogenous calcium and elevated PTH levels calcium is increasingly resorbed from bone. Indeed the contribution to poor absorption of calcium and subsequent increase in the risk of osteoporosis and metabolic bone disease by gastric surgery has been documented [22]. |
| An additional factor frequently commented on in the development of reduced bone mass after bariatric surgery was the menopause. It was noted that post-menopausal women, particularly those who did not receive HRT had significantly higher rates of osteopaenia and osteoporosis [33]. |
| In one study 1.7% of patients had osteopaenia at the femoral neck and 10.2% had osteopaenia at the lumbar spine prior to surgery though 36 months after RYBG this increased to 15.3% at the femoral neck and 30.5% at the lumbar spine and several (8.5%) developed osteoporosis [34]. Interestingly when the individuals with low BMD were compared to others in the study the differences in BMI, weight loss, calcium, vitamin D or PTH did not reach statistical significance. Rather these women were older, had lower levels of lean mass and were more likely to be postmenopausal. |
| These findings reinforce the idea that loss of BMD in the post bariatric patient is multifactorial and metabolic deficiency may only be one component. In fact the paper suggests that menopause is the single most important variable implicated in bone disease and may be aggravated by the reduced conversion of androgens to oestrogens by adipose aromatases. This is important to consider when discussing metabolic bone disease, as a large proportion of patients studied are female. |
| Conclusion |
| Bone disease after bariatric surgery is not well understood and though several studies have been conducted few provide long-term follow up data. Whilst these studies are scant, they have provided a valuable insight into the metabolic consequences of bariatric surgery and an awareness that pre-existing nutritional deficiencies can be exacerbated by reduced intake, decreased absorption, poor compliance with vitamin and mineral supplements and the menopause. |
| Aggressive and thorough evaluation with long-term follow up is mandatory in order to prevent potentially serious consequences in these groups. To achieve this, a multidisciplinary approach is required involving surgeons, dieticians, endocrinologists, gastroenterologists and primary care practitioners. The authors hope that this article will provide a heightened awareness of metabolic bone disease following bariatric surgery, which will assist in both the prevention of associated complications and the earlier recognition and treatment of any resultant pathology. |
References
- Statistics on Obesity, Physical Activity and Diet, England, 2010
- Hamdan K, Somers S, Chand M (2011) Management of late postoperative complications of bariatric surgery. Br J Surg 98: 1345-1355.
- Burns EM, Naseem H, Bottle A, Lazzarino AI, Aylin P, et al. (2010) Introduction of laparoscopic bariatric surgery in England: observational population cohort study. BMJ 341: c4296.
- Zhao Y, Encinosa W, Bariatric Surgery Utilization and Outcomes in 1998 and 2004: Statistical Brief #23.
- Snow JM, Severson PA (2011) Complications of adjustable gastric banding. Surg Clin North Am 91: 1249-1264
- Al Harakeh AB (2011) Complications of laparoscopic Roux-en-Y gastric bypass. Surg Clin North Am 91: 1225-1237.
- Abell TL, Minocha A (2006) Gastrointestinal complications of bariatric surgery: diagnosis and therapy. Am J Med Sci 331: 214-218.
- Williams SE (2011) Metabolic bone disease in the bariatric surgery patient. J Obes 2011: 634614.
- Valderas JP, Velasco S, Solari S, Liberona Y, Viviani P, et al. (2009) Increase of bone resorption and the parathyroid hormone in postmenopausal women in the long-term after Roux-en-Y gastric bypass. Obes Surg 19: 1132-1138.
- Goldberg TB, da Silva CC, Peres LN, Berbel MN, Heigasi MB, et al. (2009) Calcium intake and its relationship with risk of overweight and obesity in adolescents. Arch Latinoam Nutr 59: 14-21.
- Strohmayer E, Via MA, Yanagisawa R (2010) Metabolic management following bariatric surgery. Mt Sinai J Med 77: 431-445.
- DiGiorgi M, Daud A, Inabnet WB, Schrope B, Urban-Skuro M, et al. (2008) Markers of bone and calcium metabolism following gastric bypass and laparoscopic adjustable gastric banding. Obes Surg 18: 1144-1148.
- Viégas M, Vasconcelos RS, Neves AP, Diniz ET, Bandeira F (2010) Bariatric surgery and bone metabolism: a systematic review. Arq Bras Endocrinol Metabol 54: 158-163.
- de Campos CD, Dalcanale L, Pajecki D, Garrido AB, Halpern A (2008) Calcium intake and metabolic bone disease after eight years of roux-en-y gastric bypass. Obes Surg 18: 386-390
- Soleymani T, Tejavanija S, Morgan S (2011) Obesity, bariatric surgery, and bone. Curr Opin Rheumatol 23: 396-405.
- Hamrick MW, Ferrari SL (2008) Leptin and the sympathetic connection of fat to bone. Osteoporos Int 19: 905-912.
- Fulzele K, Riddle RC, DiGirolamo DJ, Cao X, Wan C, et al. (2010) Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell 142: 309-319.
- Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, et al. (2009) Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 122: 248-256.
- Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF (2000) Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 72: 690-693.
- Goldenberg L (2011) Mechanisms of metabolic bone disease in bariatric surgery patients. Bariatric Times 8: 28-29
- Kumar R, Lieske JC, Collazo-Clavell ML, Sarr MG, Olson ER, et al. (2011) Fat malabsorption and increased intestinal oxalate absorption are common after Roux-en-Y gastric bypass surgery. Surgery 149: 654-661.
- Sipponen P, Härkönen M (2010) Hypochlorhydric stomach: a risk condition for calcium malabsorption and osteoporosis? Scand J Gastroenterol 45: 133-138.
- Bhan A, Rao AD, Rao DS (2010) Osteomalacia as a result of vitamin D deficiency. Endocrinol Metab Clin North Am 39: 321-331
- Sung CC, Lee HS, Diang LK, Lin SH (2010) Refractory diffuse bony pain 20 years after jejunoileal bypass. South Med J 103: 570-573.
- Love C, Din AS, Tomas MB, Kalapparambath TP, Palestro CJ (2003) Radionuclide bone imaging: an illustrative review. Radiographics 23: 341-358.
- Goldner WS, O'Dorisio TM, Dillon JS, Mason EE (2002) Severe metabolic bone disease as a long-term complication of obesity surgery. Obes Surg 12: 685-692.
- Basha B, Rao DS, Han ZH, Parfitt AM (2000) Osteomalacia due to vitamin D depletion: a neglected consequence of intestinal malabsorption. Am J Med 108: 296-300.
- Fleischer J, Stein EM, Bessler M, Della Badia M, Restuccia N, et al. (2008) The decline in hip bone density after gastric bypass surgery is associated with extent of weight loss. J Clin Endocrinol Metab 93: 3735-3740.
- De Prisco C, Levine SN (2005) Metabolic bone disease after gastric bypass surgery for obesity. Am J Med Sci 329: 57-61.
- Collazo-Clavell ML, Jimenez A, Hodgson SF, Sarr MG (2004) Osteomalacia after Roux-en-Y gastric bypass. Endocr Pract 10: 195-198.
- Berarducci A, Haines K, Murr MM (2009) Incidence of bone loss, falls, and fractures after Roux-en-Y gastric bypass for morbid obesity. Appl Nurs Res 22: 35-41.
- Haria DM, Sibonga JD, Taylor HC (2005) Hypocalcemia, hypovitaminosis d osteopathy, osteopenia, and secondary hyperparathyroidism 32 years after jejunoileal bypass. Endocr Pract 11: 335-340.
- Bano G, Rodin DA, Pazianas M, Nussey SS (1999) Reduced bone mineral density after surgical treatment for obesity. Int J Obes Relat Metab Disord 23: 361-365.
- Vilarrasa N, San José P, García I, Gómez-Vaquero C, Miras PM, et al. (2011) Evaluation of bone mineral density loss in morbidly obese women after gastric bypass: 3-year follow-up. Obes Surg 21: 465-472.
- Halverson JD, Haddad JG, Bergfeld M, Teitelbaum SL (1989) Spontaneous healing of jejunoileal bypass-induced osteomalacia. Int J Obes 13: 497-504.
- Mechanick JI, Kushner RF, Sugerman HJ, Gonzalez-Campoy JM, Collazo-Clavell ML, et al. (2008) Executive summary of the recommendations of the American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery medical guidelines for clinical practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient. Endocr Pract 14: 318-336.
- Williams SE, Cooper K, Richmond B, Schauer P (2008) Perioperative management of bariatric surgery patients: focus on metabolic bone disease. Cleve Clin J Med 75: 333-334.
- Heber D, Greenway FL, Kaplan LM, Livingston E, Salvador J, et al. (2010) Endocrine and nutritional management of the post-bariatric surgery patient: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 95: 4823-4843.
- Srikanth MS, Oh KH, Fox SR (2011) Revision to malabsorptive Roux-en-Y gastric bypass (MRNYGBP) provides long-term (10 years) durable weight loss in patients with failed anatomically intact gastric restrictive operations: long-term effectiveness of a malabsorptive Roux-en-Y gastric bypass in salvaging patients with poor weight loss or complications following gastroplasty and adjustable gastric bands. Obes Surg 21: 825-831.
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
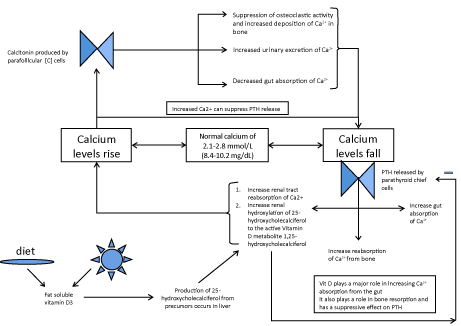 |
| Figure 1 | Figure 2 |
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 13254
- [From(publication date):
specialissue-2013 - Oct 27, 2025] - Breakdown by view type
- HTML page views : 8734
- PDF downloads : 4520
