18-Glycyrrhetinic Acid Chalcones and Its Salts with Their Mass Fragmentation Pattern
Received: 06-Feb-2021 / Editor assigned: 01-Jan-1970 / Reviewed: 01-Jan-1970 / Revised: 01-Jan-1970 / Accepted Date: 20-Mar-2021 / Published Date: 27-Mar-2021
Abstract
This work reveals the synthesis of chalcones which are derived from 18β-glycyrrhetinic acid, through its keto derivative. First of all by means of Jones oxidation the hydroxyl group of glycyrrhetinic acid at position 3 oxidized to keto group. Then α, β-unsaturated ketones (enones) are produced when this keto group is treated with different benzaldehyde through Claisen Schmidt reaction. We also prepared potassium and sodium salts of all these compounds. By mean of thin layer chromatography reactions are monitored and by means of chromatographic techniques reaction products are purified. By using spectroscopic techniques like MS, 1HNMR and IR 18β-glycyrrhetinic acid derivatives are characterized. By the presence of all the possible peaks 1HNMR spectra confirms the products formation. The Claisen Schmidt synthesis has been done successfully this is shown by the existence of aromatic proton. Their fragmentation pattern and mass spectra of all the compounds discussed in detail with schemes. Almost same fragmentation pattern is shown by all the compounds. Fragments had shown by all the compounds at m/z 262, 55, 135 and 303 along with other fragments. Formation of potassium and sodium salts of compound 3, its keto derivative and of chalcones confirmed by the IR of salts.
Keywords
18β-glycyrrhetinic acid, Derivatives, Synthesis, Chalcones, Claisenschmidt synthesis
Introduction
Licorice (Leguminosae, Glycyrrhizaglabra) is a naturally occurring perennial plant and cultivated in Asia (especially the central and Europe (the Mediterranean) and South-Western parts. It was used in Indian cultures, and ancient Assyrian, Chinese, Egyptian and appreciated by ancient Greeks and Romans. It also, used as a medicine for diseases in the respiratory, cardiovascular, gastrointestinal, genitalurinary systems, as well as for eye, skin and other diseases. It was used in Arabic medicine, and, from the Middle Ages onwards, in Germany, Italy, Spain and England. The root of this plant (Radix Glycyrrhizae (Liquirtiae), has a sweet flavor, now is generally used for the treatment purpose of gastric ulcer disease and upper respiratory tract diseases. It also used as a flavoring agent and sweetening in the tobacco, food industries and pharmacy. The active ingredient of Licorice is the glycyrrhizin, triterpenoidsaponin, mixture of potassium, calcium and magnesium salts of GA (Glycyrrhizic acid) [1].
GA consist of 18β-glycyrrhetinic acid an aglycone, GE(glycyrrhetinic acid) also exists in two form 18β (cis) and 18α (Trans) stereoisomers [2]. The chemical structure of the glycyrrhetinic acid (GE) molecule has great potentials for variations leading to derivatives [3]. For the generation of new drugs many structural conversions have been planned, involving alkylation, esterification, and reduction reaction [3- 5]. GA or glycyrrhetinic acid but in some cases, both are used in many in vivo and in vitro studies, antibacterial antiulcer antiviral neuroprotective activities, anti-inflammatory, anticancer, hepatoprotective, antileishmanial and also used as cell-protective and chemo preventive effects [6,7]. Blood coagulation factor Xa inhibited by anticoagulant activity that is shown by glycyrrhetinic acid and its analogues, also have properties against adult worms of Brugiamalayi and microfilariae [8,9]. Glycyrrhetinic acid and its analogues are also recognized, as inhibitors of adipogenic differentiation via the phosphorylation of protein kinase B/Akt, and as stimulators of lipolysis through hormone-sensitive lipase/ HSL activation, due to their antiobesity activity [10]. Now a day, they are marketed on the basis of promising pharmacological activity of this active constituent of Glycyrrhizaglabra L. It involve e.g. preparations of glycyrrhizin for patients with liver disease [1,11].
Licorice root extracts have been discovered by men in early times due to their antiviral properties. Fiore et al have been reviewed antiviral effects of Glycyrrhizaglabra L. the derivatives of glycyrrhetinicasiatic acid, oleanolic acid have exposed their activity against Human enterovirus 71 (EV71). Western blot and reverse transcription (RT)-PCR analyzed the inhibitory effects of this derivative on EV71 replication. The influenza A virus infection have been revealed by the interesting antiviral activity of these compounds which are in vitro. Simultaneously treating them with different concentrations of glycyrrhetinic acid derivatives and by infecting MDCK cells with influenza A H3N2 virus the antiviral activity of the glycyrrhetinic acid derivatives was determined. Against several DNA viruses these analogues have also exhibited high activity. Against Hepatitis C virus (HCV), Van Rossum et al and Kumada et al. reported the potential of glycyrrhetinic acid while against Hepatitis B virus (HBV) Wang et al. offered the biological evaluation, structure-activity relationships and synthesis of glycyrrhetinic acid derivatives [12].
In current years, against resistant microorganisms and antibiotic susceptible the phytochemicals and antimicrobial activity of plant extracts have been estimated. Antimicrobial properties was recognized by glycyrrhetinic acid [13].
By using the agar diffusion method Licorice root extracts have demonstrated the significance of gram-negative bacteria (Pseudomonasaeruginosa, Escherichia coli) and anti-bacterial activities against gram-positive (Staphylococcus aureus, Bacillus subtilis) [14]. The synthesis of RNA and DNA, proteins without membrane distraction was inhibited by glycyrrhetinic acid [15]. Peptic ulcers are also treated by Licorice and glycyrrhizin. Against clarithromycinresistant strains glycyrrhetinic acid exhibited effectiveness. In an additional study against 32 hospital strains of Actinobacillus and 55 hospital strains of S. aureus, evaluation of glycyrrhetinic acid activity was performed [16]. Also MIC of streptomycin, isoniazid, rifampicin and 18β-glycyrrhetinic acid or its derivative, against drug‐resistant Mycobacterium bovis have been significantly decreased by the the synergistic effect of 18β-glycyrrhetinic acid [17].
Anti-parasitic properties of selectivity and amazing efficacy revealed by phenolicsalkaloids and terpenes derived from natural plantproducts. Against certain parasites e.g. diterpenes (cryptotanshinone), simple monoterpenes (espintanol), iridoids (amarogentin), sesquiterpenes (artemisinin), and limonoids (nimbolide) terepenes, have been demonstrated their activity. In order to increase effectiveness of the resistant anti-malarial drugs by using GE with anti-malarials are supported against drug resistant strains [18].
Materials and Methods
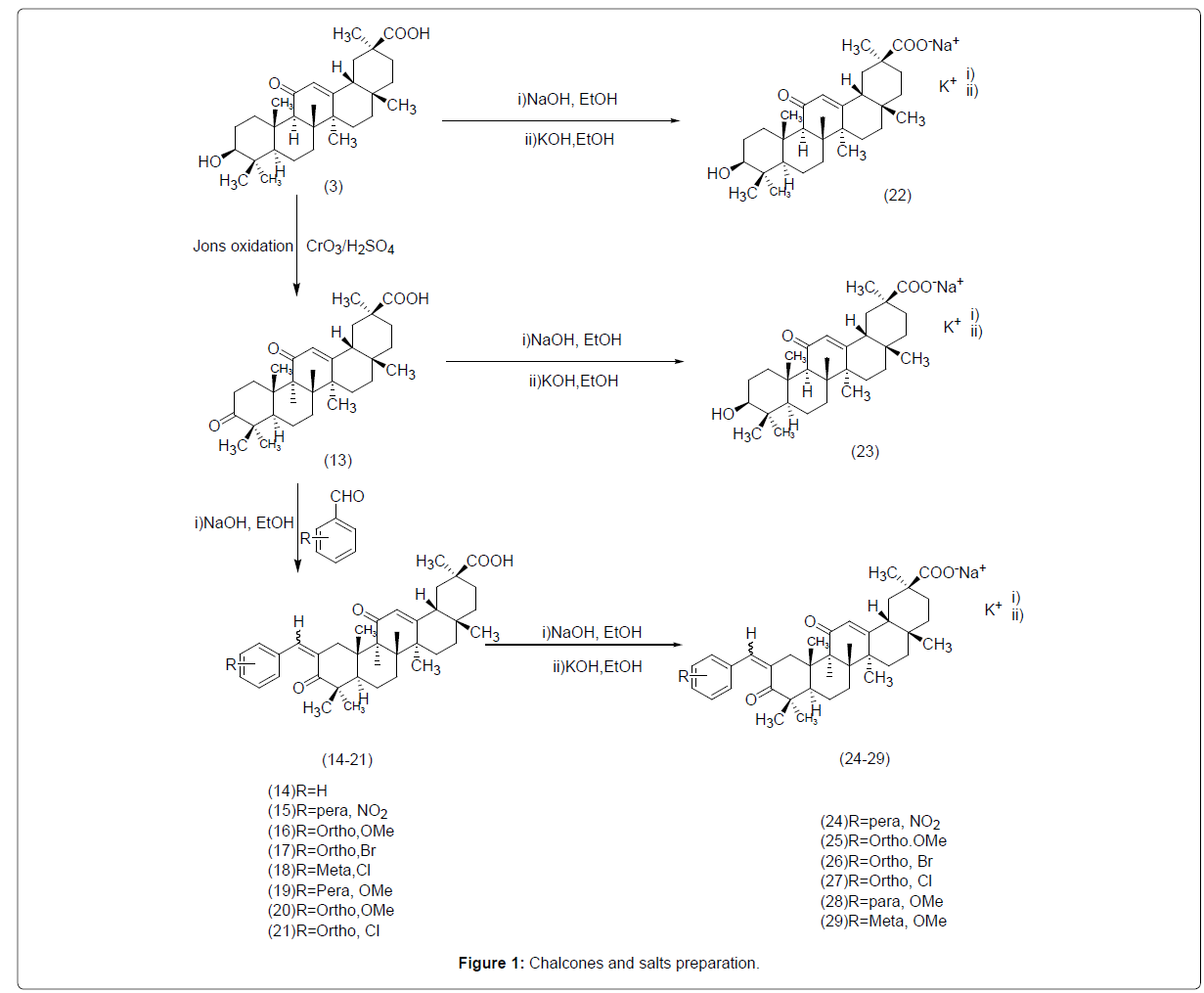
Various compounds construction by oxidation of compound 3 at carbon-3 position followed by its conjugation with various compounds like dehydrozingeron [19]. Amino-alkyl derivatives of glycyrrhetinic acid exhibits excessive cytotoxicity and synthesized derivatives i.e., ester and ethers have extensive variety of biological activities [20]. Similarly, amino acids derivatives showed increased cytotoxicity against several human cancer cells and current work is the modification of compound 3 to form chalcones and its potassium and sodium salts [21].
Synthesis of Chalcone Derivatives of compound 3
Jones oxidation leads to keto derivative which through Clasien Schmidt synthesis with different benzaldehyde to produce chalcones (14-21) as shown in figure. IR, HNMR spectroscopy and MS spectrometry helps in determining the structure of all compounds and mass to charge ratio value of each fragment is discussed in detail.
Scheme for the Chalcones and Salts Preparation
(Figure 1)
HNMR Data
In HNMR spectra methyl proton appeared in the range of 0.76- 1.53ppm while olefinic proton appear in the range of 5.44-5.96 ppm and aromatic proton appear in the range of 6.84 to 8.21 ppm. Comparison of 1HNMR Data Compound (3) and Compound (13)
In compound 3, 3H-proton appears as triplet at 3.20 ppm with (3J = 10.2 Hz and 2J = 4.2 Hz) and this peak disappear after jones oxidation by converting hydroxy to oxo-compounds. Compound (14-21)
In the form of multiplet Chalcone protons appear, triplets and doublet and very downfield for aromatic ring. Compound (14) is produced by the Claisen Schmidt synthesis of 13 with benzaldehyde. At 0.84 to 1.40 ppm seven methyl groups are appeared in product. At position 9 Singlet of proton is appeared at 2.53 ppm. At position 18 Doublet of proton with J= 16.8 Hz is appeared at 4.23 ppm. At 7.44 ppm Proton appeared at position 1″. Doublet are shown by HNMR spectra of two protons at 7.27 ppm with J= 7.2 Hz. At 7.36 ppm triplet for one proton is appeared whose 2J value is 7.2 Hz and 3J value is 15 Hz at 7.46 another triplet appeared for two proton whose 2J value is 7.5 Hz and 3J value is 14.1 Hz
. In compound 15 at position 1″ singlet of proton appears at 7.44 ppm. J= 8.7 Hz at 8.21 ppm doublet of two protons appear and at 7.58 ppm with J=8.7 Hz. In compound 16, at 7.37 ppm one proton appears and at 6.94 ppm multiplet of three protons appears. At position 1″ singlet of proton appears at 8.02 ppm. This pattern is observed in all the chalcones compounds.
Mass Fragmentation
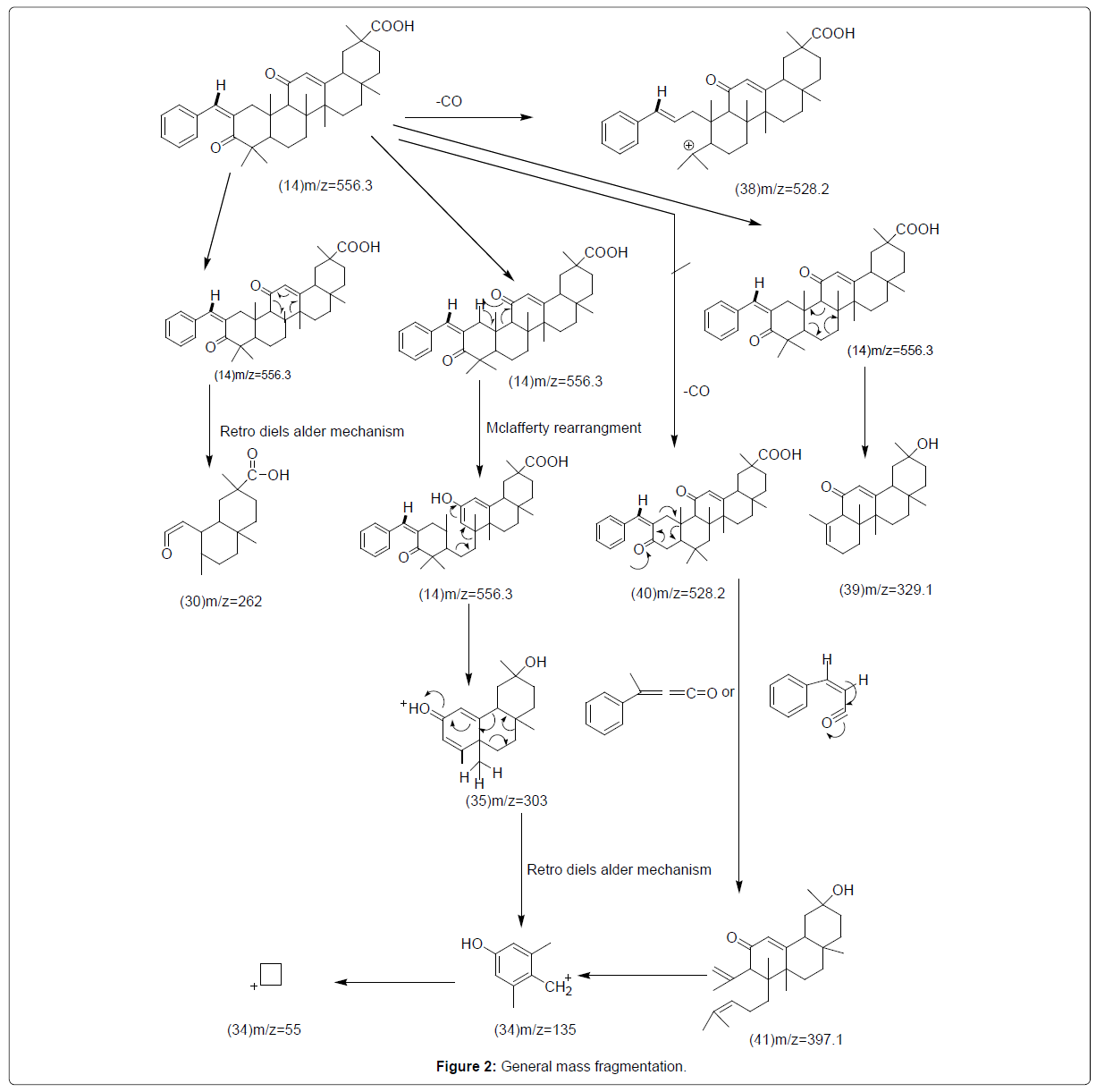
Virtually the similar mass fragmentation pattern is followed by all the glycyrrhetinic acid, keto derivative and its chalcones. Molecular ion peaks are absent in compound 17, 20 and 21. The base peak in compound 20 and in some compounds peak at 55 is present. While the base peaks are at m/z of 135 in compound 3, 13, 14 and 17. Base peak of compound 18, 21 presents at mass to charge ratio of 138. Fragment of m/z 262 is produce through Mclaferrty rearrangement in all compounds.
Next, the removal of formic acid in compound 3 the fragment of mass to charge ratio of 262 converted into the fragment of mass to charge ratio of 216 while further fragmentation does not take place in all other compounds. Common fragment with mass to charge ratio of 303 produced by retro Diels alder rearrangement of all the compounds. Fragment 135 produced by the fragment of mass to charge ratio 303 in compounds 3, 13, 14, 17, 19, and 20 through Diels alder rearrangement, through the bond cleavage of fragment 135 produce fragment of mass to charge ratio of 55. Fragment at mass to charge ratio 303, 55, 135 and 262 shown by the Compound 3 having mass to charge ratio of 470.68. The fragment 31 of mass to charge ratio of 216 is produced after the removal of formic acid in compound 3 from the fragment 30 of mass to charge ratio of 262 while further fragmentation does not take place in all other compounds. After the removal of formic acid Compound 13 with mass to charge ratio of 468 also shows fragmentation beside all fragments at mass to charge ratio 303, 55, 135 and262 converted into fragment 36 with mass to charge ratio 422. With the removal of carbon monoxide fragment 37 is produced with m/z 440 by this compound 13. Compound 14 is converted to fragment 38 with mass to charge ratio 528.2 with the removal of carbon monoxide from position 3. The fragment having mass to charge ratio of 528.2 is also produced when carbon monoxide removed from position 30, fragment 41 with m/z 397.1 is produced from the Diels alder rearrangement of this fragment. Fragment with mass to charge ratio 329.1 is produced by cleavage and rearrangement of Fragment 41. Molecular ion peak at 635.67 is absent in compound 17. With the removal of Br beside all the fragments at mass to charge ratio 303, 55, 135 and 262 it also shows fragment 42 at mass to charge ratio 555.4 with the removal of formic acid which undergoes further fragmentation at mass to charge ratio 511. Fragment of 303 produced from Compound 18 with mass to charge ratio of 591 through mclafferty rearrangement. Fragment 44 with mass to charge ratio of 301 produced through bond cleavage of Compound 18, with the removal of Cl fragment 46 produced with mass to charge ratio of 266. Fragment 45 with mass to charge ratio 138 produced from further breakdown of Fragment 44 fragment 47, 34 and 48 produced from continuous cleavage of this fragment 45 with mass to charge ratio of 110, 55 and 75. Through diels alder and mclaferrty rearrangement Same fragments of mass to charge ratio 303, 135, 262 and 55 shown by the Compound 19 with mass to charge ratio of 586. When MeO removed this compound also shows fragment 49 with mass to charge ratio 555.9. When benzaldehyde group removed from Compound 19 fragment 50 produced with mass to charge ratio of 453.3 when formic acid removed fragment 51produed with mass to charge ratio of 407. In compound 20 with mass to charge ratio of 586.3 molecular ion peak is absent and shows fragments of mass to charge ratio 303, 552, 135 and 62 but differ from other compounds, through bond cleavage and rearrangement fragment 262 produce fragment 5 and 53 with mass to charge ratio of 175 and 234. In compound 21 molecular ion peak is also absent. Through Diels alder and mclaferrty rearrangement compound 21 with mass to charge ratio of 590.3 shows fragments of mass to charge ratio 303, 135, 55 and 262. In this compound fragment with mass to charge ratio of 311 and 427.6 are also present. With the removal of Cl fragment at 555.3 mass to charge ratio shown by this compound.
General Mass Fragmentation Schemes
(Figure 2)
IR Spectroscopy
By means of IR spectroscopy the different functional groups present on compounds can be easily determined. With respect to IR the most important functionality is the Carboxylic (-COOH) group and the characteristic functional groups are α, β-unsaturated carbonyl (-C=C-C=O) group, and secondary hydroxyl (-OH group).
The carboxyl groups (-COOH) of these molecules are produced from the carbonyl (CO) and hydroxyl (OH) groups having different infrared (IR) spectral signals. In carboxylic acids and their salts, the OH stretching bands appear at 3700 cm-1 to 1700 cm-1. The absorption is not zero even at the lower-limit region, but it is weak.
The OH stretching absorption goes below 1700 cm-1 while weak absorptions on top of these bands in the 2900 cm-1 region as the CH stretching bands and combination bands are below 2800 cm-1. At 3100- 2800 cm-1 the aliphatic CH stretch bands are located [22-24].
The average carbonyl double bond (C=O) position for the acid species is 1723 ± 12 cm-1. At 1257 ± 20 cm-1 the average single carboxyl bond (C-O) position is situated.
The high position of (C-O) band indicates that for all these species R-C(=O)-OH group is not ionized. Salts produced are ionized groups (R-C=OO-) and between the two O atoms the negative charge resonates.
At 3441 cm-1 the secondary hydroxyl (-OH) and carboxylic group appears, while the aliphatic CH shows the signal at 2943 cm-1 which is adjacent to the 12-position of carbonyl group. In literature it is found that aliphatic CH comes below 3000 cm-1 while the sp2-CH comes above 3000 cm-1 but this carbonyl has sp2-CH at11-position. Signals at 1685 cm-1 and 1454 cm-1 is the characteristic signal of carbonyl group (-C=O). We have two carbonyl groups, one is carboxylic group and other isα, β-unsaturated carbonyl group.α, β-unsaturated carbonyl (-C=O) group appears at lower stretching frequency While the stretching frequency of carboxylic group appear at 1195 cm-1.
C-OH hydroxyl group appears at 1010 cm-1. The Hydroxyl (OH) group in potassium salt (22) appears at 3306 cm-1, in compound 3 it was appeared at 3441 cm-1 while aliphatic CH shows signal at 2931 cm-1. For compound (3) low frequency of 1628 cm-1 shows the formation of salt at 1685cm-1 carbonyl signal of carboxylic acid appeared. Now at 1408 cm-1 carbonyl group of α,β-unsaturated carbonyl appears which was previously at 1454 cm-1. As compared to its starting material the frequency is lowered in the salt. The disappearance of signal for (C-O) of C-OH hydroxyl group in glycyrrhetinic acid at 1195 cm-1 confirms the salt formation. Signal of (C-O) glycyrrhetinic acid appeared at 1010 cm-1 but now they are appeared at 991 cm-1.
Results and Discussions
3-Hydroxy-11-oxo-18-olean-12-ene-29-oic acid (3)
Compound is white solid, 1HNMR, 300 MHz (CDCl3)): δ (ppm) 0.81, 0.78, 0.98, 1.12, 3.20 (2J = 4.2, H-3, 3J = 10.2, 1H, t),1.35 (7-CH3), 1.14, 2.32 (H-9,1H, s), 1.20, 5.67 (H-12,1H, s), EIMS m/z (rel. int. %): m/z 303, 216, 135,470(M+), 55, 262, IR (potassium bromide) cm-1: 2943 (CH), 1010 (C-O of C-OH), 1454 (C=O), 1685 (C=O), 3441 (OH).
3,11-Dioxo-olean-12-ene-29-oic acid (13)
Compound is grey solid, yield 98%, 1HNMR 300 MHz(CDCl3): δ (ppm) 1.08, 1.25, 1.36 (7-CH3), 1.15, 2.42 (H-9,1H, s), 1.04, 5.72(H-12,1H, s), 0.83, 1.21, EIMS m/z (rel. int. %): m/z 422, 135, 303, 440.1, 55, 468 (M+), 262, IR(potassium bromide) cm-1: 1685 (CO), 2947 (CH), 1654 (CO), 1161 (C-O), 3317 (OH).
2-Benzylidene-3,11-dioxo-olean-12-ene-29-oic acid (14)
Compound is white solid: yield:53%, 1HNMR 300 MHz, (CDCl3): δ(ppm) 1.14, 1.21, 1.13, 1.19, 1.16, 0.84, 1.40 (7-CH3), 5.78 (H-12, s,1H), 7.27 (J = 7.2, d, 2H), 4.23 (1H, d, J = 16.8, H-18), 7.44 (H-1′′, s,1H), 7.46 (3J = 14.1, t, 2J = 7.5,2H), 2.53 (H-9, s, 1H), 7.36 (2J =7.2, t, 1H, 3J= 15), EIMS m/z (rel. int. %): m/z 329, 135, 528.2, 303, 556.3 (M+), 55, 397, 262.
4′-Nitro-2-benzylidene-3,11-dioxo-olean-12-ene-29-oic acid (15)
Compound is dark brown solid: yield 45%, 1HNMR 300 MHz, (CDCl3): δ (ppm) 1.19, 1.16, 1.21, 0.84, 1.41, 1.16, 1.21, 1.40 (7-CH3), 7.58 (J = 8.7, d, 2H), 4.24(H-18, J = 16.5, d,1H), 8.21(J = 8.7, d,2H), 7.44 (s, H-1′′,1H), 2.52 (s, H-9,1H), 7.44 (s, H-1′′,1H), IR (potassium bromide) cm-1: 1400 (CH=CH), 1631 (CO), 2931 (CH).
2′-Methoxy-2-benzylidene-3,11-dioxo-olean-12-ene-29-oic acid (16)
Compound is brownish yellow solid: yield 56%, 1HNMR 300 MHz, (MeOD): δ (ppm) 1.18, 1.15, 1.38, 1.16, 0.83, 1.52 (7-CH3), 5.72 (H- 12, s, 1H), 8.02 (H-1′′, s, 1H), 4.13(J = 16.2, d, 1H, H-18), 6.94 (M, 3H), ), 3.41 (H-9, s, 1H), 7.37 (M, 1H), IR (potassium bromide) cm-1: 2951 (CH), 1462 (CH=CH), 1157 (OMe), 1284 (C-O), 1616 (CO), 3159 (COOH), 1211 (C-O ), 1689 (CO).
2′-Bromo-2-benzylidene-3,11-dioxo-olean-12-ene-29-oic acid (17)
Compound is white solid: yield 87%, 1HNMR 300 MHz, (MeOD): δ(ppm) 1.12, 1.18, 1.09, 1.17, 0.81, 1.16, 7.22 (2J = 1.8, t, 1H, 3J = 8.7), 1.85 (H-9, s,1H), 5.71 (H-12, s,1H), 7.63 (J = 7.82, d, H), 7.44 (s, H-1′′,1H), 3.92 (J = 7.8, d, 1H, H-18), 7.35(M,1H), EIMS m/z (rel. int. %): 555.4, 303, 634.27 (M+), 511, 135, 55, 262.
3′-Chloro-2-benzylidene-3,11-dioxo-olean-12-ene-29-oic acid (18)
Compound is brown solid: yield: 61%, 1HNMR 300 MHz, (DMSO): δ(ppm), 1.18, 1.22, 0.77, 1.33, 1.09, 0.98, 1.41 (7-CH3), 5.46 (H-12, s,1H), 7.53 (M, 3H), 3.93 (J = 17.1, d, 1H, H-18,), 7.31 (s, 1H), 7.87 (H-1′′,s,1H), 2.71 (1H, s, H-9), EIMS m/z (rel. int. %): m/z 55, 266, 303, 75, 591 (M+), 301, 110, 138, IR (potassium bromide) cm-1: 1693 (CO), 1184 (C-O), 1435 (CH=CH, CO), 2951 (CH), 898 (C-Cl), 3375 (OH), 1284 (C-O).
4′-Methoxy-2-benzylidene-3,11-dioxo-olean-12-ene-29-oic acid (19)
Compound is light yellow solid: yield 82%, 1HNMR (300 MHz, DMSO): δ (ppm) 1.08, 1.00, 0.97, 1.13, 0.76, 1.07, 1.36 (7-CH3), 5.44 (H-12, s,1H,), 3.85(J = 14.4, d, H-18,1H), 7.33 (H-1′′, s,1H), 3.77 (H-9, s,1H,), 7.47 (J = 8.7, d,2H,), 3.77 (H-9, s,1H,), 7.03 (J = 18.3, d,2H,), EIMS m/z (rel. int. %): m/z 555.9, 407, 262, 586.3 (M+), 55, 453.3, 135, 303, 135.
3′-Methoxy-2-benzylidene-3,11-dioxo-olean-12-ene-29-oic acid (20)
Compound is dark brown solid: yield 48%, 1HNMR 300 MHz, (DMSO): δ (ppm) 1.14, 1.21, 1.13, 1.16, 0.83, 1.39 (7-CH3), 5.74 (H- 12, s,1H,), 7.07 (J = 5.7, d,2H,), 4.25(J = 12.6, d, 1H, H-18,), 7.00 (s, 1H), 7.40 (H-1′′,s,1H,), 2.51 (H-9, s,1H,), 6.84 (J = 6, d,1H,), EIMS m/z (rel. int. %): m/z 407, 55, 175, 586.3 (M+), 262, 453.3, 234, 303, 135, IR (potassium bromide) cm-1: 1620 (CO), 1014 (OMe), 1442 (CH=CH), 3348 (OH), 1215 (C-O ), 2947 (CH), 1261 (C-O).
2′-Chloro-2-benzylidene-3,11-dioxo-olean-12-ene-29-oic acid (21)
Compound is dark brown solid: yield 35%, 1HNMR 300 MHz, (DMSO): δ (ppm) 1.18, 0.82, 1.18, 1.24, 1.19, 1.36 (7-CH3), 7.62 (1H, d, J = 15), 4.03(J = 17.1, d,1H, H-18,), 7.39 (M, 3H, ),
10.47(H-1′′, s, 1H,), 5.96 (H-12, s,1H,), 2.45 (H-9, s,1H,), EIMS m/z (rel. int. %): m/z 331, 262, 427.6, 590.3 (M+), 427.6, 555, 303, IR(potassium bromide) cm-1: 1701 (CO), 1249 (CO), 3055 (OH), 1188 (C-O), 2958 (CH), 1450 (CH=CH).
3-Hydroxy-11-oxo-18-olean-12-ene-29-oic acid potassium salt (22)
Compound is white solid: yield 78%, IR (potassium bromide) cm-1: 1628 (COO-), 3306 (OH), 991 (CO), 2931 (CH), 1408 (COO-).
3,11-Dioxo-olean-12-ene-29-oic acid potassium salt (23)
Compound is white solid: yield 65%, IR (potassium bromide) cm-1: 1396 (C=O), 2935 (CH), 3406 (OH), 1600 (CO).
4′-Nitro-2-benzylidene-3,11-dioxo-olean-12-ene-29-oic acid potassium salt (24)
Compound is dark brown solid: yield 62%, IR (potassium bromide) cm-1: 1400 (CH=CH, NO2), 1878 (CO), 2931 (CH), 1631 (CO).
2′-Methoxy-2-benzylidene-3,11-dioxo-olean-12-ene-29-oic acid sodium salt (25)
Compound is Gummy yellow solid: yield 43%, IR (potassium bromide) cm-1: 1195 (OMe), 1693 (COO-), 1593 (COO-), 3425 (OH), 1431 (CH=CH), 3012 (CH).
2′-Bromo-2-benzylidene-3,11-dioxo-olean-12-ene-29-oic acid sodium salt (26)
Compound is white solid: yield 63%, IR (potassium bromide) cm-1: 1670 (CO), 3356 (OH), 1195 (C-O), 2981 (CH), 1462 (CH=CH).
3′-Chloro-2-benzylidene-3,11-dioxo-olean-12-ene-29-oic acid sodium salt (27)
Compound is light orange solid: yield 56%, IR (potassium bromide) cm-1: 879 (C-Cl), 1674 (CO), 1211 (C-O), 2993 (CH), 1458 (CH=CH).
4′′-Methoxy-2-benzylidene-3,11-dioxo-olean-12-ene-29-oic acid potassium salt (28)
Compound is white solid: yield 43%, IR (potassium bromide) cm-1: 1404 (CH=CH), 2935 (CH), 1639 (CO), 3089 (OH).
3′-Methoxy-2-benzylidene-3,11-dioxo-olean-12-ene-29-oic acid sodium salt (29)
Compound is light orange solid: yield 52%, IR (potassium bromide) cm-1: 1450 (CH=CH), 1701 (CO), 3001 (CH), 1651 (CO), 1701 (CO).
Conclusion
We have completed modification for the synthesis of chalcones and potassium and sodium salts from 18β-glycyrrhetinic acid at C-29. Keto group is produced from hydroxyl group at 3-position with the help of Jones oxidation. Chalcones are produced through Claisen Schmidh reaction of different benzaldehydes with this 3-keto-18β-glycyrrhetinic acid. With the help of IR, 1HNMR and MS characterization of 18β-glycyrrhetinic acid derivatives was determined. Claisen Schmidt synthesis has been completed successfully this is indicated by the presence of aromatic protons. Aromatic protons are confirmed by the presence of peak in very downfield region. Formation of sodium and potassium salts of compound 3, its chalcones and keto derivative are confirmed by the IR data of its salts. Same pattern of fragmentation shown by almost all the compounds along with other fragments m/z 262,55,303 and 135 shown by all the compounds.
References
- Langer DB, Czarczynska-Goslinska B, Goslinski T (2016) Glycyrrhetinic acid and its derivatives in infectious diseases. Curr Issues Pharm Med Sci 29:118-123.
- Vampa G, Benvenuti S (1991) Separtion of 18α-and 18β-glycyrrhetinic acid by high-performance thin-layer chromatographic densitometry. J Chromatography A 543:479-482.
- Kang L, Li X, Chen C,Wand F (2014) Research Progress on Structure Modification and Biological Activity of 18β-Glycyrrhetinic Acid. Curr Opin Complement Altern Med 1:34-44.
- Hu J, Wu J, Lu J, Ju Y (2012) A dual-responsive macrocycle based on glycyrrhetinic acid. Tetrahedron Letters 53:6705-6709.
- Tolstikov G, Baltina L, Serdyuk N (1998)Glycyrrhetic acid (a review). Pharmaceutical Chemistry Journal 32:402-412.
- Akman T, Guven M, Aras AB, Ozkan A, Sen H, et al. (2015)Theneuroprotective effect of glycyrrhizic acid on an experimental model of focal cerebral ischemia in rats. Inflammation 38:1581-1588.
- Zhou J, Cai W, Jin M, Xu J, Wang Y, et al. (2015) 18β- glycyrrhetinic acid suppresses experimental autoimmune encephalomyelitis through inhibition of microglia activation and promotion of remyelination. Scientific reports 5:13713.
- Jiang L, Wang Q, Shen S, Xiao T, Li Y (2014) Discovery of glycyrrhetinic acid as an orally active, direct inhibitor of blood coagulation factor xa. Thromb Res 133:501-506.
- Kalani K, Kushwaha V, Verma R, Murthy PK,Srivastava SK (2013)Glycyrrhetinic acid and its analogs: a new class of antifilarial agents. Bioorg Med Chem Lett 23:2566-2570.
- Moon MH, Jeong JK, Lee YJ, Seol JW, Ahn DC, et al. (2012) 18β-Glycyrrhetinic acid inhibits adipogenic differentiation and stimulates lipolysis. Biochem Biophys Res Commun 420:805-810.
- Semalty A, Semalty M, Rawat MSM, Franceschi F (2010)Supramolecular phospholipids–polyphenolics interactions: The PHYTOSOME® strategy to improve the bioavailability of phytochemicals. Fitoterapia 81:306-314.
- Wang LJ, Geng CA, Ma YB, Huang XY, Luo J, et al. (2012) Synthesis, biological evaluation and structure–activity relationships of glycyrrhetinic acid derivatives as novel anti-hepatitis B virus agents. Bioorg Med ChemLett 22:3473-3479.
- Nascimento GG, Locatelli J, Freitas PC, Silva GL (2000) Antibacterial activity of plant extracts and phytochemicals on antibiotic-resistant bacteria. Braz J Microbiol 31:247-256.
- Nitalikar MM, Munde KC, Dhore BV, Shikalgar SN (2010) Studies of antibacterial activities of Glycyrrhizaglabra root extract. Int J Pharm Tech Res 2:899-901.
- Kim HK, Park Y, Kim HN, Choi BH, Jeong HG, et al. (2002) Antimicrobial mechanism of β-glycyrrhetinic acid isolated from licorice, Glycyrrhizaglabra. Biotechnology Letters 24:1899-1902.
- Salari M, Eshraghi S, Noroozi M (2001) Antibacterial effect of glycyrrhetinic acid on 55 hospital strains of staphylococcus aureus and 32 actinobacillus actinomycetemcomitans. DARU J PharmaSci 9:37-39.
- Zhou X, Zhao L, Liu X, Li X, Jia F, et al.(2012)Antimycobacterial and Synergistic Effects of 18β‐Glycyrrhetinic Acid or Glycyrrhetinic acid‐30‐piperazine in Combination with Isoniazid, Rifampicin or Streptomycin against Mycobacterium bovis. Phytother Res 26:253-258.
- Roy A, Saraf S (2006) Limonoids: overview of significant bioactive triterpenes distributed in plants kingdom. Biol Pharm Bull 29:191-201.
- Liu D, Song D, Guo G, Wang R, Lv J, et al. (2007) The synthesis of 18β-glycyrrhetinic acid derivatives which have increased antiproliferative and apoptotic effects in leukemia cells. Bioorg Med Chem 15:5432-5439.
- Tatsuzaki J, Taniguchi M, Bastow KF, Nakagawa-Goto K, Morris-Natschke S, et al. (2007) Anti-tumor agents 255: Novel glycyrrhetinic acid-dehydrozingerone conjugates as cytotoxic agents. Bioorg Medicinal Chem 15:6193-6199.
- Schwarz S,Csuk R (2010) Synthesis and antitumour activity of glycyrrhetinic acid derivatives. Bioorg Med Chem 18:7458-7474.
- Alpert NL, Keiser WE, Szymanski HA (2012) IR: theory and practice of infrared spectroscopy. Springer Science & Business Media.
- Parul R, Kundu SK, Saha P (2013) In vitro nitric oxide scavenging activity of methanol extracts of three Bangladeshi medicinal plants. The Pharma Innovation 1:83.
- Atta-ur-Rahman CM, Thomsen J (1999) Manual of bioassay techniques for natural product research.
Citation: Kousar S, Shahzadi S, Bibi F, Irfan M, Mudassir MA (2021) 18β-Glycyrrhetinic Acid Chalcones and Its Salts with Their Mass Fragmentation Pattern. Clin Pharmacol Biopharm 10: 213.
Copyright: © 2021 Kousar S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 2698
- [From(publication date): 0-2021 - Dec 06, 2025]
- Breakdown by view type
- HTML page views: 1870
- PDF downloads: 828