A Case of Severe Respiratory Failure with All-Trans-Retinoic-Acid (ATRA) Syndrome Successfully Improved By Bilateral Pleural Effusions Drainage and Postural Drainage with Non-invasive Positive Pressure Ventilation (NPPV)
Received: 22-Oct-2018 / Accepted Date: 09-Nov-2018 / Published Date: 16-Nov-2018
Keywords: Severe respiratory failure; Immunocompromised host; Pleural effusions drainage; Postural drainage
Abbreviations
ATRA: All-Trans-Retinoic-Acid; NPPV: Non- Invasive Positive Pressure Ventilation; APL: Acute Promyelocytic Leukemia
Introduction
All-trans-retinoic acid (ATRA) is a differentiation agent, which can induce complete remission in most patients with acute promyelocytic leukemia (APL). The most serious and potentially fatal adverse effect of ATRA is named “ATRA syndrome”, which is characterized by fever and respiratory distress, along with weight gain, pleural or pericardial effusions, peripheral edema, thromboembolic events, and intermittent hypotension. It is estimated to occur in up to 26% of the patients who receive ATRA. In previous years, 30% of ATRA patients developed lethal organ dysfunction, but currently, the mortality has been reduced to approximately 2% by treatment with dexamethasone or methylprednisolone [1,2]. Symptoms usually appear 10 days after the beginning of ATRA treatment, although they may occur as early as the second day. For early diagnosis of ATRA syndrome and administration of intravenous corticosteroids, hemodynamic monitoring and ventilatory assistance are critical [1]. The lungs appear to be the most affected organ. Therefore, Non-invasive positive pressure ventilation (NPPV) can offer effective ventilatory support and improve gas exchange in the treatment of acute respiratory failure (ARF) in hematological malignancy patients [3]. We report a case of retinoic acid syndrome in a 55-year-old male who developed severe respiratory failure while being treated with ATRA for acute promyelocytic leukemia. Our case was successfully treated with intravenous corticosteroids, and NPPV was used without intubation. After the concurrent chemotherapy, he achieved complete remission.
Case Report
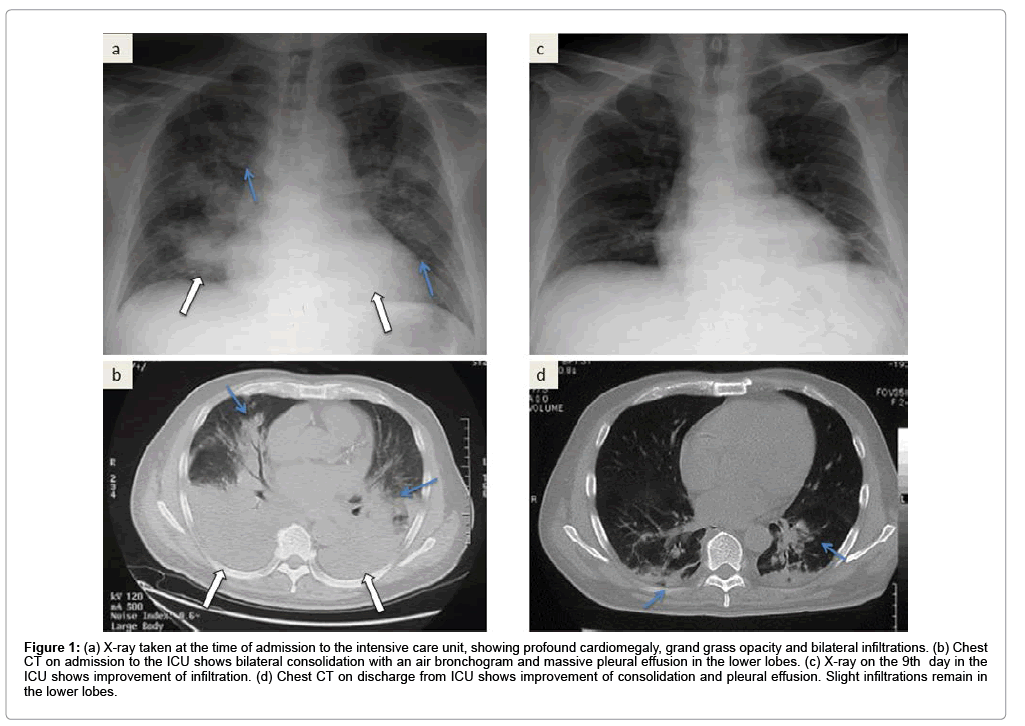
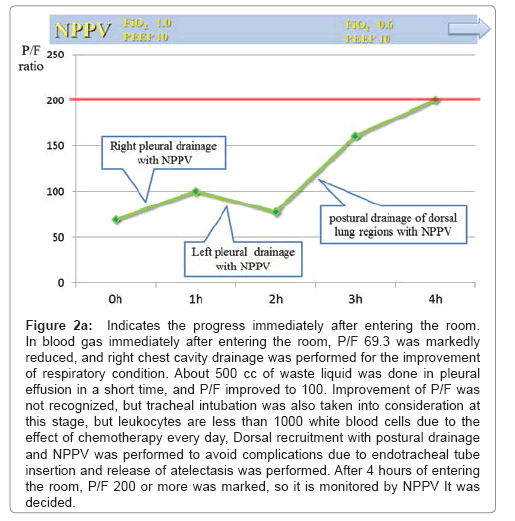
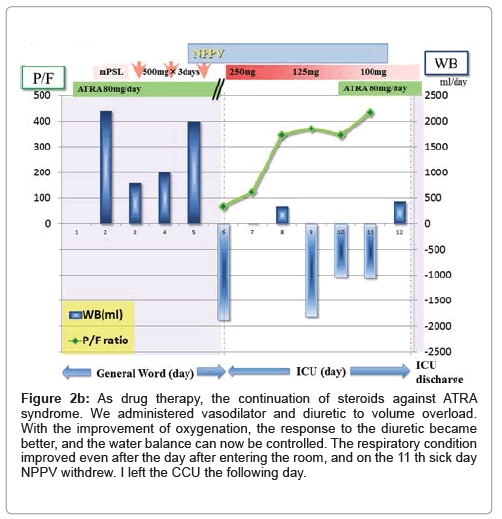
A 55-year-old male was referred to our hospital because of bloody stool, leukocytosis, blast cells in peripheral blood and thrombocytopenia. His symptoms were general fatigue, appetite loss, and shortness of breath. The patient’s underlying diseases had only hypertension and no other history of metabolic syndrome such as hyperlipidemia and diabetes. The patient had a history of acute hepatitis but there was no history of liver steatosis. The patient had not any special family history such as stroke, CAD, PAD, He was never smoker and to drink occasionally. His height was 167 cm, and his body weight was 70.8 kg with a body mass index of 25.4 kg/m2. His white blood cell count was 21,400/μl, hemoglobin was 8.9 g/dl, and platelet count was 15,000/μl on admission. His peripheral blood smear revealed leukoblastic cells (1.5% blast cells and 87% promyelocyte cells). Clinical and laboratory investigations led to the diagnosis of acute promyelocytic leukemia, which was confirmed by bone-marrow biopsy. His leukemic cells contained the translocation t (15;17) (q22;q11.12), suggesting that he should respond to an antineoplastic regimen with ATRA. He immediately underwent treatment with idarubicin (17 mg/ kg body weight) and ATRA (80 mg/kg body weight) to prevent ATRA syndrome because his white blood cell count was higher than 10,000/ μl. He was also complicated by disseminated intravascular coagulation (DIC); therefore, fresh frozen plasma and platelet transfusions were employed. Three days after chemotherapy, he had respiratory distress with bilateral infiltrates in the lung. Moreover, he showed weight gain and generalized edema because of over hydration due to transfusion blood products every day. ATRA syndrome was diagnosed based on clinical and radiological evaluation. The hematology team prescribed oxygen, intravenous methylprednisolone and temporary interruption of ATRA while continuing conventional chemotherapy. Our patient’s condition did not improve after being treated with intravenous diuretics, carperitide and nitroglycerin for fluid overload every day. The patient's respiratory status worsened despite the treatments, and therefore, he was admitted to our intensive care unit (ICU) because of severe respiratory failure (dyspnea, desaturation and severe hypoxemia). On physical examination at the ICU, his weight was 76.0 kg (6 kg above normal), blood pressure was 148/78 mmHg, pulse rate was 80 bpm and regular, body temperature was 37.0°C, and respiratory rate was 31 breaths per minute. On auscultation, breathing sounds revealed coarse crackles in the upper fields and they were decreased in the bilateral dorsal fields. Peripheral edema appeared. A blood test in the patient in the ICU showed pancytopenia, slight renal insufficiency, coagulopathy with DIC and severe respiratory failure, the brain natriuretic peptide (BNP) level was increased to 1,230 pg/ml, and the arterial pO2/fraction of inspired oxygen (P/F) ratio was decreased to 69.3 (Table 1). An X-ray revealed infiltration in bilateral lower fields, cardiomegaly (the cardiothoracic ratio was 58.3%), and chest computed tomography (CT) revealed grand glass opacity in the upper lobe and consolidation with an air bronchogram in the lower lobe associated with bilateral pleural effusion (Figures 1a and 1b). Congestive heart failure was suspected because of the increasing BNP levels. Echocardiography showed normal left ventricular wall motion, and therefore, an increase in BNP levels appeared to be associated with diastolic dysfunction with volume overload. Initially, we started NPPV to improve gas exchange, but the P/F ratio was still poor at 69.3 (CPAP mode, positive end-expiratory pressure: 10 cm H2O; fraction of inspired oxygen: 1.0). We then performed bilateral chest drainage to improve ventilation. After chest drainage, right pleural effusion was 1,150 ml of drainage and left pleural effusion was 550 ml of drainage, and the P/F ratio was temporally improved to 100. To obtain further improvement of lower lobe ventilation and to prevent intubation, we attempted postural drainage of the dorsal lung region with NPPV. Four hours after starting therapy oxygenation, there was a dramatic improvement, and the P/F ratio was over 200 (CPAP mode, positive end-expiratory pressure: 10 cm H2O; fraction of inspired oxygen: 0.6) (Figure 2a). Water balance was also improved with diuretic therapy. On the 6th day, the patient was weaned from NPPV and on the 9th day he was discharged from the ICU (Figure 2b). An X-ray on the 9th day in the ICU showed improvement of infiltration. A chest CT on discharge from the ICU revealed improvement of consolidation and pleural effusion. Slight infiltrations remained in the lower lobes (Figures 1c and 1d). After the patient respiratory condition was getting better, we accommodate the amount of steroid reduced gradually and finally off as soon as possible. After 2 months, he achieved full remission from his APL and was discharged with no complications.
Figure 1: a) X-ray taken at the time of admission to the intensive care unit, showing profound cardiomegaly, grand grass opacity and bilateral infiltrations. (b) Chest CT on admission to the ICU shows bilateral consolidation with an air bronchogram and massive pleural effusion in the lower lobes. (c) X-ray on the 9th day in the ICU shows improvement of infiltration. (d) Chest CT on discharge from ICU shows improvement of consolidation and pleural effusion. Slight infiltrations remain in the lower lobes.
Figure 2a: Indicates the progress immediately after entering the room. In blood gas immediately after entering the room, P/F 69.3 was markedly reduced, and right chest cavity drainage was performed for the improvement of respiratory condition. About 500 cc of waste liquid was done in pleural effusion in a short time, and P/F improved to 100. Improvement of P/F was not recognized, but tracheal intubation was also taken into consideration at this stage, but leukocytes are less than 1000 white blood cells due to the effect of chemotherapy every day, Dorsal recruitment with postural drainage and NPPV was performed to avoid complications due to endotracheal tube insertion and release of atelectasis was performed. After 4 hours of entering the room, P/F 200 or more was marked, so it is monitored by NPPV It was decided.
Figure 2b: As drug therapy, the continuation of steroids against ATRA syndrome. We administered vasodilator and diuretic to volume overload. With the improvement of oxygenation, the response to the diuretic became better, and the water balance can now be controlled. The respiratory condition improved even after the day after entering the room, and on the 11 th sick day NPPV withdrew. I left the CCU the following day.
| Hematology | Serology | Immune serum | |||
|---|---|---|---|---|---|
| WBC | 1500/µl4 | AST | 22 U/L | β-D glucan | <5.0 pg/ml |
| RBC | 231 × 10 /µl | ALT | 21 U/L | Procalcitonin | (-) |
| Hb | 7.5 g/dl | LDH | 461 U/L | Candida Ag | (-) |
| Ht | 21.70% | T-Bil | 0.6 mg/dl | Aspergillus Ag | (-) |
| Plt | 8.6 × 10 /µl4 | AMY | 63 U/L | -- | -- |
| Coagulation | Alb | 4.5 g/dl | Atrial blood gas | ||
| (CPAP FiO2 1.0/PEEP 10) | |||||
| PT-INR | 1.73 | UA | 11.1 mg/dl | pH | 7.456 |
| APTT | 26.7 sec | BUN | 48.4 mg/dl | PCO2 | 43.2 mmHg |
| Fib | 156 gm/dl | Cr | 0.75 mg/dl | PO2 | 69.3 mmHg |
| ATⅢ | 107.60% | Na | 146 mEq/L | HCO3 | 30.0 mmol/l |
| TAT | 8.4 µlg/ml | K | 3.8 mEq/L | BE | 6.0 mmHg |
| PIC | 5.1 µlg/ml | Cl | 103 mEq/L | SaO2 | 94.60% |
| D-dimer | 10.7 µlg/ml | CRP | 1.48 mg/dl | Lac | 21 mg/dl |
| FDP | 26.7 µlg/ml | BNP | 1230 pg/ml | P/F ratio | 69.3 |
Note: WBC: White Blood Cell; RBC: Red Blood Cell; Hb: Hemoglobin; Ht: Hematocrit; Plt: Platelet; Fib: Fibrinogen; TAT: Thrombin-Antithrombin Complex; PIC: Plasmin-Α2 Plasmin Inhibitor Complex; FDP: Fibrin/Fibrinogen Degradation Products; AST: Aspartate Aminotransferase; ALT: Alanine Aminotransferase; LDH: Lactate Dehydrogenase; T-Bil: Total Bilirubin; AMY: Amylase; Alb: Albumine; UA: Uric Acid; BUN: Blood Urea Nitrogen; Cr: Creatinine; Na: Sodium; K: Potassium; Cl: Chloride; CRP: C-Reactive Protein; BNP: Brain Natriuretic Peptide; Ag: Antige; HCO3-: Bicarbonate Ion; BE: Base Excess; SaO2: Oxygen Saturation; Lac: Lactate; P/F: Arterial PO2/Fraction of Inspired Oxygen
Table 1: Laboratory data at ICU admission because of acute respiratory failure due to all-trans-retinoic-acid syndrome.
Discussion
Retinoic acid syndrome was first described in 1992 by Frankel and colleagues. This syndrome is an unpredictable but frequent complication, which may develop after administration of ATRA, most commonly in patients with APL [1]. De Botton and colleagues published the largest case series of ATRA-induced syndrome [2-4]. These authors described 413 patients with newly diagnosed APL, 64 (15%) of which developed ATRA syndrome. The median time for the development of ATRA syndrome after starting ATRA was 7 days (range, 1-35 days) [4]. The latest review shows that ATRA syndrome occurs in approximately 25 percent of patients with APL during induction therapy [5]. ATRA syndrome means that differentiated APL cells adhere to vascular endothelial cells and cause vascular hyper permeability accompanying release of cytokines such as IL-8 and MMP-9, and they develop symptoms such as pulmonary edema and pleural effusion or ascites.
The primary symptoms were fever and respiratory distress. Respiratory distress (89%) and fever (81%) were the most common signs of ATRA syndrome followed by pulmonary infiltrates (81%) and weight gain (50%). Other findings included lower-extremity edema, pleural or pericardial effusions diffuse alveolar hemorrhage, cerebral hemorrhage, headaches, elevated intracranial pressure, nausea and vomiting, skin reactions, bone pain, tonsillar or cervical lymphadenopathy, pericarditis, intermittent hypotension, and thromboembolism [4,5]. These phenomena are a cytokine release syndrome, sometimes called "cytokine storm," and all of the pathophysiologic consequences result from the release of inflammatory cytokines from malignant promyelocytes, probably independent of their differentiation to segmented neutrophils and hyper leukocytosis. Establishing an accurate diagnosis of ATRA syndrome is difficult because of the similar toxicities and complications associated with APL therapy. The diagnosis is made based on clinical features and/or findings in the absence of other causes. At least three of the following signs and / or symptoms should be present to diagnose ATRA syndrome [2,3,6]. In general, it is recommended to treat this syndrome with prompt highdose corticosteroids and symptom-directed supportive care including empiric antibiotics, cautious diuresis, supplemental oxygen, and sometimes, mechanical ventilation for respiratory failure. Prophylactic steroids are not recommended, but prompt administration of steroids at the first sign of unexplained dyspnea, fever, weight gain or pulmonary infiltrate, is critical [5]. Previously, the mortality was reported as approximately 30%, but recently, Patatanian and colleagues reviewed that the mortality has improved in recent years [2]. The overall incidence of ATRA syndrome is slightly lower than the most widely reported numbers in the literature [1,2]. This may reflect the increasing recognition of the syndrome and prompt institution of steroid therapy. As is this case, acute respiratory failure (ARF) is one of the most severe complications in immunocompromised patients, which still causes a relatively high mortality under the current medical status [7]. NPPV is considered as an alternative to IMV in more severe patients or a means of avoiding IMV in less severe patients [8]. Recently, NPPV has been used increasingly to treat ARF. The best indications for its use are ARF in patients with chronic obstructive pulmonary disease exacerbations, acute pulmonary edema, and immunocompromised states. For these indications, multiple controlled trials have demonstrated that therapy with NPPV avoids intubation and reduces mortality [9,10]. Noninvasive ventilation (NIV) is commonly used as first-line therapy for immunocompromised patients with ARF and it has been recommended as the first line strategy in treating ARF in immunocompromised patients by several national guidelines [11]. The representative study shows that in a randomized trial of patients with hypoxemic respiratory failure following solid-organ transplantation, NPPV treatment was demonstrated to decrease intubation rate (20% vs. 70%, p<0.002) and ICU mortality (20% vs. 50%, p<0.05) compared with conventional therapy with oxygen [12]. Moreover, Hilbert and colleagues also observed fewer intubations (46% vs. 77%, p<0.05) and a lower mortality rate (50% vs. 81%, p<0.05) among immunocompromised patients with ARF randomized to NPPV as opposed to conventional therapy [13]. As these studies in immunocompromised patients with acute hypoxemic respiratory failure report that compared with invasive mechanical ventilation or oxygen (typically low flow oxygen), NPPV is associated with decreased ICU mortality, intubation rate, and ICU length of stay [14-16]. NPPV is a safe and effective tool for severe respiratory failure induced with immunocompromised hosts because it can prevent nosocomial or ventilator associated pneumonia (VAP) and subsequently reduces mortality. In fact, as same as in this case, Andrea and colleagues, Bassani and colleagues reported the efficacy of NPPV with respiratory failure from ATRA syndrome, respectively [3,17]. On the other hand, according to this case, we considered that the bad conditions such as a ventilatory capacity and hemodynamic impairments due to bilateral massive pleural effusion from volume overload which was characteristic of ATRA syndrome had also a baneful influence on ARF. This situation affected poor diuretic response and induced ARF, so we needed to perform pleural effusion drainage in addition to NPPV management. As the result, the patient's respiratory condition was getting better. In general, pleural effusions can affect the cardiorespiratory system, exercise capacity. Gas exchange worsens with pleural effusions and improves following thoracentesis [18]. Furthermore, Razazi and colleagues reported that drainage of large (>500 ml) pleural effusion in mechanically ventilated patients improves oxygenation and end-expiratory lung volume. Oxygenation improvement correlated with an increase in lung volume and a decrease in trans pulmonary pressure [19]. These effects discuss that chest wall expansion and displacement of the diaphragm are the principal mechanisms by which the effusion is accommodated; deflation of the thoracic cage and restoration of diaphragmatic function after thoracentesis may be important mechanisms by which breathlessness improves [18]. In summary, ATRA syndrome is said to have a good prognosis to treat with steroids therapy but we think that this case did not respond to steroid therapy because of not only the respiratory condition worsened but also deteriorated into the general conditions due to volume overload. As a result of intensive care of systemic management in ICU, such as NPPV treatment, bilateral pleural drainages, frequent postural drainage and adequate of hemodynamics administrate, his respiratory condition was immediately improved and it led to us to be able to escape from the cytokine storm in order to have been improved steroid reactivity.
Limitations
In general, when it cannot achieve to improve P/F ratio initial treatment with NPPV, we have to consider changing to IMV. But regard to immunocompromised hosts, NPPV management in acute respiratory failure is known to be the benefits for the mortality and the evidence level is high. The institution to be familiar to treat with NPPV treatment can be possible not require endotracheal intubation by multidisciplinary treatments like this case, and as a result, it is expected to reduce the treatment invasion, the treatment period in ICU, ventilation-free days, and the mortality rate compared with IMV treatment. In our country, ICU unit in a lot of other hospitals tends to consist of anesthesiology department or emergency department alone, but our hospital was a special unit to be consisted by several specialists like a cardiology, anesthesiology and respiratory departments, so this situation can be done these multidisciplinary treatments. In addition, our hospital has been aggressively used NPPV for acute respiratory failure, so we are accustomed to NPPV therapy to treat with acute respiratory failure. The experience contributes to being a successful treatment of NPPV for this case.
Future Directions
IUC unit need multidisciplinary treatments. It seems the best situation to consist of multi-special team. If it comes true to configure multi-department specialists in ICU, it can utilize each expert knowledge and experience. And then this situation can be achieved further improved in survival rate.
Conclusion
NPPV can offer effective ventilatory support and improve gas exchange in the treatment of acute respiratory failure in hematologic patients. In addition, the efficacy of NPPV on immunocompromised adult patients with acute hypoxemic respiratory failure has been reported in numerous studies. So the hospital which is accustomed to NPPV therapy can decrease the risk of life-threatening complications associated with endotracheal intubation and conventional mechanical ventilation in patients with hematologic malignancies.
References
- Frankel SR, Eardley A, Lauwers G, Weiss M, Warrell RP Jr (1992) The "retinoic acid syndrome" in acute promyelocytic leukemia. Ann Intern Med 117: 292-296.
- Patatanian E, Thompson DF (2008) Retinoic acid syndrome: A review. J Clin Pharm Ther 33: 331-338.
- Bassani MA, De Oliveira AB, Oliveira Neto AF (2009) Noninvasive ventilation in a pregnant patient with respiratory failure from all-trans-retinoic-acid (ATRA) syndrome. Respir Care 54: 969-972.
- De Botton S, Dombret H, Sanz M, Miguel JS, Caillot D, et al. (1998) Incidence, clinical features, and outcome of all trans-retinoica acid syndrome in 413 cases of newly diagnosed acute promyelocytic leukemia. The European APL Group Blood 92: 2712-2718.
- Cardinale L, Asteggiano F, Moretti F, Torre F, Ulisciani S, et al. (2014) Pathophysiology,clinical features and radiological findings of differentiation syndrome/all-trans-retinoic acid syndrome. World J Radiol 6: 583-588.
- Montesinos P, Bergua JM, Vellenga E, Rayón C, Parody R, et al. (2009) Differentiation syndrome in patients with acutepromyelocytic leukemia treated with all-trans retinoic acid and anthracycline chemotherapy:characteristics,outcome, and prognostic factors. Blood 113: 775-783.
- Lemiale V, Resche-Rigon M, Azoulay E (2014) Early non-invasive ventilation for acute respiratory failurein immunocompromised patients (IVNIctus): Â A study protocol for a multicenter randomized controlled trial. Trials 15: 372
- Wang T, Liu G, He K, Lu X, Liang X, et al. (2017) The efficacy of initial ventilation strategy for adult immunocompromised patients with severe acute hypoxemic respiratory failure: A study protocol for a multicentre randomized controlled trial (VENIM). BMC Pulm Med 17: 127.
- Liesching T, Kwok H, Hill NS (2003) Acute applications of non-invasive positive pressure ventilation. Chest 124: 699-713.
- Rochwerg B, Brochard L, Elliott MW, Hess D, Hill NS, et al. (2017) Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J 50
- Keenan SP, Sinuff T, Burns KE, Muscedere J, Kutsogiannis J, et al. (2011) Clinical practice guidelines for the use of noninvasive positive-pressure ventilation and noninvasive continuous positive airway pressure in the acute care setting. CMAJ 183: E195-E214
- Garpestad E, Brennan J, Hill NS (2007) Noninvasive ventilation for critical care. Chest 132: 711-720.
- Hilbert G, Gruson D, Vargas F, Valentino R, Gbikpi-Benissan G, et al. (2001) Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acuterespiratory failure. N Engl J Med 344: 481-487.
- Wang T, Zhang L, Luo K, He J, Ma Y, et al. (2016) Noninvasive versus invasive mechanical ventilation for immunocompromised patients with acute respiratory failure: A systematic review and meta-analysis. BMC Pulm Med 16: 129
- Huang HB, Xu B, Liu GY, Lin JD, Du B (2017) Use of noninvasive ventilation in immunocompromised patients with acute respiratory failure: A systematic review and meta-analysis. Crit Care 21: 4.
- Liu J, Bell C, Campbell V, DeBacker J, Tamberg E, et al. (2017) Noninvasive Ventilation in Patients With Hematologic Malignancy. J Intensive Care Med 1: 885066617690725.
- Cogliati AA, Conti G, Tritapepe L, Canneti A, Rosa G (2002) Noninvasive ventilation in the treatment of acute respiratory failure induced by all-trans retinoic acid (retinoic acid syndrome) in children with acute promyelocytic leukemia. Pediatr Crit Care Med 3: 70-73.
- Thomas R, Jenkins S, Eastwood PR, Lee YC, Singh B (2015) Physiology of breathlessness associated with pleural effusions. Curr Opin Pulm Med 21: 338-345.
- Razazi K, Thille AW, Carteaux G, Beji O, Brun-Buisson C, et al. (2014) Effects of pleural effusion drainage on oxygenation, respiratory mechanics, and hemodynamics in mechanically ventilated patients. Ann Am Thorac Soc 11: 1018-1024.
Citation: Suzuki M, Yamamoto T, Hosokawa Y, Nakazato K, Akutsu K, et al. (2018) A Case of Severe Respiratory Failure with All-Trans-Retinoic-Acid (ATRA) Syndrome Successfully Improved By Bilateral Pleural Effusions Drainage and Postural Drainage with Non-invasive Positive Pressure Ventilation (NPPV). J Respir Med 2: 110.
Copyright: © 2018 Suzuki M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 3949
- [From(publication date): 0-2018 - Dec 09, 2025]
- Breakdown by view type
- HTML page views: 2988
- PDF downloads: 961