A case of Spinal Neurocystercercosis - Python Eggs in the Spine
Received: 02-Dec-2022 / Manuscript No. JNID-22-82991 / Editor assigned: 05-Dec-2022 / PreQC No. JNID-22-82991(QC) / Reviewed: 20-Dec-2022 / QC No. JNID-22-82991 / Revised: 26-Dec-2022 / Manuscript No. JNID-22-82991(R) / Published Date: 30-Dec-2022 DOI: 10.4172/2314-7326.1000430
Introduction
Neurocysticercosis (NCC)is the most common parasitic infection of the central nervous system(CNS) (1), The larval form of the Taenia solium is the cause of the disease. The Cysticercal larvae typically involves the intracranial subarachnoid space. The subarachnoid space acts as a sieve that filters out the larvae, preventing them from entering the parenchyma and trapping them to grow in the subarachnoid space(2) The isolated involvement of spinal intradural area is extremely rare even in endemic areas. Here we report a 21-year-old female who presented with low back ache and radiating pain along the left lower limb, which is eventually diagnosed as NCC involving from L1 to L5 level. This case report is on the surgical experience with the case.
Case Report
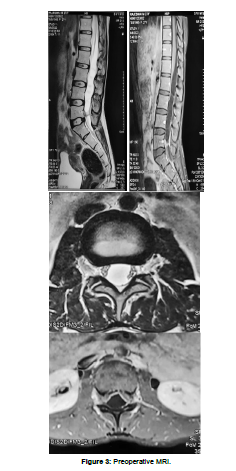
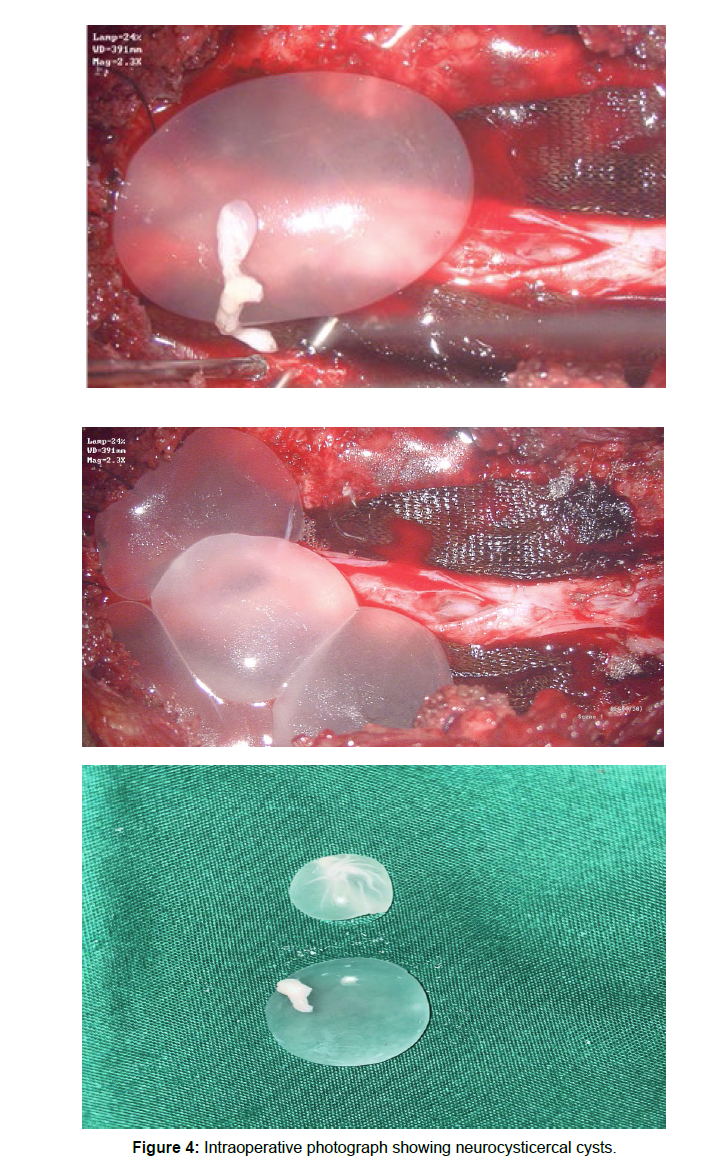
A 27 years old female patient presented with Low back pain for three months, which is radiating to left lower limb. The pain is sharp and shooting type aggravated on walking and on prolonged standing. The lower limb tone is increased on either side, The power in left lower limb is slightly decreased,the deep tendon reflexes are exaggerated in both knee and ankle. With these clinical findings An MRI of the lumbar spine with whole spine screening was done initially which revealed a well defined intradural extramedullary altered signal intensity lesions which are multiple and has a fluid signal that is hyperintense on T2/ STIR, hypointense on T1 showing thin patchy peripheral contrast enhancement noted in the spinal canal from L1 vertebral body level up to L5 level. An altered signal intensity which is iso to slightly hyperintense on T1 and slightly hyperintense on T2 noted in the inferior aspect of the lesion at the L5 vertebral body level and showed heterogenous enhancement. The lesion is displacing the nerve roots towards the periphery and conus anteriorly. NCC was suspected with these radiological findings, An MRI screening of the brain was normal. She underwent L5 and S1 laminectomy and opening of the dura, upon opening the dura multiple fluid-filled cysts popped out and all of them were intact, they were carefully collected without any rupture in a separate bowl, and there appeared to be a thickened arachnoid which was sent for biopsy after haemostasis the head end of the table was raised and Valsalva was given with dura open to retrieve any remanent cysts, a gentle saline wash was given and dura is closed in water tight fashion. On gross examination each cyst was found to be round to oval shaped with whitish gelatinous wall and near clear fluid and an area of whitish patch over the wall suggestive of scolex, scolex was not seen in some cysts.Postoperatively the patient was relieved of her complaints, she was started with Albendazole for 8 weeks and was discharged. She was followed up after 8 weeks and a repeat MRI scan was done which showed the spinal cord was decompressed and there were no residual cysts. The enzyme-linked immunosorbent assay (ELISA) test showed no evidence of infection.
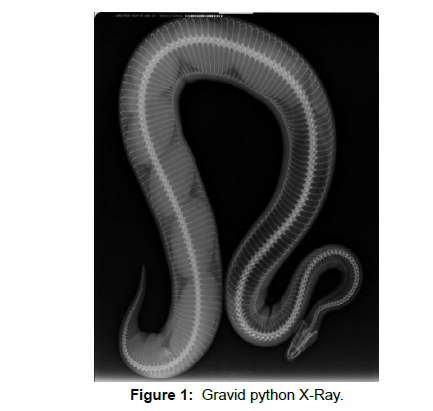
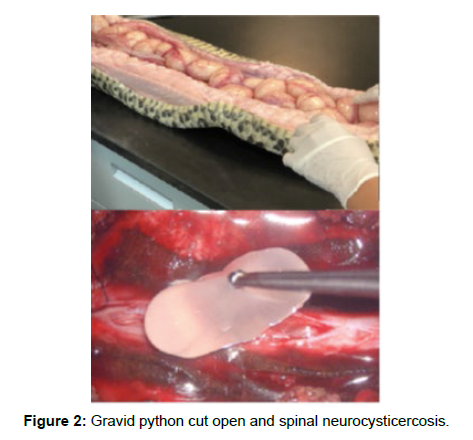
Comparative images of python eggs and spinal Neurocystercercosis (Figures 1-4)
Discussion
Despite the high incidence of NCC in endemic areas, spinal NCC is very rare compared with intracranial NCC. Indeed, spinal cysticercosis (SC) accounts for 1.2–5.8% of NCC cases [1]. There have been fewer than 200 cases of spinal NCC reported in the world literature [2]. The cysticercal involvement of the brain is more common than the spine because the brain draws more blood supply than the spine [3]. More than 50% of patients with spinal cysticercosis have evidence of Taenia solium infection elsewhere [4]. Spinal NCC can be both intra dural and extra Dural, in intra dural type it can be intra medullary or extra medullary. Spinal Intradural cysticercosis usually involves the subarachnoid space compared to the intra medullary area [5]. Intramedullary cysticercosis might be due to hematogenous dissemination, whereas the leptomeningeal form, via the CSF dissemination [6]. The CSF dissemination may be due to the effect of gravity, where the larvae migrate into the spinal canal from the ventricles of the brain under the influence of gravity [7]. Classically, the NCC of brain are located more frequently in the fourth ventricle, less commonly in the third, and least frequently in the lateral ventricles [8]. Associated arachnoiditis is also reported in the spinal NCC [9] Spinal NCC-presents with symptoms of myelopathy, progressive weakness, paraesthesia’s, bowel and bladder disturbances. In this case, neuroimaging showed no evidence of intracranial lesion, no skeletal lesion was identified. The diagnosis of spinal NCC is extremely challenging and involves the radio imaging and Enzyme-linked immunosorbent assay (ELISA) testing of serum and CSF. Detection of antibodies specifc for Taenia solium, has been reported to be the best diagnostic tool with a specifcity of 100% and a sensitivity of 94%-98% for patients with two or more cystic or enhancing lesions [10,11]. Magnetic resonance imaging with contrast with thin sections is the diagnostic study of choice for evaluating spinal NCC. The imaging pattern depends on the stage of the parasite [12]. Vesicular stage lesions appear hypodense on computed tomography and hyperintense on T2 image, with an eccentric scolex. Colloidal vesicular stage moderate edema is present due to an inflammatory reaction from the parasite. In the granulonodular stage there is a thickening of the capsule and calcium deposition; The fourth stage is a calcified lesion. Contrast Enhancement is seen in the vesicular and granulonodular stages. The racemose (grape-like) form presents as multiple, multicystic elongated or ovoid lesions, which do not calcify, whereas no scolices are demonstrated. They follow CSF signal intensity and are better identified on FLAIR sequences [9].
Albendazole and praziquantel are the drug of choice for the treatment of NCC [13] Simultaneous steroid usage will reduce the inflammation of the spinal cord caused by the larva, also usage of steroids along with albendazole has proven the increased CSF penetration of Albendazole [14].
Whenever the patients experience severe and progressive neurological dysfunction surgical removal of the parasite is to be done. Arachnoiditis due to the inflammatory reaction makes the removal of the cysts difficult, a careful but sharp dissection must be carried out. The key to preventing recurrence lies in the unruptured removal of the cysts, A watertight closure of dura has to be achieved, and in case of a ruptured cyst three per cent saline wash can be given. Postoperative usage of Albendazole for a prolonged period is to be carried out.
Conclusion
The diagnosis of Spinal neurocysticercosis is challenging and suspicion of the disease has to be there in endemic countries like India, the radiological imaging is the key to diagnosis of the disease. A complete brain imaging is necessary as the isolated spinal NCC is rare, Immunological diagnosis has to be obtained if necessary. Surgical decompression of the spinal cord and retrieval of intact cysts and usage of Albendazole for 8 weeks is the treatment of choice.
References
- Themes UFO (2017) Parasitic Diseases of the Central Nervous System. Radiology Key.
- Dhar A, Dua S, Singh H (2021) Isolated Intramedullary Lumbar Spine Neurocysticercosis: A Rare Occurrence and Review of Literature. Surg J Dec 15: e327- 336.
- Carydakis C, Baulac M, Laplane D, Schuller E, Philippon J (1984) [Pure spinal cysticercosis. Note on the cerebrospinal fluid]. Rev Neurol 140: 590-593.
- Izci Y, Moftakhar R, Salamat MS, Baskaya MK (2008 ) Spinal intramedullary cysticercosis of the conus medullaris. WMJ 107: 37-39.
- Parmar H, Shah J, Patwardhan V, Patankar T, Patkar D, et al. (2001) MR imaging in intramedullary cysticercosis. Neuroradiology 43: 961-967.
- Leite CC, Jinkins JR, Escobar BE, Magalhães AC, Gomes GC, et al. (1997) MR imaging of intramedullary and intradural-extramedullary spinal cysticercosis. AJR Am J Roentgenol 169: 1713-1717.
- Colli BO, Assirati Júnior JA, Machado HR, dos Santos F, Takayanagui OM (1994) Cysticercosis of the central nervous system. II. Spinal cysticercosis. Arq Neuropsiquiatr 52: 187-199.
- Zee CS, Go JL, Kim PE, DiGiorgio CM (2000) Imaging of neurocysticercosis. Neuroimaging Clin N Am 10: 391-407.
- Paterakis KN, Kapsalaki E, Hadjigeorgiou GM, Barbanis S, Fezoulidis I, et al. (2007) Primary spinal intradural extramedullary cysticercosis. Surg Neurol 68: 309-311.
- Brutto OHD, Rajshekhar V, Jr ACW, Tsang VCW, Nash TE, et al. (2001) Proposed diagnostic criteria for neurocysticercosis. Neurology 57: 177-183.
- Machado LR, Livramento JA, Vaz AJ, Bueno EC, Mielli SR, Bastouly V, et al. (2002) IgG intrathecal synthesis and specific antibody index in patients with neurocysticercosis. Arq Neuropsiquiatr 60: 395-399.
- .Rahalkar MD, Shetty DD, Kelkar AB, Kelkar AA, Kinare AS, et al. (2000) The many faces of cysticercosis. Clin Radiol 55: 668-674.
- Qi B, Ge P, Yang H, Bi C, Li Y (2011) Spinal Intramedullary Cysticercosis: A Case Report and Literature Review. Int J Med Sci 8: 420-423.
- Sotelo J, Del Brutto OH (2002) Review of neurocysticercosis. Neurosurg Focus 12: 1-7.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Reddy KC, Reddy KS (2022) A case of Spinal Neurocystercercosis - Python Eggs in the Spine. J Neuroinfect Dis 13: 430. DOI: 10.4172/2314-7326.1000430
Copyright: © 2022 Reddy KC. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 1883
- [From(publication date): 0-2022 - Dec 13, 2025]
- Breakdown by view type
- HTML page views: 1460
- PDF downloads: 423