Research Article Open Access
A Gelatin-Based Prophylactic Sealant for Bowel Wall Closure, Initial Evaluation in Mid-rectal Anastomosis in a Large Animal Model
Yael Kopelman1,2, Yael Nir3, Yariv Siman-Tov4, Benjamin Person2,5, Oded Zmora6,7, Hagit Tolchinski7,8, Amir Szold9 and Doron Kopelman2,10*1Gastroenterology Institute, Hillel-Yaffe Medical Center, Hadera, Israel
2Rappaport Faculty of Medicine of the Technion – Israel institute of technology, Haifa, Israel
3LifeBond Medical Technologies Ltd., Caesarea, Israel
4Pre-clinical Research Unit at Assaf Harofe Medical Center, Zrifin, Israel
5Department of Surgery, Carmel medical center, Haifa, Israel
6Department of Surgery, Sheba Medical Center, Tel Hashomer, Israel
7Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
8Department of Surgery, Tel Aviv Medical Center, Tel Aviv, Israel
9Assia Medical Group, Tel Aviv, Israel
10Deaprtment of Surgery, HaEmek Medical Center, Afula, Israel
- *Corresponding Author:
- Prof. Doron Kopelman
Head Dept. of surgery, "HaEmek" Medical Center
Yitzhak Rabin Av. Afula, Israel
Tel : 972-547-390939
E-mail: kopelmand@bezeqint.net
Received date: November 17, 2014; Accepted date: February 27, 2015; Published date: March 10, 2015
Citation: Kopelman Y, Nir Y, Siman-Tov Y, Person B, Zmora O, et al. (2015) A Gelatin-Based Prophylactic Sealant for Bowel Wall Closure, Initial Evaluation in Mid-rectal Anastomosis in a Large Animal Model . J Gastrointest Dig Syst 5:258. doi:10.4172/2161-069X.1000258
Copyright: © 2015 Kopelman Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Background: Leakage is the most significant complication of gastrointestinal surgery and advanced endoscopic procedures. Sealants applied over the closure lines may help in the prevention of leakage by reinforcing the anastomosis during the initial susceptible healing period, allowing the natural healing process additional time by mechanically supporting the bowel edges.
Objective: To evaluate the safety and performance of a gelatin-based sealant in a porcine model.
Design: A prospective double arm, randomized study of 21 pigs, 12 in the sealant study group and 9 controls.
Setting: Animal laboratory
Main Outcome Measurements: Animal wellbeing, radiological contrast studies, gross intra-abdominal pathology and histological evaluation at post-operative days 5, 7, and 10.
Intervention: Transection and re-anastomosing of the mid-rectum
Results: In all 12 sealant arm animals, bowel motility was restored within 24 hours. No adverse effects were detected. No significant difference was noted in type or severity of adhesions. All but one demonstrated a full staple line coverage, transparency, flexibility and perfect adherence of the sealant. Contrast studies did not show leakage. The local wound healing process in both groups was identical, as assessed by histology. The tissue reaction to the sealant was characterized by a capsular formation on the outer surface, mimicking a serosal layer.
Limitation: Differences between porcine and human colorectal anatomy
Conclusions: A gelatin-based liquid sealant is safe to use on colorectal closure in a swine model and shows a favorable performance profile. Clinical studies are required in order to evaluate its efficacy in reducing the rate of gastrointestinal anastomotic leakage.
Abstract
Background: Leakage is the most significant complication of gastrointestinal surgery and advanced endoscopic procedures. Sealants applied over the closure lines may help in the prevention of leakage by reinforcing the anastomosis during the initial susceptible healing period, allowing the natural healing process additional time by mechanically supporting the bowel edges.
Objective: To evaluate the safety and performance of a gelatin-based sealant in a porcine model.
Design: A prospective double arm, randomized study of 21 pigs, 12 in the sealant study group and 9 controls.
Setting: Animal laboratory
Main Outcome Measurements: Animal wellbeing, radiological contrast studies, gross intra-abdominal pathology and histological evaluation at post-operative days 5, 7, and 10.
Intervention: Transection and re-anastomosing of the mid-rectum
Results: In all 12 sealant arm animals, bowel motility was restored within 24 hours. No adverse effects were detected. No significant difference was noted in type or severity of adhesions. All but one demonstrated a full staple line coverage, transparency, flexibility and perfect adherence of the sealant. Contrast studies did not show leakage. The local wound healing process in both groups was identical, as assessed by histology. The tissue reaction to the sealant was characterized by a capsular formation on the outer surface, mimicking a serosal layer.
Limitation: Differences between porcine and human colorectal anatomy
Conclusions: A gelatin-based liquid sealant is safe to use on colorectal closure in a swine model and shows a favorable performance profile. Clinical studies are required in order to evaluate its efficacy in reducing the rate of gastrointestinal anastomotic leakage.
Keywords
Anastomosis; Colorectal anatomy
Introduction
Anastomotic and bowel closure line leakage is the most significant complication of gastrointestinal surgery and a major obstacle towards successful advanced endoscopic resections of bowel lesions. The application of a biocompatible and well conforming sealant around the anastomotic closure line may help in the prevention of leakage by reinforcing the closure line during the initial susceptible healing period, enhancing healing by mechanically supporting the bowel edges and preventing bacteria leaking into the peritoneal cavity. Numerous studies have been undertaken on the role of tissue adhesives as gastrointestinal (GI) sealants, with inconsistent results [1]. Various commercially available sealants have been evaluated in recent small animal studies with mixed results [2]. The aim of this study was to evaluate the safety and performance of a novel gelatin-based sealant in a porcine model of colo-rectal transection and re-anastomosis.
Material and Methods
The Sealant
The closure line anastomotic sealant evaluated in this study, (LifeSeal™, LifeBond, Caesarea, Israel) is intended for use as an adjunct for staple-line reinforcement during the construction of a gastrointestinal anastomosis and as an adjunct for other endoscopic techniques of bowel wall closure. The sealant slowly degrades as the natural healing processes take place.
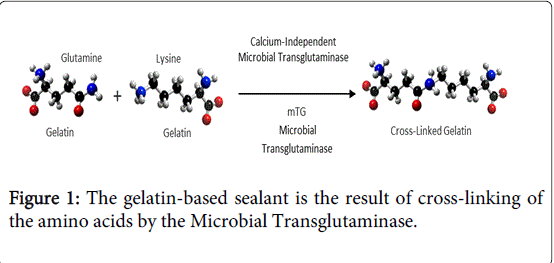
The sealant is comprised of a porcine gelatin solution component and an enzyme catalyst solution component. When the two components are mixed together, the gelatin solution rapidly polymerizes in-situ to form a sealing gel. The enzyme is a microbially synthesized transglutaminase (mTG). The mTG enzyme functions as a catalyst to the process of the gelatin stabilization. The porcine gelatin component is naturally stabilized by formation of inter- and intramolecular chemical bonds between the gelatin molecules. The bonds are created between the g-carbonyl group of a glutamine residue and the e-amino group of a lysine residue (Figure 1).
Animal care and anesthesia
21 healthy female pigs, Sus scrofa domestica, approximately 3.5 months old, weighing an average of 45.9 kg (42.6 kg to 52 kg) were used. The study was approved by the Institutional Animal Care and Use Committee of Assaf Harofe Medical Center (#10-2011,44-2010). The animals were randomized to either a study group (12 animals) or a control group (9 animals). The swine were acclimated for at least 5 days prior to commencement of treatment while being fed their standard diet and water ad libidum. A restricted commercially available pig-mix (Nir Oz mixture institute, Nir Oz, Israel) was given to the pigs prior and following surgery. 3 days before the surgery day, paraffin oil was added to the diet. Animals were fasted for 24-36 hours (with free access to water) before surgery. Mechanical bowel preparation was given by an oral laxative solution (Soffodex, Dexxon Ltd, Or-Akiva, Hadera, Israel) 24 hours prior to the surgery. A cleaning enema was performed two hours prior to surgery using 2 x 250 mL (EasyGoEnema, Gilco Pharm Ltd, Rishon Le Ziyon, Israel).
A single dose of prophylactic antibiotics Cefazoline-sodium (Merck, Darmstadt, Germany) 30 mg/kg and vetrimoxine LA veterinary (Ceva Sante Animale S.A., Libourne, France) 1 gr was given intravenously. 20-30 minutes before initiation of the surgery. Preemptive analgesia (intramuscular Dipyron 1 gr) was given to all any amounts prior to surgery. The animals were anesthetized with a combination of Ketamine 22 mg/mL and Xylazine injection (Apectrum Chemicals and Laboratory Products, Gardena, California) 1 mg/kg IM. Midazolam (5-15 mg) was given IV upon veterinarian discretion. Endotracheal intubation was performed and anesthesia maintained by inhalation of Isoflurane 2-3 % (Abbott Laboratories Ltd, Queenborough Kent, England) and spontaneous breathing of oxygen 2 L/minute. The pigs were infused during the procedure with 35 mL/hour of normal saline solution and monitored for Oxygen saturation and heart rate throughout the surgical procedure.
The procedure of bowel transection and stapled re-closure anastomosis
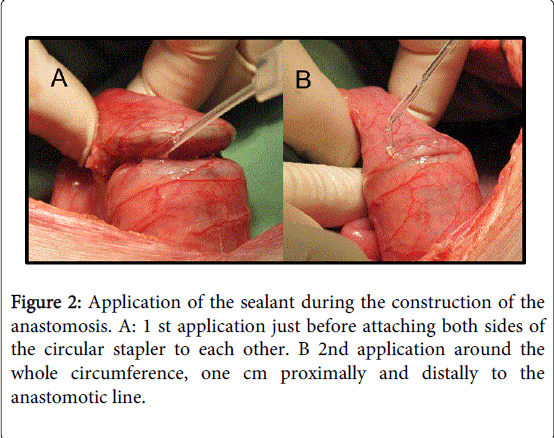
The anesthetized animals were placed in dorsal recumbence position. A ventral midline abdominal incision was made to expose the mid-rectum. The mid rectum was dissected free of its retroperitoneal and perineal attachments, so that it would remain freely mobile on the mesorectum. A 28 mm circular stapler (Covidien, Westbury, MA, USA) was introduced trans-anally with the anvil still attached to the shaft of the stapler and positioned at the lower rectum. The stapler was then opened to allow a gap between the anvil and the chamber of the shaft. A silk knot was tied around the shaft connecting the anvil to the distal segment of the device. The stapler was partially closed, leaving a 1 cm gap between the anvil and the main chamber of the stapler. There was no resection of a bowel segment prior to the construction of the anastomosis (Figure 2). The only resected segments were the two "rings" of rectum which were resected by the circular stapler itself. Approximately 1.5 ml of either the surgical sealant (in the device group) or normal saline (in the control group) was applied inside the formed gap (Figure 2). The anvil was immediately closed and fired and an additional layer of approximately 2.5 ml of sealant or saline was applied to the circumference of the tissue with approximately 1 cm margins from the anastomotic line on both sides of the anastomosis (Figure 2). Immediately after firing, the stapler was partially opened to allow relaxation of the tissue and the sealant was left to cure. Following curing of the sealant and rinsing with saline, the stapler was gently withdrawn.
After forming the anastomotic closure line the abdominal wall was closed by layers with continuous nylon loop for the linea alba, continuous Vicryl 2/0 for the subcutaneous fascia and metal (titanium) staples for the skin.
Outcome Measures
Ease of use
All procedures were performed by a team of two surgeons. At the end of the procedure the surgeon was asked to evaluate the ease of use in either three possible answers: 1) cumbersome, difficult to perform; 2) requires special yet reasonable effort; 3) easy to apply.
Animal follow up
The animals were allowed to recover and were followed daily by an experienced veterinarian. The clinical follow up evaluation included behavioral evaluation and general health status, GI tract function and fecal excrement and two evaluations of weight gain (at the beginning and at the end of the study). Blood samples were taken on days 5, 7 or 10 post-operatively.
Fluoroscopy
The pigs were sacrificed 10 days postoperatively. The previous laparotomy incision was opened and the abdominal cavity was evaluated for signs of possible infection, leaks, bowel obstruction or adhesions.
At day of sacrifice, under general anesthesia, contrast fluoroscopy was conducted using a C-arm (General Electric Company, Fairfield, CT, USA) to evaluate for signs of leaks or narrowing of the anastomotic site. The visualization of the staple line via x-ray was followed by insertion of a rectal tube and injection of 50mL of water soluble contrast medium and 50mL saline. The entire passage of the medium through the staple line was recorded as video data. All GI radiography data were analyzed with respect to the following:
Closure line leakage.
Narrowing of the anastomotic segment.
Determination of narrowing was independently performed by each of the 3 surgeons in a blind manner. The surgeons received the data and determined “Yes” for narrowing, “No” when no narrowing was observed and “Maybe” when inconclusive.
Blood work
Blood samples were collected under anesthesia upon the animals' arrival to the study facility on day 0 and on postoperative days 5, 7, 10. Hematological and biochemistry tests were conducted. A minimum of 3mL of whole blood was drawn from the animal’s pre-cava vein. Using automated routine hematology (ADVIA 120, Siemens, Tarrytown, NY) or clinical chemistry (Modular P-800, Roche-Hitachi, Indianapolis, IN) analyzers, the samples were evaluated for complete blood count, liver functions, kidney functions, electrolytes, glucose, and lipids contents. All tests were performed at American Medical Laboratories, Herzeliya, Israel.
Pathology and Histological evaluation
The pigs were sacrificed after either 5 (n=7), 7 (n=7), or 10 (n=7) days postoperatively. The previous laparotomy incision was opened and the abdominal cavity was evaluated for signs of possible infection, leaks, bowel obstruction or adhesions.
At the time of sacrifice, a segment of the rectum including the anastomotic line and 3cm on each side were resected. The specimens were fixed in 10% neutral buffered formalin (4% formaldehyde solution) for 48 hours fixation period. Each specimen was sectioned into 5-6 segments, 1cm thick. Staples were gently removed using tweezers. Dehydrated segments were embedded into paraffin blocks, with each paraffin block sectioned at three different thicknesses: 0, 1.5 and 3 microns. The sections were fixated on glass slides and stained with Hematoxylin & Eosin by an automated process. The following parameters were evaluated by an experienced pathologist blinded to allocation of the animal:
Characterization of local tissue response to the sealant.
Sealant’s effect on tissue’s natural healing processes.
For possible aberration of the normal healing process, the anastomotic area was evaluated by using the following parameters:
Gross observation
The general appearance of the sealant was evaluated and recorded as opaque or clear. The presence of adhesions and specifically adhesions of the surrounding tissue to the anastomotic site was recorded. Adhesion severity was graded as follows [3].
None
Thin filmy, divided by blunt dissection
Thin vascular, easily divided by sharp dissection
Extensive thick vascular, requires division by sharp dissection
Dense, bowel is at risk of injury with division
Sealant application site of each treated animal was evaluated for the following parameters:
Staple line coverage by sealant – macro and micro pathology were used to assess the percent of staple line covered with sealant. The macroscopic evaluation was performed when the rectal specimen was flat and the staple line linear
Sealant integrity – was evaluated by examining the number of discontinuations in the sealant layer over the staple line
Sealant flexibility – was evaluated using a manual bending test. Accordingly, sealant delaminating or lose of full integrity upon staple line bending was tested. Test was performed on 3 different areas of the staple line. It was recorded as pass – sealant maintained its integrity following the bending test, or fail – sealant failed to maintain its integrity. The results of the three repetitions were averaged for each animal.
Microscopic evaluation
Degree of maturation of the fibrous tissue – indicates organization of the granulation tissue from no maturation with markedly reactive fibroblast and a minimal amount of collagen to mature connective tissue with fewer fibroblasts and an abundance of collagen. Foreign body reaction – either no reaction or a prominent reaction with the presence of microgranulomas, macrophages and giant cells. Inflammation - infiltration by either neutrophils, eosinophils or lymphocytes in the area of the anastomotic line. Degree of epitheliazation – attempts of mucosa adjacent to the anastomotic line to cover the defect on the mucosal surface, either continuous epithelium and lamina propria or an ulcerated surface with no epitheliazation.
Results
A total of 21 animals were included in the study. All sealant arm animals restored bowel motility within the first 24 hours. 4 of the control group animals restored bowel function only after 72 hours. Two animals in the treatment group developed postoperative complications, compared to none in the control group. One animal deteriorated during follow-up and was found at sacrifice to have a volvulus of the small intestine with near complete blockage of passage of bowel content. An additional animal developed a ventral hernia at the surgical incision. The bodyweight change of animals in both groups throughout the study was within the expected normal range of the clinical context of the study, with no significant difference between the two groups.
Ease of use
All procedures were performed by two surgeons. Both reported their satisfaction from the ergonomic application device and ease of the sealant application.
Blood tests
Blood tests results were within the normal ranges. No indication of adverse systemic effects was detected. No significant differences were noted between the device and control groups except in the animal that developed the small bowel volvulus that presented hemoconcentration and mild leucocytosis.
Gross pathology
On visual examination of the anastomotic site, all animals except one, showed a normal anastomotic area. One animal in the control group showed visible narrowing of the anastomotic segment. Adhesions to the staple line were found in 4 animals in the sealant group and in a single animal of the control group. Adhesions to other organs were observed in 9 cases in the treatment group and 6 cases in the control group. No significant difference was noted between the sealant group and the control group in terms of the type or severity of adhesions. The average adhesion score was 1.5 (range 0-2) in the device group and 1.1 (range 0-2) in the control group. All these differences were statistically insignificant.
A limited minor retroperitoneal hematoma was found in 4 animals, 2 in the control group and 2 in the sealant arm.
Sealant performance evaluation
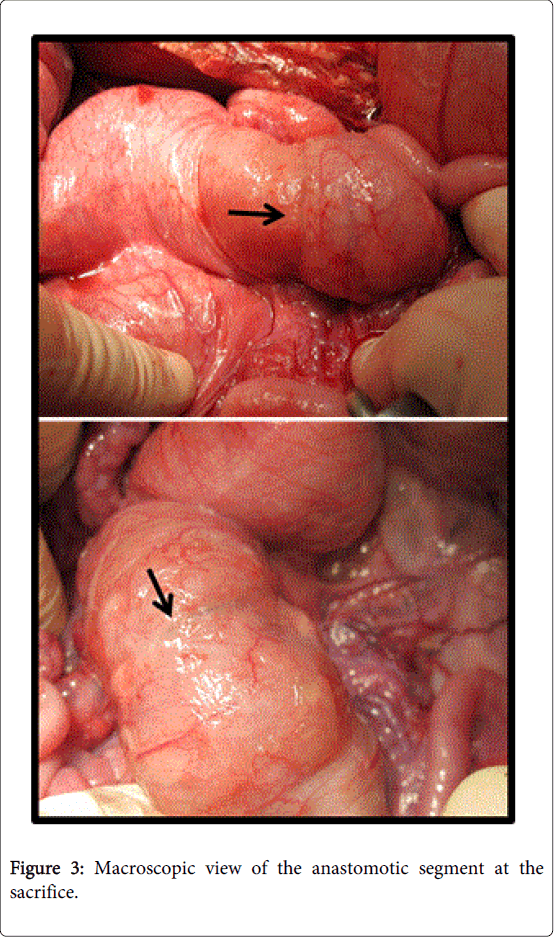
Staple line coverage: On macroscopically assessments at the time of sacrifice, all but one of the sealant arm animals demonstrated a full (100%) staple line coverage on visual inspection. In one animal it macroscopically appeared to be only 40-60% coverage . Of note, on histological examination of this anastomosis it was found that an extended capsule formation hindered the visual coverage which was in fact 100% (Figure 3).
Sealant transparency: In all, but one of the device arm animals, the sealant was transparent. In a single animal, reduced transparency was observed.
Sealant flexibility and adherence: the sealant fully maintained its flexibility and adherence in all the device group animals. No signs of delamination or cracking were found in the bending test in any of the device animals.
Fluoroscopy
GI radiography did not show any signs of leakage in the anastomotic region for any of the animals in both study groups. The animals in the sealant group did not show signs of anastomotic segment narrowing. Two animals of the control group had positive radiographic findings: one showed mild narrowing 2-3cm distal to the anastomosis and the other could not be assessed due to a complete blockage of the anastomotic segment at the level of the staple line. Visual inspection indicated a significant narrowing at the staple line due to hematoma and marked edema and fecal accumulation on the proximal side of the anastomosis.
Histological evaluation
In the control group the local tissue response to the staples was similar in the 7 days and 10 days time points, following the anastomosis formation: Minimal to mild foreign body granulomatous reaction surrounded the staples cavities, consisting of inner layer composed of minimal to mild infiltration of macrophages, few neutrophils and minimal presence of amorphous material (potential fibrin). The outer layer consisted of minimal to mild fibrosis. Only very sporadic multinucleated giant cells were noted. In the device group, in which sealant was found to be adjacent to the staples, no tissue response differences were identified. The nature and severity of the granulomatous reaction in the staples cavities were of exactly the same grade as seen in the control animals. The local tissue response surrounding the titanium staples used for the anastomosis formation was identical in both sealant and control groups, suggesting that the interaction between the sealant and the staples had no effect on the local tissue response surrounding the staples. In both groups, a reactive lymphoid hyperplasia was found (Figure 4).
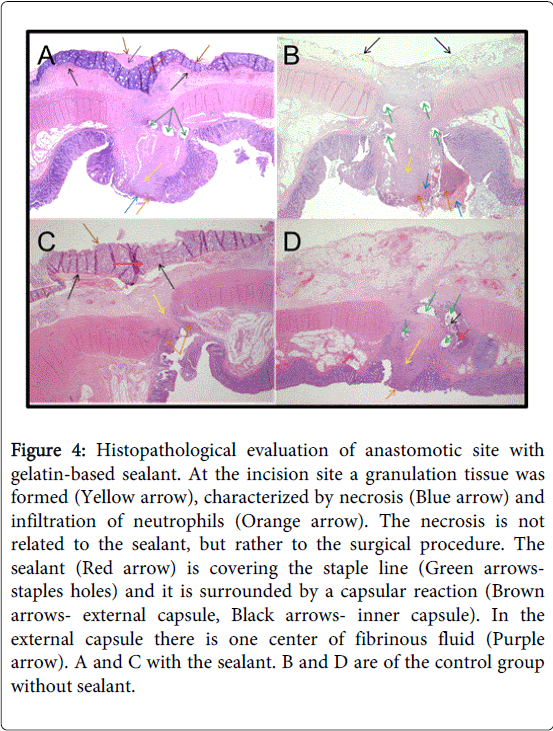
Figure 4: Histopathological evaluation of anastomotic site with gelatin-based sealant. At the incision site a granulation tissue was formed (Yellow arrow), characterized by necrosis (Blue arrow) and infiltration of neutrophils (Orange arrow). The necrosis is not related to the sealant, but rather to the surgical procedure. The sealant (Red arrow) is covering the staple line (Green arrowsstaples holes) and it is surrounded by a capsular reaction (Brown arrows- external capsule, Black arrows- inner capsule). In the external capsule there is one center of fibrinous fluid (Purple arrow). A and C with the sealant. B and D are of the control group without sealant.
The local wound healing process in the sealant group was found to be identical to the local healing process of the control group. The tissue reaction to the sealant was characterized by a capsular formation on the outer surface, mimicking a serosal layer. The sealant did not cause any adverse effect: no presence of abscess formation or any indication of necrosis related to the sealant was found. In both groups a mild well circumscribed foreign body reaction to the staples was noted.
Discussion
Endoscopic leakage from closure of defects in bowel wall, as well as surgical anastomotic leak is one of the most devastating complications in gastrointestinal procedures, leading to significant morbidity and occasional mortality. Leak often results in septic complications, reoperations, creation of stoma, or the formation of enterocutaneous fistula. On top of the significant economic burden of anastomotic leak, it is also associated with significant moral impact on both patients and surgeons. For this reason, the quest towards the "leak proof closure and anastomosis" is an appealing field of research in gastrointestinal endoscopy and surgery. Multiple causative factors and etiologies play a role in leakage such as: systemic and regional infection, bowel wall blood perfusion, tissue oxygenation, tissue tension, nutritional status, medications and other therapeutic toxicities, surgical technique, prolonged operation, and other intra-operative events such as blood transfusion, instrumental malfunction, use of drains etc. [4-6]. Despite this multifactorial process, there are currently several new techniques and devices being developed and tested in order to overcome drawbacks in current practice, making further risk reduction in colorectal anastomosis of great future promise [1,2,7]. Among these new technologies, the use of glues at the closure or anastomotic site is appealing, owing to its ease of use and low risk. Glues may theoretically act to reduce leakage rate by three main pathways. The first and probably most important is the temporary mechanical sealing of the closure line. Closure made by staples, clips or sutures connects the two sides of the bowel wall in multiple sites. This technique may leave tiny gaps between the sutures or staples, which may potentially allow bacteria and infection to penetrate through. Even if the anastomosis is completely tight at the time of closure, local edema and ischemia associated with the wound healing process may form such tiny gaps at the postoperative course. A second pathway may theoretically involve some mechanical support which may somewhat reduce tension from the closure line, or at least make it more tolerable to pressure at the post-procedural course, due to peristalsis or passage of bowel content for instance. The third pathway may include the formation of an acellular scaffold into which inflammatory cells and fibroblasts may migrate, to enhance the wound healing process around the closure line.
Different types of surgical glues and sealants have been presented so far with no high level evidence as to their efficacy and none have gained popularity as a useful device for this indication nor have they become the standard of care [8]. The main parameters according to which a good sealant should be evaluated are: Toxicity in both systemic and local tissue reactions, adherence to the union site without causing any interference with resumption of normal gastrointestinal function: motility, bowel movements etc., or interference with normal wound healing processes especially at the anastomotic site, possible impact on adjacent bowel loops and organs, such as the degree of peritoneal adhesions, ease of use and cost. A non-human source, gelatin-based sealant may be a good candidate for anastomotic sealant in terms of toxicity and tissue reaction. In this animal model the gelatin-based sealant has shown promising favorable results in the above mentioned parameters. No systemic adverse or toxic effects were noted. The post-operative morbidity presented by two animals: one with volvulus of a small bowel loop and another with a ventral hernia at the surgical incision, was deemed as not related to the application of the sealant. The level of post-operative peritoneal adhesions is reasonably low, was not increased by the use of glue, and was in full accordance with the level of manual handling of the bowel during the primary procedure. The local performance of the sealant in terms of full coverage of the closure line, along the entire study period is very encouraging since it is mandatory for an ideal sealant to reinforce the entire union line during the most vulnerable period of any type of closure or anastomosis, (i.e. 4 to 10 days post-operative). The added reinforcement layer around the closure line should remain flexible in order to not crack and lose comprehensive protection over the entire circumference on the one hand, and not to act as a rigid belt that interferes with the motility of that segment, or even promote stricture formation in cases of circular closure lines, which is considered to be a risk even without applying any agent following the stapling act itself. The histopathological results support our expectations as for the sealant acting as an inert, non-toxic, protecting shield around the anastomosis.
Few limitations of this study should be noted: The most important probably are the obvious differences between human and porcine bowel anatomy and tissue healing properties. This will justify further clinical studies. Animal model do not perfectly simulate anastomotic wound healing in humans, as the anastomotic leak rate may differ between the human and a swine. The relatively small number of subjects in each group may be underpowered to detect small differences between the two groups. Another potential drawback of this study is the length of follow-up. Anastomotic stricture is usually a late result of wound healing process and 10 days are not enough in order to exclude the risks of anastomotic stricture.
The very same liquid gelatin-based sealant can be applied in future studies via endoscopic instruments. Despite that, this is the first study assessing the use of a gelatin based sealant for anastomotic and closure line reinforcement, with favorable results, which justify further preclinical and clinical evaluation of this technology for the indication of prevention of leakage.
Acknowledgements
Part of this animal study, materials and animals, was funded by Life-Bond Ltd.
References
- Vakalopoulos KA, Daams F, Wu Z, Timmermans L, Jeekel JJ, et al. (2013) Tissue adhesives in gastrointestinal anastomosis: a systematic review. J Surg Res. 180: 290-300.
- Slieker JC, Vakalopoulos KA, Komen NA, Jeekel J, Lange JF (2013) Prevention of leakage by sealing colon anastomosis: experimental study in a mouse model. J Surg Res 184:819-824.
- Beck DE, Ferguson MA, Opelka FG, Fleshman JW, Gervaz P, et al. (2000) Effect of previous surgery on abdominal opening time.Dis Colon Rectum 43: 1749-1753.
- Klein M (2012) Postoperative non-steroidal anti-inflammatory drugs and colorectal anastomotic leakage. NSAIDs and anastomotic leakage.Dan Med J 59: B4420.
- Morse BC, Simpson JP, Jones YR, Johnson BL, Knott BM, et al. (2013) Determination of independent predictive factors for anastomotic leak: analysis of 682 intestinal anastomoses. Am J SurgS0002-9610:00430-00433.
- Buchs NC, Gervaz P, Secic M, Bucher P, Mugnier-Konrad B, et al. (2008) Incidence, consequences, and risk factors for anastomotic dehiscence after colorectal surgery: a prospective monocentric study. Int J Colorectal Dis 23:265-270.
- Boccola MA, Lin J, Rozen WM, Ho YH (2010) Reducing anastomotic leakage in oncologic colorectal surgery: an evidence-based review. Anticancer Res 30:601-617.
- Pommergaard HC, Achiam MP, Rosenberg J (2012) External coating of colonic anastomoses: a systematic review.Int J Colorectal Dis 27: 1247-1258.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 15786
- [From(publication date):
April-2015 - Aug 20, 2025] - Breakdown by view type
- HTML page views : 11110
- PDF downloads : 4676