A Multi Gene Targeting Approach to Treating Liver Diseases with Metadichol®
Received: 27-Dec-2018 / Accepted Date: 07-Jan-2019 / Published Date: 14-Jan-2019
Abstract
Liver diseases are becoming a major health concern. In the developing countries it is due to microbial infection. In the rest of the developed world it is due to alcohol abuse. Chronic liver disease and cirrhosis are a significant health concern in western countries. It is the fifth most common cause of death, after heart disease, cancer, stroke, and chest disease. The liver is capable of regeneration, but it can be overwhelmed leading to liver diseases like cirrhosis and hepatocellular cancer (HCC).
Vitamin D levels are low in most patients with liver diseases, and this suggests possible therapeutic benefits with use of vitamin D or its analogues. Vitamin D, through the vitamin D nuclear-40 receptor (VDR) plays a crucial role in mineral ion homeostasis. The liver is central in vitamin D synthesis and there is a need for an agent that will not lead to hypercalcemia. Metadichol, a nano emulsion of long-chain alcohols derived from food, is an inverse agonist of Vitamin D.
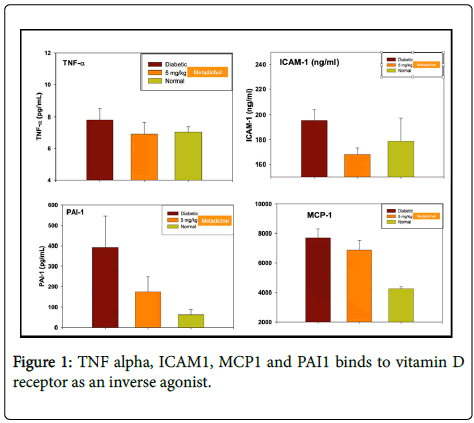
In Diabetic rat studies, it inhibits TNF alpha, ICAM1 (intracellular adhesion molecule), CCL2 (chemokine C-C motif) is also referred to as monocyte chemoattractant protein 1 (MCP1). All these cytokines, chemokines are known to have important role in liver diseases. We show that Metadichol indeed does work in liver disease patients by normalizing essential liver enzymes ALT, AST and ALP, and GGT. This approach is an example where Metadichol targets multiple genes and via multiple pathways to bring about homeostasis of the liver and is a useful, safe, nontoxic product in treating liver diseases and alleviating a global threat.
Keywords: Liver; Cirrhosis; Hepatocellular cancer; Hepatic failure; NAFLD; NASH; TNF; CCL2; MCP1; PAI1; ICAM1; Inverse agonist; VDR; Gilbert syndrome
Abbreviations
NAFLD: Non-Alcoholic Fatty Liver Disease; NASH: Nonalcoholic Steatohepatitis; TNF: Tumor Necrosis Factor; CCL2: Chemokine Ligand 2; MCP1: Monocyte Chemoattractant Protein 1; PAI1: Plasminogen Activation Inhibitor; ICAM1: Intercellular Adhesion Molecule 1; VDR: Vitamin-D Nuclear Receptor
Introduction
Liver diseases are a worldwide problem. The number of drugs for treating liver diseases is small in number. There is a need for a safe and therapeutic treatment with new molecular entities [1,2,3].
The liver helps purify the blood. It produces albumin as well as the proteins that cause blood clotting. The liver stores sugar appropriately, fats and vitamins until they are needed elsewhere in the body and also manufactures fat, cholesterol, and protein bilirubin. An inflamed liver does not perform these functions well, which brings about many of the symptoms, signs, and problems associated with any hepatitis.
Liver diseases
NAFLD (Non-alcoholic fatty liver disease is a condition when there is an excess of fat in the liver of people who do not consume alcohol [4]. The standard form of NAFLD is a benign condition called fatty liver when fat accumulates in the liver cells. NAFLD leads to hepatic steatosis NAFL (Nonalcoholic fatty liver disease), and affects about 20% of patients and also is present in type 2 diabetes patients.
NASH [5] is a condition where the liver sustains substantial damage, and the liver cells are gradually replaced by scar tissue impairing liver function. Some patients who develop cirrhosis may eventually require a liver transplant to remove the damaged liver.
Hepatitis A B and C are viruses [6] that can impair liver function. The diseases caused by them are similar and [7] leads to liver inflammation that can be severe or even life-threatening. There are effective vaccines for hepatitis A and B but not for type C. Liver failure and hepatocellular carcinoma (HCC) are caused by alcohol abuse and obesity the leading causes of chronic liver diseases especially in the developed world.
In advanced liver diseases, Perez et al. [7] showed that liver cirrhosis is caused by HBV/HCV infection. Prevention of liver cirrhosis and HBV/HCV infection is necessary as HCC can result. The solution is to avoid alcohol, and drugs but treating the underlying causes i.e. is alcoholism and HBV/HCC related infections.
HCC [8] is a serious problem because no treatment is available. The underlying causes for development of HCC are type 2 diabetes, alcoholism and chronic HBV/HCV infections lthat can lead to liver cirrhosis.
Nuclear receptors, cytokines, chemokines and liver diseases
Nuclear receptors (NRs) express genes that are involved in clearing toxic biliary constituents that are the hall mark of cholestasis [9]. Activation of NRs like VDR and FXR modulates many biological processes like inflammation, carcinogenesis. Break down of NR signaling leads to cholestasis disorders. NRs activation has the potential to modulate processes in cholestatic liver diseases [10]. Drugs used today exert their beneficial effects in cholestasis via NR activation for e.g. FXR activation (obeticholic acid) [11].
VDR activation in liver cells leads to anti-inflammatory and antifibrotic effects [12]. Targeting of VDR leads to improved bone density. Cholestasis leads to low vitamin D levels in Osteoporosis. 1, 25-Vit D3 actions on VDR results in reduced fibrosis in rat [13] and also lowered levels of pro-inflammatory cytokines in mice studies [14]. Vitamin D supplementation can help patients with liver diseases.
TNF α
Cirrhosis is considered an advanced stage of liver fibrosis. It causes over 1 million deaths per year, being the 14th leading cause of death worldwide [15]. Liver fibrosis leads to accumulation of collagen, elastin, and fibronectin, a consequence of hepatocyte death because of liver inflammation [16]. Serum levels of TNF-α is directly related to the severity of hepatic dysfunction in liver Cirrhosis [17]. TNFα enhances HSC (Hepatic stellate cella) survival leading to enhanced liver fibrosis. TNF alpha inhibitors in the market today have serious side effects [18,19]. Clinical trials using anti-TNFα antibody have been failed in alcoholic hepatitis [20]. Therefore, targeting of specific TNFα signaling pathways could be considered as a new therapeutic approach for the liver fibrosis.
ICAM1
Intercellular adhesion molecule 1 (ICAM-1) belongs to immunoglobulin supergene family that promotes intercellular adhesion. Present on the cell surface glycoprotein it is an important early marker of response to inflammatory mediators and immune activation [21,22].
Capra et al. [23] showed that patients with chronic HCV-related hepatitis show higher levels of sICAM-1 than do control subjects. The increase in sICAM-1 level is a result of HCV activity, which damages both hepatocytes and sinusoidal vessels [24] and, in particular, endothelial cells. Inflammation and cytolysis lead to an up regulation in the expression of tissue ICAM-1, which is mediated by proinflammatory cytokines [25,26].
In knockout mice ICAM-1 and infiltrating leukocytes play essential roles in early alcohol-induced liver injury [27]. They suggest that it is most likely caused by free radicals from NADPH oxidase in the Kupffer cell increasing NF-kB activation, that induces ICAM-1 expression via mechanisms involving TNF-a.
Chen et al. [27] showed that ICAM-1is a critical biomarker associated with development of future HCC incidence in chronic liver disease in patients with various chronic liver diseases that were free of HCC at baseline. Their findings held across diverse etiologies of liver disease and in patients with and without cirrhosis, were independent of established clinical risk factors.
CCL2
CCL2 (also called as MCP1) is a chemokine involved in immunoregulatory and inflammatory processes in the development of several acute and chronic liver diseases, inflammation and regulation of immune response, Chemokines have been shown to regulate diverse conditions such as oncogenesis and cancer [28]. Expression of CCL2 results in the infiltration of monocytes that predominately express the receptor CCR2.
In liver diseases CCL2 and CCR2 levels are high and results in increased inflammation, fibrosis, and steatosis [29]. Continuing inflammation leads to chronic liver diseases, like hepatitis C and nonalcoholic steatohepatitis. CCL2 has a role in alcohol-related damage, because its liver and plasma levels were associated with disease severity and with inflammation, including neutrophil infiltration, but not with steatosis, in patients with the alcoholic liver disease. [30,31]. Plasma levels and hepatic expression of CCL2 have been shown to be increased in ALD (alcoholic liver disease) patients CCL2 overexpression is associated with parameters of disease severity in all live diseases. Activation of pro-inflammatory pathways induced by alcohol was controlled in CCL2-deficient mice. Besides genes of fatty acid metabolism were induced in livers of alcohol-fed CCL2-KO mice, [32,33,34].
PAI1
PAI-1 (also known as SERPINE1) is the primary inhibitor of plasminogen activators playing a significant role in fibrinolysis [35]. PAI-1 has a major role in mediating fibrosis during cholestasis. Hyper fibrinolysis and hypo-fibrinolysis are the result of elevated PAI-1 levels and also the development of alcoholic liver disease (ALD). This results in inflammation, and necrosis (steatohepatitis), a ultimately leads to fibrosis and cirrhosis. Inhibition of PAI-1 could mitigate alcoholinduced liver damage fibrosis and cirrhosis). It has been shown that hepatic fibrosis is eliminated in mice that are deficient in PAI-1 [36].
No therapy is available to treat ALD. Treatment goal is on reducing effects of disease and or transplantation of livers of individuals with terminal cirrhosis. Hepatic steatosis can be blocked by inhibiting PAI-1 activation [37]. PAI-1 level is an indication of the disease severity [38].
Initially Hepatic changes caused by alcohol lead to steatosis. That initiation, then leads to ALD [39]. For example, in fatty livers the degree of fatty infiltration correlates with severity ALD fibrosis and cirrhosis [40,41]. Steatosis is caused by alcohol metabolism [42] that induces (TNF-α production which up regulates PAI-1 expression) [43].
Gilbert syndrome a liver disorder that results when the body cannot process bilirubin a waste product of red cells [44] caused by or structural liver damage. This results in elevated levels of bilirubin caused by a lack of liver enzymes needed to elimination of bilirubin [45,46,47].
Individuals may only exhibit mild yellowing of the skin and whites of the eyes (jaundice). Gilbert syndrome is seen more often in males than females. The disorder affects approximately 6-7% of individuals in the general population. Gilbert syndrome affects individuals of all races. Similar to Gilbert's syndrome are Crigler-Najjar, Rotor syndrome and Dubin-Johnson syndrome. All these are disorders are due to high levels of bilirubin in the blood [48].
Levels of liver enzymes serum aminotransferases alanine and aspartate aminotransferases (ALT and AST), alkaline phosphatase (ALP) and gamma-glutamyltransferase (GGT) [49,50,51] is what is used to detect liver diseases and their severity. A high level of serum ALT, AST is directly related to damaged liver tissues. Alcoholic liver disease the AST: ALT ratios are also used in determining presence of damaged liver diseases [52].
Alkaline phosphatase levels has a serum range of 20 to 140 U/L. The ALP test is used in identifying conditions such as hepatitis, cirrhosis, inflammation of the gallbladder blockage of bile ducts (from a gallstone, inflammation, or cancer. The enzyme alkaline phosphatase is a vital serum analyte, and its elevation in serum is seen in bone, liver and other diseases [53] ALP levels lead to bile ducts obstruction. Elevated ALP could indicate also (hyperparathyroidism vitamin D deficiency, or damaged liver cells [54]. Levels are elevated in people with untreated Celiac disease [55]. Over expression of ALP genes is seen in cancer patients [55,56], tumorigenesis [57,58] and in breast cancer patients [59-62].The number of drugs for treating useful liver drugs is small that there is a void that needs to be filled for safe and efficient therapeutic agents [63]. Compounds and or extracts from plants have been used in targeting liver diseases liver but have not been well studied and there is a need for additional research to justify their use in as it relates so safety and efficacy. Steroids have been used but have failed to show any significant results [64].
IFN-α treatment in viral hepatitis is the standard of care today [65]. It has a drawback as serious side effects such as depression and also nephrotic syndrome, retinal ischemia and decreased visual acuity have been reported. No FDA-approved drug treatment exists for the nonalcoholic fatty liver disease.
Metadichol® is a nano emulsion of long chain alcohols derived from food-based sources. It is safer than salt and sugar and has no known reported side effects. We have shown that it is a TNF α, ICAM1, CCL2 (also known as MCP1) and PAI1 (Serpine1) inhibitor (Figure 1).
We have shown that it binds to Vitamin D receptor as an inverse agonist [66]. Given the importance of these cytokines/chemokines described above in liver diseases, we present case studies to confirm its effects on liver diseases.
From US patent 8,722,093, Zucker diabetic fatty rat Metadichol dosage 5 mg/kilo.
Case Studies
Patient No. 1
A 29-year-old female on kidney dialysis for last 9 years after first child birth. Not on any liver medication, very high Alkaline Phosphatase levels and elevated GGT levels. Treated with Metadichol @ 5 mg.
Twice a day, her ALP levels increased in the first weeks before a steady decrease began over the next few weeks. There were also other improvements in her biomarkers RDW (Red Cell Distribution Width) which we have documented in other kidney patients [67]. Patient is still on dialysis as she awaits a donor and also Metadichol and showing slowly but steady improvement in other biomarkers (Figure 2).
Patient No 2
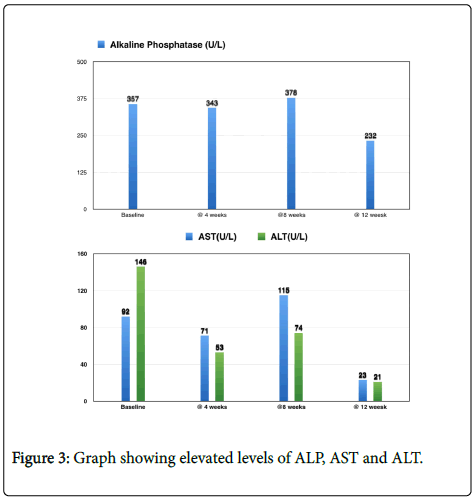
A 25-year-old Male diagnosed with Autoimmune Hepatitis and possibly primary schlerosing cholangitis (PSC) since the age of 15 and elevated ALP, AST and ALT levels 90 (Figure 3). Suffered from GI and other Colon, food allergies and repeated infections. Tried various treatments with drugs like prednisone and Imuran and Remicade all were of no use. Having tried and failed with known therapies the patient started on Metadichol orally @ 5 mg twice a day. Patient was on no other treatment, during this 12th week treatment. The patient reports improved energy levels, and for the first time in 10 years, his AST and ALT returned to normal. He underwent a liver transplant soon after and is now normal.
Patient No 3
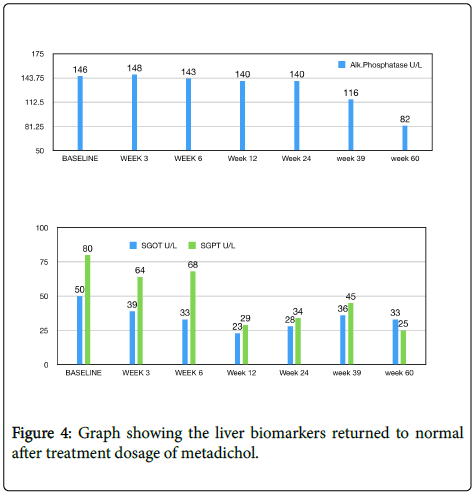
A 47-year-old female diabetic for 6 years mildly elevated liver enzymes. Metadichol treatment 5 mg twice a day. The liver biomarkers returned to normal (Figure 4).
Patient No 4
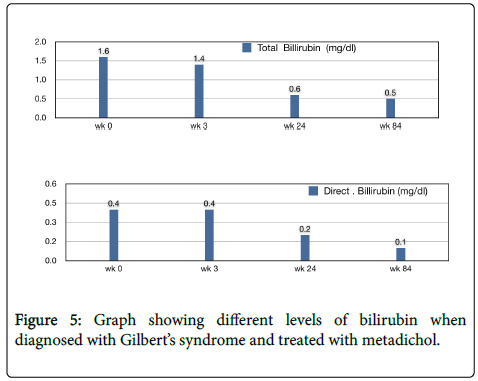
A 35-year-old female, elevated levels of bilirubin, diagnosed as Gilbert's syndrome. Metadichol @ 5 mg twice a day. Her levels returned to normal in 24th week (Figure 5).
Discussion and Conclusion
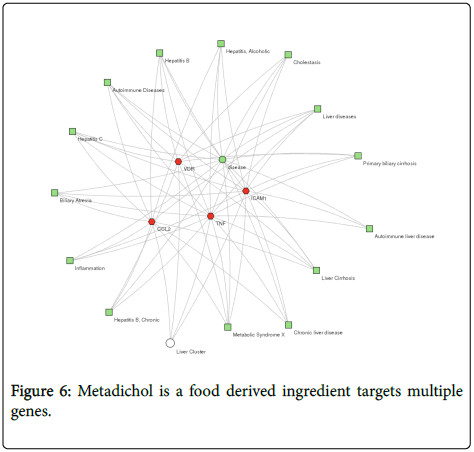
The application of Metadichol in all the cases presented and rapidly resolution of the liver biomarkers suggests that it could be modulating diseases through multiple pathways, binding to VDR and activating innate immunity pathways and Inhibiting TNF alpha, ICAM1, and CCL2. Most of the known therapies for today target one gene and there is a need for a comprehensive targeting of clusters of genes to target liver and other diseases via multiple disease pathways. Metadichol is a food derived ingredient targets multiple genes as shown in Figure 6.
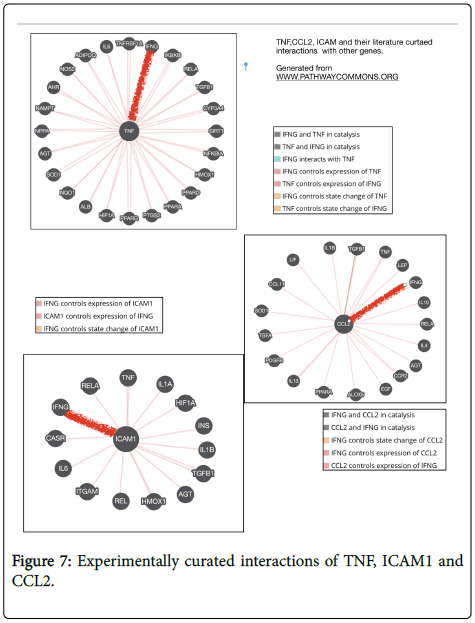
Metadichol binding to VDR and inhibition of TNF, ICAM1 and CCL2 can be analyzed using a software program like ToppCluster [68,69] shows how these clusters of genes target many liver-related diseases. Using Pathway Commons program [70] has one can further extend this approach by looking at the known experimentally curated interactions of TNF, ICAM1, and CCl2 shown in Figure 7. We see that IFNG (interferon gamma) is regulated by TNF, ICAM1, and CCL2. IFNG plays an important role in inflammation and autoimmune disease [71]. Increased levels of IFN-γ are directly related to liver disease severity [72] and were associated with the progression of liver disease [73]. T-helper cell (Th17) cells a subset of T cells are critical to inflammation [74]. VDR leads to a TH2 response, and Metadichol is a inverse of ROR gamma thus blocking the Th17 pathway [74]. Given that CCL2, TNF and ICAM 1 are all inhibited and thus IFN gamma the whole process is driven towards a VDR driven Th2 pathway [75].
Diseases are connected through closely related gene networks, and this is an approach that can be exploited to modulate multiple targets to enhance therapeutic effect as ligands today are focused on a single target and limited by their efficacy. Metadichol is a safe therapeutic that target multiple genes, pathways and multiple diseases that confirms the relevance of network-based approach of polypharmacology [76] and this has been demonstrated by our studies on other diseases [77-91].
References
- Rowe IA (2017) Lessons from Epidemiology: The Burden of Liver Disease. Dig Dis 35: 304-309.
- Udompap P, Kim D, Kim WR (2015) Current and Future Burden of Chronic Nonmalignant Liver Disease. Clin Gastroenterol Hepatol 13: 2031-2041.
- Uhl P, Fricker G, Haberkorn U, Mier W (2014) Current Status in the Therapy of Liver Diseases. Int J Mol Sci 15: 7500-7512.
- Benedict M, Zhang X (2017) Non-alcoholic fatty liver disease: An expanded review. World J Hepatol 9: 715-732.
- Wattacheril J, Issa D, Sanyal A (2018) Nonalcoholic Steatohepatitis (NASH) and Hepatic Fibrosis: Emerging Therapies. Annu Rev Pharmacol Toxicol 58: 649-662.
- Wood NJ (2011) Viral hepatitis: progress and promise. Nat Rev Gastroenterol Hepatol 8: 239.
- Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP (2006) The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol 45: 529-538.
- Waller LP, Deshpande V, Pyrsopoulos N (2015) Hepatocellular carcinoma: A comprehensive review. World J Hepatol 7: 2648-2663.
- Halilbasic E, Baghdasaryan A, Trauner M (2013) Nuclear Receptors as Drug Targets in Cholestatic Liver Diseases. Clin Liver Dis 17: 161-189.
- Rudraiah S, Zhang X, Wang L (2016) Nuclear Receptors as Therapeutic Targets in Liver Disease: Are We There Yet? Annu Rev Pharmacol Toxicol 56: 605-626.
- Wagner (2011) Hepatology, Nuclear Receptors in Liver Disease. 53: 1023-1024.
- Abramovitch S, Dahan-Bachar L, Sharvit E, Weisman Y, Ben Tov A, et al. (2011) Vitamin D inhibits proliferation and profibrotic marker expression in hepatic stellate cells and decreases thioacetamide-induced liver fibrosis in rats. Gut 60: 1728-1737.
- Ogura M, Nishida S, Ishizawa M, Sakurai K, Shimizu M, et al. (2009) Vitamin D3 modulates the expression of bile acid regulatory genes and represses inflammation in bile duct-ligated mice. JÂ Pharmacol Exp Ther 28: 64-570.
- Elangovan H, Chahal S, Gunton JE (2017) Vitamin D in liver disease: Current evidence and potential directions. Biochimica et Biophysica Acta 1863: 907-916.
- Tsochatzis EA, Bosch J, Burroughs AK (2014) Liver cirrhosis. Lancet 383: 1749-1761.
- Bataller R, Brenner DA (2005) Liver fibrosis. J Clin Invest 115: 209-218.
- Sabry HS, El-Hendy AK, Mohammed HI, Essa AS, Abdel-Aziz AS (2015) Study of serum tumor necrosis factor-α in patients with liver cirrhosis. Menoufia Med J 28: 525-531.
- Aggarwal BB, Gupta SC, Kim JH (2012) Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood 119: 651-665.
- Brenner D, Blaser H, Mak TW (2015) Regulation of tumor necrosis factor signaling: live or let die. Nat Rev Immunol 15: 362-374.
- Naveau S, Chollet-Martin S, Dharancy S, Mathurin P, Jouet P, et al. (2014) A double-blind, randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology 39: 1390-1397.
- Springer TA (1990) Adhesion receptors of the immune system. Nature 346: 425-434.
- Seth R, Raymond FD, Makgoba MW (1991) Circulating ICAM-1 isoforms: diagnostic prospects for inflammatory and immune disorders. Lancet 38: 83-84.
- Capra F, Maria ED, Lunardi C, Marchiori L, Mezzelani P, et al. (2000) Serum Level of Soluble Intercellular Adhesion Molecule 1 in Patients with Chronic Liver Disease Related to Hepatitis C Virus: A Prognostic Marker for Responses to Interferon Treatment. J Infect Dis 181: 425-431.
- Banner BF, Allan C, Savas L, Baker S, Barnard G, et al. (1997) Inflammatory markers in chronic hepatitis C. Virchows Arch 431: 181-187.
- Adams DH, Mainolfi E, Burra P, Neuberger JM, Ayres R, et al. (1992) Detection of circulating intercellular adhesion molecule–1 in chronic liver disease. Hepatology 16: 810-814.
- Zöhrens G, Armbrust T, Pirzer U, Meyer zum Büschenfelde KH, Ramadori G (1993) Intercellular adhesion molecule–1 concentration in sera of patients with acute and chronic liver disease: relationship to disease activity and cirrhosis. Hepatology 18: 798-802.
- Chen VL, Le AK, Podlaha O, Estevez J, Li B, et al. (2017) Soluble intercellular adhesion molecule-1 is associated with hepatocellular carcinoma risk: Multiplex analysis of serum markers. Nature Scientific reports 7: 11169.
- Schwabe RF, Wiley JW, Section Editors (2014) Roles for Chemokines in Liver Disease. Gastroenterology 147: 577-594.
- O’Connor T, Borsig L, Heikenwalder M (2015) CCL2-CCR2 Signaling in Disease Pathogenesis. Endocr Metab Immune Disord Drug Targets 15: 105-118. Â
- Marra F, DeFranco R, Grappone C, Milani S, Pastacaldi S, et al. (1998) Increased expression of monocyte chemotactic protein-1 during active hepatic ï¬brogenesis: correlation with monocyte inï¬ltration. Am J Pathol 152: 423-430.
- Heymann F, Trautwein C, Tacke F (2009) Monocytes and macrophages as cellular targets in liver ï¬brosis. Inflamm Allergy Drug Targets 8: 307-318.
- Marra F, Valente AJ, Pinzani M, Abboud H E (1993) Cultured human liver fat-storing cells produce monocyte chemotactic protein-1. Regulation by proinflammatory cytokines. J Clin Invest 92: 1674-1680.
- Marra F, Romanelli RG, Giannini C, Failli P, Pastacaldi S, et al. (1999) Monocyte chemotactic protein-1 as a chemoattractant for human hepatic stellate cells. Hepatology 29: 140-148.
- Degre D, Lemmers A, Gustot T, Ouziel R, Trépo E, et al. (2012) Hepatic expression of CCL2 in alcoholic liver disease is associated with disease severity and neutrophil inï¬ltrates. Clin Exp Immunol 169: 302-310.
- Seki E, de Minicis S, Inokuchi S, Taura K, Miyai K, et al. (2009) CCR2 promotes hepatic ï¬brosis in mice. Hepatology 50: 185-197.
- Arteel GE (2008) New role of plasminogen activator inhibitor-1 in alcohol-induced liver injury. J Gastroenterol Hepatol 23: S54-S59.
- Bergheim I, Guo L, Davis MA, Duveau I, Arteel GE (2006) Critical Role of Plasminogen Activator Inhibitor-1 in Cholestatic Liver Injury and Fibrosis. J Pharmacol ExpTher 316: 592-600.
- Beier JI, Arteel GE (2012) Alcoholic liver disease and the potential role of plasminogen activator inhibitor-1 and fibrin metabolism. Exp Biol Med (Maywood) 237: 1-9.
- Tran-Thang C, Fasel-Felley J, Pralong G, Hofstetter JR, Bachmann F, et al. (1989) Plasminogen activators and plasminogen activator inhibitors in liver deficiencies caused by chronic alcoholism or infectious hepatitis. Thromb Haemost 62: 651-653.
- Yang SQ, Lin HZ, Lane MD, Clemens M, Diehl AM (1997) Obesity increases sensitivity to endotoxin liver injury: implications for the pathogenesis of steatohepatitis. Proc Natl Acad Sci USA 4: 2557-2562.
- Sorensen TI, Orholm M, Bentsen KD, Hoybye G, Eghoje K, et al. (1984) Prospective evaluation of alcohol abuse and alcoholic liver injury in men as predictors of development of cirrhosis. Lancet 2: 241-244.
- Teli MR, Day CP, Burt AD, Bennett MK, James OF, et al. (1995) Determinants of progression to cirrhosis or fibrosis in pure alcoholic fatty liver. Lancet 346: 987-990.
- Pessayre, Mansouri A, Fromenty B (2002) Nonalcoholic steatosis and steatohepatitis. vs Mitochondrial dysfunction in steatohepatitis. Am J Physiol Gastrointest Liver Physiol 282: G193-G199.
- Fearns C, Loskutoff DJ (1997) Induction of plasminogen activator inhibitor one gene expression in murine liver by lipopolysaccharide. Cellular localization and role of endogenous tumor necrosis factor-alpha. Am J Pathol 150: 579-590.
- Rossi F, Francese M, Iodice RM, Falcone E, Vetrella S, et al. (2005) Inherited disorders of bilirubin metabolism. Minerva Pediatr 57: 53-63.
- Monaghan G, McLellan A, McGeehan A, Li Volti S, Mollica F, et al. (1999) Gilbert’s syndrome is a contributory factor in prolonged unconjugated hyperbilirubinemia of the newborn. J Pediatr 134: 441-446.
- Bosma PJ, Chowdhury JR, Bakker C, Gantla S, de Boer A, et al. (1995) The genetic basis of the reduced expression of bilirubin UDP- glucuronosyltransferase 1 in Gilbert’s syndrome. N Engl J Med 333: 1171-1175.
- Aono S, Adachi Y, Uyama E, Yamada Y, Keino H, et al. (1995) Analysis of genes for bilirubin UDP glucuronosyltransferase in Gilbert’s syndrome. Lancet 345: 958-959.
- Maier KP (2002) Rare, but important chronic liver diseases. Praxis (Bern 1994) 91: 2077-2085.
- Kuntz E Laboratory diagnostics (2001) In: Kuntz E, Kuntz HD, eds. Hepatology, principles, and practice. Heidelberg: Springer -Verlag. 78-112.
- Pratt DS, Kaplan MM (2000) Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med 342: 1266-1271.
- Rosalki SB, Tarlow D, Rau D (1971) Plasma gamma-glutamyl transpeptidase elevation in patients receiving enzyme-inducing drugs. Lancet 2: 376-377.
- Sharma U, Pal D, Prasad R (2014) Alkaline Phosphatase: An overview. Indian J Clin Biochem 29: 269-278.
- Wolf PL (1999) Biochemical diagnosis of liver disease. Indian J Clin Biochem 14: 59-90.
- Sorbi D, Boynton J, Lindor KD (1999) The ratio of aspartate aminotransferase to alanine transferase: potential value in differentiating nonalcoholic steatohepatitis from alcoholic liver disease. Am J Gastroenterol 94: 1018-1022.
- Epstein E, Kiechle FL, Artiss JD, Zak B (1986) The clinical use of alkaline phosphatase enzymes. Clin Lab Med 6: 491-505.
- Rodan GA, Rodan SB (1984) In: Peck WA, editor. Advances in bone and mineral research annual II. Amsterdam: Excerpta Medica 244-285.
- Preussner HT (1998) Detecting coeliac disease in your patients. Am Fam Physician 57: 1023-1034.
- Sharma U, Singh SK, Pal D, Khajuria R, Mandal AK, et al. (2012) Implication of BBM lipid composition and fluidity in mitigated alkaline phosphatase activity in renal cell carcinoma. Mol Cell Biochem 369: 287-293.
- Prasad R, Lambe S, Kaler P, Pathania S, Kumar S, Attri S, et al. (2005) Ectopic expression of alkaline phosphatase in proximal tubular brush border membrane of human renal cell carcinoma. Biochim Biophys Acta 1741: 240-245.
- Lange PH, Millan JL, Stigbrand T, Vessella RL, Ruoslahti E, et al. (1982) Placental alkaline phosphatase as a tumor marker for seminoma. Cancer Res 42: 3244-3247.
- Niedermayer I, Feiden W, Henn W, Steilen-Gimbel H, Steudel WI, et al. (1997) Loss of alkaline phosphatase activity in meningiomas: a rapid histochemical technique indicating progression associated deletion of a putative tumor suppressor gene on the distal part of the short arm of chromosome 1. J Neuropathol Exp Neurol 56: 879-886.
- Kim JM, David Kwon CH, Joh JW, Park JB, Ko JS, et al. (2013) The effect of alkaline phosphatase and intrahepatic metastases in sizeable hepatocellular carcinoma. World J Surg Oncol 11: 40.
- Muriel P, Rivera-Espinoza Y (2008.) Beneï¬cial drugs for liver diseases. J Appl Toxicol 28: 93-103.
- Rambaldi A, Iaquinto G, Gluud C (2002) Anabolic-androgenic steroids for alcoholic liver disease: a Cochrane review. Am J Gastroenterol 97: 1674-1681.
- Yeh WS, Armstrong EP, Skrepnek GH, Malone DC (2007) Peginterferon alfa-2a versus peginterferon alfa-2b as initial treatment of hepatitis C virus infection: a cost-utility analysis from the perspective of the Veterans affairs health care system. Pharmacotherapy 27: 813-824.
- Raghavan PR (2017) Metadichol® and Red Cell Distribution Width (RDW) in CKD patients. J Stem Cell Res Ther 7: 392.
- Chen J, Bardes EE, Aronow BJ, Jegga AG (2009) ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res 37: W305-311.
- Kaimal V, Bardes EE, Tabar SC, Jegga AG, Aronow BJ (2010) ToppCluster: a multiple gene list feature analyzer for comparative enrichment clustering and network-based dissection of biological systems. Nucleic Acids Res 38: W96-102.
- Cerami EG, Gross BE, Demir E, Rodchenkov I, Babur O, et al. (2011) Pathway Commons, a web resource for biological pathway data. Nucleic Acids Res 39: D685-690.
- Horras CJ, Lamb CL, Mitchell KA et al. (2011) Regulation of Hepatocyte Fate by Interferon-γ. Cytokine Growth Factor Rev 22: 35-43.
- Attallah AM, El-Far M, Zahran F, Shiha GE, Farid K, et al. (2016) Interferon-gamma is associated with hepatic dysfunction in fibrosis, cirrhosis, and hepatocellular carcinoma. J Immunoassay Immunochem 37: 597-610.
- Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL (1986) Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol 136: 2348-2357.
- Raghavan PR (2017) Metadichol, A Novel ROR Gamma Inverse Agonist and Its Applications in Psoriasis. J Clin Exp Dermatol Res 8: 433.
- Székely JI, Pataki à (2012) Effects of Vitamin D on Immune Disorders With Special Regard to Asthma, COPD and Autoimmune Diseases. A Short Review. Expert Rev Respir Med 6: 683-704.
- Zhang W, Bai Y, Wang Y, Xiao W (2016) Polypharmacology in Drug Discovery: A Review from Systems Pharmacology Perspective. Curr Pharm Des 22: 3171-3181.
- Raghavan PR (2018) Umbilical Cord Cells Treatment with Metadichol®. IRS Proteins and GLUT4 Expression and Implications for Diabetes. J Stem Cell Res Ther 8: 6.
- Raghavan PR (2018) Metadichol® and CD34 Expression in Umbilical Cord Cells. J Stem Cell Res Ther 8: 409.
- Raghavan PR (2018) Metadichol®, Vitamin C and GULO Gene Expression in Mouse Adipocytes. Biol Med 10: 426.
- Raghavan PR (2017) Metadichol ®. A Novel Inverse Agonist of Aryl Hydrocarbon Receptor (AHR) and NRF2 Inhibitor. J Cancer Sci Ther 9: 661-668.
- Raghavan PR (2017) Metadichol® Induced High Levels of Vitamin C: Case Studies. Vitam Miner 6: 169.
- Raghavan PR (2017) Metadichol® and Vitamin C Increase In Vivo, an Open-Label Study. Vitam Miner 6: 163.
- Raghavan PR (2017) Rheumatoid Arthritis and Osteoporosis: A Case Study. J Arthritis 6: 240.
- Raghavan PR (2017) Systolic and Diastolic BP Control in Metabolic Syndrome Patients with Metadichol® a Novel Nano Emulsion Lipid. J Cardiol & Cardiovasc Ther 5: 555660.
- Raghavan PR (2017) Metadichol® A Novel Nano Lipid; GPR 120 Agonist. Int J Diabetes Complications 1: 1-4.
- Raghavan PR (2017) Metadichol® and MRSA Infections: A Case Report, J Infect Dis Ther 5: 2
- Raghavan PR (2017) Improving Longevity with Metadichol ® by inhibiting Bcat-1 Gene. Aging Sci 5: 174.
- Raghavan PR (2016) In vitro Inhibition of Zika Virus by Metadichol®. A Novel Nano Emulsion Lipid. J Immunol Tech Infect Dis 5: 4.
- Raghavan PR (2016) Inhibition of Dengue and other enveloped viruses by Metadichol®, a novel Nano emulsion Lipid. J Sci Heal Out 8: 19-25.
- Raghavan PR (2016) Metadichol and Type 2 Diabetes A case report. J Sci Heal Out 8: 5-10.
Citation: Raghavan PR (2019) A Multi Gene Targeting Approach to Treating Liver Diseases with Metadichol®. J Cytokine Biol 3:126.
Copyright: © 2019 Raghavan PR. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 4568
- [From(publication date): 0-2019 - Nov 07, 2025]
- Breakdown by view type
- HTML page views: 3639
- PDF downloads: 929