A New Technique to Determine Emissions of Burley, Virginia andO riental Tobacco Using Single Puff and Puff-By-Puff Pyrolysis
Received: 23-Dec-2017 / Accepted Date: 29-Dec-2017 / Published Date: 31-Dec-2017 DOI: 10.4172/2155-9872.1000394
Abstract
In this study, we compared the pyrolysis products of the three most commonly used tobacco leaves using pyrolysis-gas chromatography coupled with mass spectrometry detection. For tobacco product regulation, it is important to understand the formation of emissions that occurs during smoking of tobacco. To this purpose, we report a simple analytical method to investigate the combustion process of tobacco leaves using a pyrolysis robot coupled to a GC-MS. This method allows for simulation of the tobacco smoking process, by taking one puff (single puff) and more puffs (puff-by-puff), and subsequent determination of the combustion products. We studied the 13 most abundant emissions formed during the pyrolysis of the three most common tobacco varieties: Burley, Virginia and Oriental. Unlike commercially available tobacco products, the tobacco leaves were not treated with any additives, allowing for an assessment of the combustion product of raw tobacco leaves. Our study shows that tobacco, when combusted, produces predominantly tobacco specific alkaloids such as nicotine, hydrocarbons (toluene, xylene and limonene) and other compounds such as acetaldehyde, phenol and furfural. Nicotine is the main chemical produced during pyrolysis followed by β-nicotyrine, acetaldehyde and toluene. For lower molecular weight components such as acetaldehyde, the amounts generally decreased with puff number. On the other hand, nicotine yields in all three tobacco leaves were found to increase with puff number.
Keywords: Pyrolysis; Tobacco; Cigarette smoke; Pyrobot; Pyroprobe; Puff-by-puff
Introduction
With 43 trillion cigarettes sold in the past 10 years and 20% of the population smoking globally, smoking cigarettes is the most popular method of consuming tobacco [1]. Smoking is the leading cause of preventable death and is associated with non-communicable diseases such as cancer, cardiovascular disease and chronic obstructive pulmonary disease (COPD) [1-3]. Cigarette smoke contains a variety of chemicals that are associated with the cause of these diseases. The composition of tobacco smoke is very complex with more than 6000 chemicals identified [1,4,5]. Among these chemicals, more than 60 have been classified as hazardous [6]. For tobacco product regulation, it is important to know the amounts and type of such components in tobacco product emissions, as well as their mechanisms of formation. The aim of this work is to compare the pyrolysates of the three different Nicotiana tabacum tobacco varieties most commonly used in the production of cigarettes: Burley, Virginia, and Oriental. Their physical and chemical properties differ due to agricultural practices, soil, stalk position, harvesting and curing processes [7-9], which will affect the pyrolysate of the tobacco. Burley tobacco is usually grown on heavier textured, more fertile soils which receive substantially higher nitrogen, resulting in relatively high amounts of nitrogen containing compounds [7-9]. Burley tobacco is air cured, resulting in leaves with low sugar but high nicotine content as compared to Virginia and Oriental. Virginia tobacco is flue-cured, removing about 97% of the moisture from the harvested leave [7,8]. The initial and most important steps in flue curing are yellowing, where chlorophyll is degraded and most carbohydrates are converted to sugars. Its’ high sugar content gives flue-cured Virginia tobacco its characteristic sweet aroma, due to the sugar combustion products. Oriental tobacco is mostly sun-cured, and contains small amounts of sugars, low protein and amino acid content, and a higher nicotine content (than the Virginia variant) [7,10]. Combustion of tobacco is often used to investigate the chemical processes occurring during smoking, either by using smoking machines or by pyrolysis. Smoking machines are used to measure the total yields of smovke emissions under a controlled environment [11,12]. The combustion processes in a burning cigarette have also been simulated with pyrolysis methods [13-15]. Pyrolysis experiments can be performed at several temperature ranges and atmospheres simulating the various burning conditions in tobacco smoking. The emissions and yields of pyrolysis have been shown to be a useful qualitative technique in the investigation of cigarette smoke [16,17]. Conventional tobacco smoke analysis uses smoking machines to collect smoke, followed by sample preparation such as extraction, separation and treatment prior to gas chromatography (GC) and high pressure liquid chromatography (HPLC) analysis. Due to the complexity of tobacco smoke, the composition can change during sample preparation. Real time measurement allows identification of reactive compounds before being degraded. Methods for online measurements using a pyrolyzer provide detailed information on the dynamic processes occurring in fresh tobacco smoke.
In this study, we performed the determination of tobacco emission on single and puff-by-puff pyrolysis experiments. During the single puff experiment, a single aliquot of tobacco is pyrolysed. The puff-bypuff experiment is conducted to mimic the smoking process, simulated using the so-called “pyrobot”. The pyrolysis set-up was coupled with gas chromatography-mass spectrometry (GC-MS) and the pyrolysate was trapped immediately after it was released. Using this “puff-by-puff” application, we studied the dynamic changes produced in fresh tobacco smoke with three different tobacco varieties, and compare their relative emissions.
Experimental
Materials
Tobacco leaves; Virginia flue cured, Burley grade A and 345 Semi- Oriental were obtained from Leaf Only (USA) from a single batch. For the pyrolysis the pyrobot and pyroprobe 5200 from CDS Analytical was used in combination with GC-MS (Varian CP-3800/Varian 225- MS ion trap). The trap of the pyroprobe 5200 contains Carboxen 572 (Sigma Aldrich, Germany) for the single puff setup and for the puff-bypuff set up, 20:35 Tenax-TA/60:80 Carboxen 1000/carbosieve SIII (CDS Analytical, United States of America). A Varian Factor Four Capillary Column VF-5 ms 30 m; 0.25 mm; 0.25 μm column was installed.
Pyroprobe and pyrobot set-up
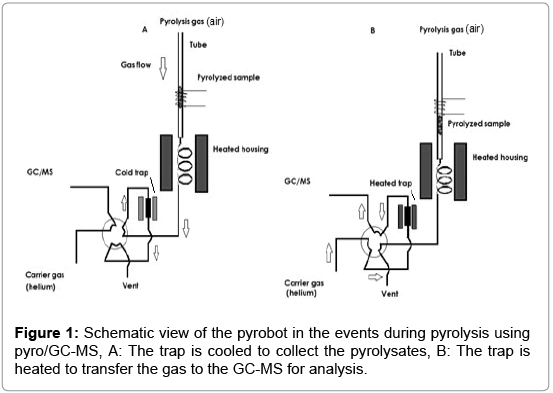
The main parts and function of this instrument are schematically depicted in Figure 1. This set-up includes a pyroprobe, equipped with a pyrolysis zone, an interface, a valve oven, and a trap. A transfer line couples the pyroprobe to the GC-MS.
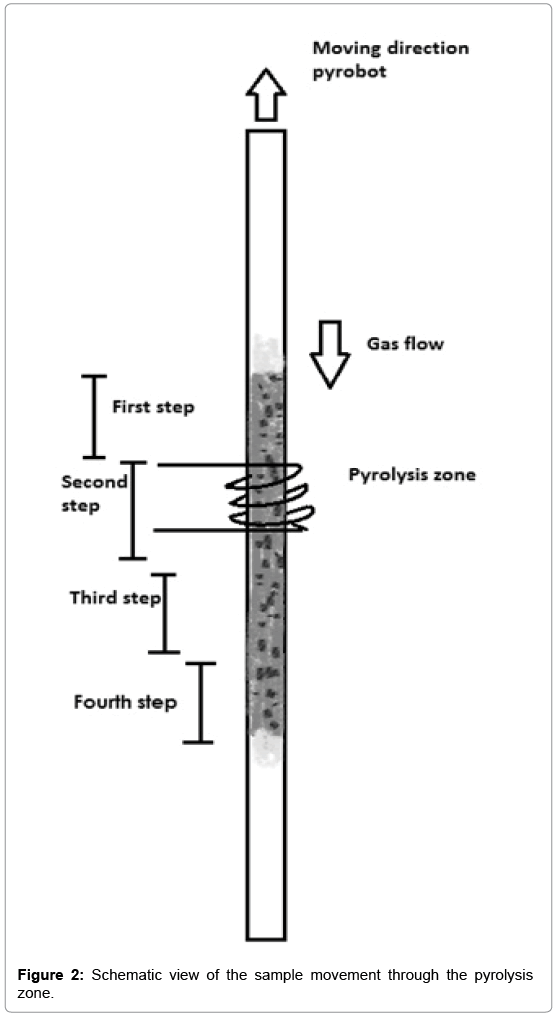
The pyroprobe 5200 works in two stages. In the first stage the sample moves through the heated pyrolysis zone, see Figure 1A. The pyrolysates are transported through the heated housing and valve oven and are collected on the trap (T<50°C). In the second stage the pyrolysates transfer to the GC-MS, see Figure 1B. In this stage the reactant gas changes from compressed air to helium and during this process the trap heats up. The pyrolysates desorb from the trap and are transferred to the GC-MS, using the valve oven and the transfer line [18,19]. The pyroprobe 5200 set-up is used to simulate a single puff (requiring a sample of less than 1 cm height in the tube). The static pyroprobe can be combined with the dynamic pyrobot, which moves along the pyrolysis tube, thus allowing for sequential pyrolysis of adjacent parts of a tobacco rod in the sample tube. In this set-up, ‘puffby- puff’ analysis can be performed, if the sample tube contains more than 1 cm sample. The gas moves downwards, in the reverse direction as the pyrobot moves upward. The pyrolysis zone is on the upper side of the tube. As the pyrobot moves upward, further pyrolysis of the sample takes place, see Figure 2. The puff-by-puff analysis continues by setting the pyrobot in the subsequent puff position at a fixed interval and position physically. This model simulation is similar to a burning cigarette process where a puff-by-puff burning takes place during a smoking process.
Sample preparation
Prior to analysis, the tobacco leaves are crushed and conditioned at 22°C with 60% relative air humidity according to ISO conditioning criteria [20]. For single puff pyrolysis, the pyrolysis tube was filled with approximately 5 mg of tobacco, and for puff-by-puff pyrolysis with approximately 7 cm of tobacco weighing around 7 mg.
Settings of pyroprobe, pyrobot and GC-MS
Measurements were carried out with settings simulating a cigarette puff [21]. Pyrolysis was performed with a temperature program from 200°C to 900°C. After heating the sample for 5 seconds at 200°C, the tobacco was heated to 900°C with a heating rate of 20°C per millisecond, and held at 900°C for 5 seconds. Air was used as reactant gas to simulate the smoking atmosphere. The pyrolysates were transported to the trap, which was held at 40°C. After 1 minute, the gas switches to helium (carrier gas for GC-MS) for analysis and the trap was heated up to 300°C for 4 minutes. Through the transfer line the pyrolysates are transferred to the GC inlet with a split flow of 1:275. The injector of the GC was set at 30°C for 4 minutes, increased with 15°C per minute to 100°C followed by another increment to 250°C, with a heating rate of 200°C per min. This temperature was held for 5 minutes after which the heating was cooled down gradually at the same rate to 25°C. The gas chromatograph was set with a constant flow rate of 1.0 ml per minute with Helium as the carrier gas. The initial GC oven temperature was set at 30°C for 4 minutes with an incremental rate of 100°C per minute to 80°C then at 8°C per minute till it reaches a temperature of 250°C. The final temperature was held for 10 minutes, which brings to the total analysis time to about 39 minutes. The mass spectrometry detection range was set from 20 to 300 amu, with an electron impact ionization of 70 eV. The ion trap was held at 180°C. The pyrolyzed products were collected using total ion chromatograms (TIC) mode.
Data analysis
Data was analysed using the GC-MS software; Varian MS workstation version 6.9.3 and the CDS 5000 pyroprobe software. The compounds were identified on the basis of comparison of their mass spectrum with the NIST version 08 mass spectrometry library. Identification was considered as relevant with match factor above 900.
Results
Single puff analysis
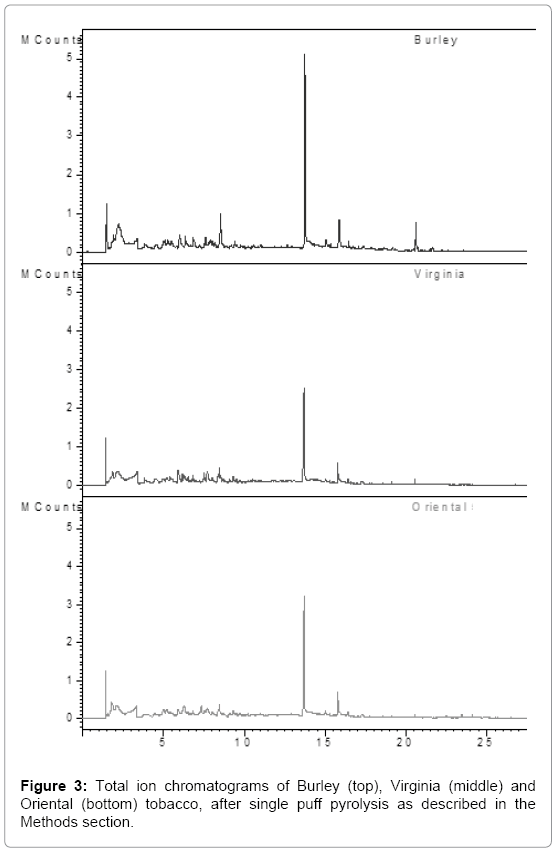
Emission profiles are shown in Figure 3, of single puff pyrolysis experiments with the three varieties of tobacco leaves, Virginia, Burley and Oriental. Thirteen peaks were identified using the NIST library. The relative amounts of these thirteen compounds, expressed as percentage of the total area of the thirteen peaks, are given in Table 1.
| Compound | Retention time | Mass(m/z) | Burley(n=3) | Virginia(n=3) | Oriental(n=3) |
|---|---|---|---|---|---|
| Acetaldehyde | 1.76 | 44.0 | 120 | 80 | 97 |
| Toluene | 5.13 | 92.1 | 88 | 43 | 99 |
| Furfural | 5.94 | 96.1 | 9 | 49 | 23 |
| P-xylene | 6.32 | 106.2 | 45 | 38 | 32 |
| 3-ethenyl-pyridine | 7.58 | 105.1 | 41 | 11 | 34 |
| Phenol | 7.65 | 94.1 | 34 | 133 | 42 |
| 2-methylbenzyl alcohol | 8.12 | 122.2 | 7 | 1 | 6 |
| Limonene | 8.41 | 136.2 | 62 | 27 | 22 |
| Nicotine | 13.62 | 162.2 | 1476 | 857 | 833 |
| Myosmine | 14.96 | 146.2 | 33 | 16 | 32 |
| ß-nicotyrine | 15.75 | 158.2 | 178 | 137 | 202 |
| Iso-nicoteine | 16.65 | 156.2 | 9 | 0 | 0 |
| Phytol | 20.53 | 296.5 | 17 | 3 | 2 |
| Nitrogen containing compound | - | - | 1737 | 1021 | 1101 |
| Total | - | - | 2120 | 1401 | 1424 |
Table 1: Relative area of the thirteen compounds emitted from raw tobacco leaf with single puff pyrolysis,(For detail reffer Supplemantry).
The results were calculated by dividing the peak absolute area with the total peak area. The total peak area was computed with the deduction of background noise. All relative standard deviation calculated from 3 replicates were below 20%. The results reported below were based on emissions obtained during the pyrolysis and were compared relatively among Burley, Virginia and Oriental. Thus, the relative peak area of the compound can be compared and the changes in their relative content can be observed.
Burley tobacco emitted the highest nicotine with 1.7 fold higher than Virginia and Oriental. Interestingly, Oriental tobacco produced the highest β-nicotyrine followed by Burley and Virginia (Table 1). Isonicoteine is not detected in Virginia and Oriental tobacco, but was found in Burley tobacco (with a relatively small area of 9). Phenol yield was found to be highest for Virginia followed by Oriental and Burley. Toluene was emitted mainly in Oriental and Burley (relative area of 99 and 88 respectively), followed by Virginia tobacco. Both Virginia and Oriental produces high amount of furfural compared to Burley, in the single puff analysis method.
Puff-by-puff pyrolysis
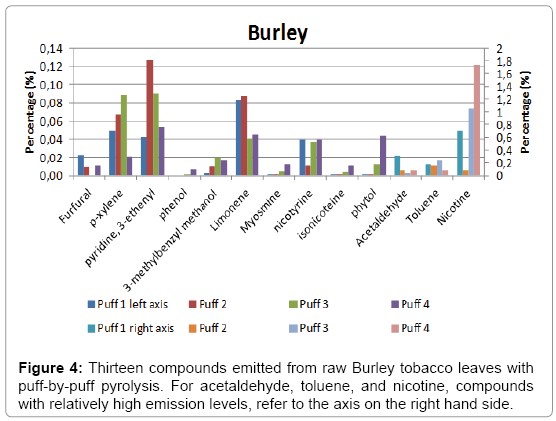
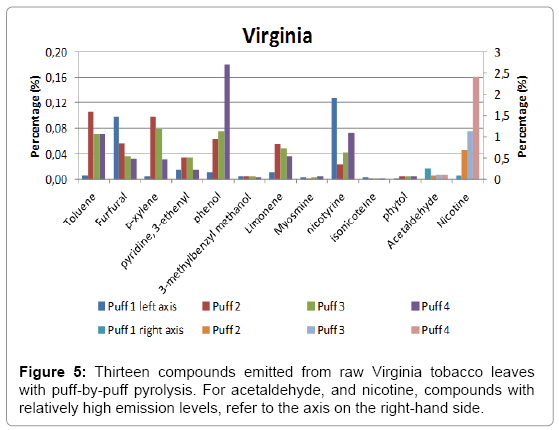
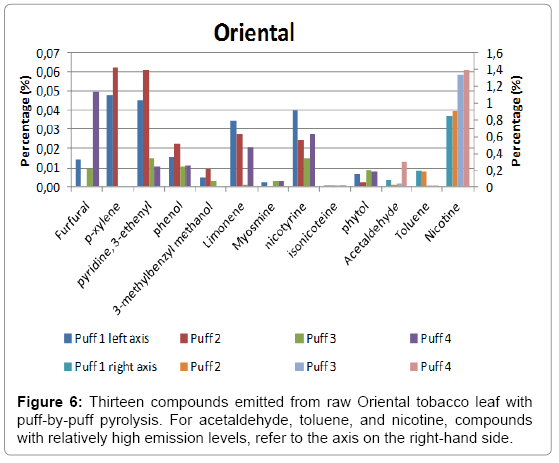
A total of four puffs were pyrolysed, and the relative amounts of the compounds identified using NIST library with a match factor above 900 in the single puff analysis are given in Figure 4 for Burley, in Figure 5 for Virginia and in Figure 6 for Oriental tobacco.
Burley: Nicotine dominates the emissions produced during the 1st puff, followed by acetaldehyde and toluene. During the 2nd puff, toluene relative yield decreased to 16, followed by acetaldehyde at 8. Between the first and the last puff the relative nicotine yield gradually increases by about 245% (71 to 174%), with an unusually low yield in the second puff. The 3rd and 4th puff for Burley were dominated by nitrogen containing compounds, contributed predominantly by nicotine. Overall, there were an increase in the total yield of the 13 pyrolysates by about 50% from the first to the fourth puff (144 to 217% respectively).
Virginia: In Virginia, the predominant compound produced in the 1st puff is acetaldehyde (relative area of 25), followed by furfural then nicotine. During the subsequent three puffs, the relative acetaldehyde amount gradually decreased (relative area of 10). Conversely, the nicotine level produced gradually increased by about 30 fold between the first puffs to the last puff (4th puff). The amount of nitrogen containing compounds produced in the Virginia pyrolysed smoke is the highest of the three tobaccos.
Oriental: We find nicotine, as expected to be the predominant compound and is the highest among the three tobacco leaves investigated. The amount of nicotine produced in the 1st puff in Oriental tobacco is the highest, followed by toluene and acetaldehyde. Similar trends were found for the subsequent three puffs with nicotine as the dominant compound.
Overall, the total yield on the thirteen peaks investigated increases with puff number. The major pyrolysis products were nicotine, acetaldehyde, toluene, furfural, xylene, limonene, 3-ethenyl-piridine and β-nicotyrine. For these compounds, we see a trend that for lower molecular weight components (m/z<100), the amounts decrease as the puff number increases. Burley tobacco was found to contain the highest level of nitrogen containing compound, followed by Oriental and Virginia at. On the other hand, we found that nicotine yields in all three-tobacco leaves increase as the puff number increases.
Discussion
In this study, we simulated cigarette smoking by pyrolysis experiments of tobacco leaves (i.e., without additives that are added during manufacturing), both single puff and puff-by-puff. We compared, semi-quantitatively, the 13 most abundant chemicals produced during the pyrolysis process of the three commonly used tobacco varieties. Four of the thirteen chemicals detected in this study, namely p-xylene, acetaldehyde, toluene and phenol, were found in the list of 60 hazardous smoke components which were assessed to be of public health interest and regulatory purposes [6]. Our study shows that raw tobacco, when combusted, produces predominantly tobacco specific alkaloids such as nicotine, hydrocarbons and other compounds such as acetaldehyde, phenol and furfural. Nicotine is the main chemical produced during pyrolysis followed by β-nicotyrine, acetaldehyde and toluene. Besides nicotine, other alkaloids found in the tobacco smoke analysis include myosmine, β-nicotyrine and iso-nicotine. Myosmine was found in all 3 tobacco leaves, and the amount of myosmine varies, depending on the tobacco species, variety and agriculture conditions [22]. Nicotine is the most dominant nitrogen-containing compound in all these three varieties of tobacco. Other tobacco alkaloids such as isonicoteine and β-nicotyrine were detected in small amounts relative to nicotine. Burley tobacco, due to its agricultural practice and curing process, produced the largest amount of nitrogen-containing compounds followed by Oriental and Virginia. These amounts are consistent with previous elemental analyses that reported the percentage of nitrogen on Burley and Oriental to be 5.22% and 2.78%. Bright tobacco leaf, similar to Virginia in its curing and agricultural process, contained 3.09% of nitrogen. Adam [10] also reports a relatively high amount of nitrogen-content in the tested Burley tobacco. The same study also describes a higher amount of carbohydrate-derived products for Virginia and Oriental. The same trend is shown in our results with higher amounts of furfural in Virginia tobacco and low furfural emission in Burley tobacco. All the three tobaccos were found to contain relatively high amount of acetaldehyde in this study, which is consistent with the finding from an earlier study [23]. A pyrolysis product of nicotine, 3-ethenylpyridine is found in all three varieties of tobacco studied. 3-ethenylpyridine is a known tobacco smoke-related air pollutant and this compound has been used as an environmental tobacco smoke marker besides nicotine [24,25]. In the puff-by-puff analysis, 3-ethenylpyridine was found to be reduced at the 4th puff in all the 3 varieties of tobacco analysed. This finding is similar to an earlier study where 3-ethenylpyridine was found to be reduced during the last cigarette smoking puffs [26]. In the puff-by-puff experiment, the filtering effect of the tobacco itself can be investigated. For lower molecular weight components (m/z<100) such as acetaldehyde, the amounts in general decreased with puff number with the level of acetaldehyde found to be higher in the first puff relative to the 2nd puff and this is consistent with published findings [27]. On the other hand, nicotine yields in all three tobacco leaves were found to increase with puff number. Besides nicotine, [28] in their study, they found abnormally high level of formaldehyde, acrolein and to a certain extent, acetaldehyde in the first puff. Our results on acetaldehyde show that the levels do fluctuate between puffs, with the first puff containing the highest among all. As acetaldehyde is a volatile compound, such fluctuation is likely to occur. It was reported that volatile pyrolysates diffuses out of the tobacco rod as the puff count increases [29-31]. When a puff is taken, the temperature closer to the burning zone is much higher than the temperature close to the filter. This results in compounds with a high boiling point to condensate and evaporates when the temperatures increase as the cigarette length is reduced.
Based on our results, it is proposed that original tobacco leaves by itself produces hazardous substances besides nicotine that is the major pyrolysates emitted. It is possible that some of the pyrolysis products were not picked up under the settings chosen in this study. Unlike a technique such as the smoking machine, the pyrolyzer coupled with GC-MS discussed here is limited to semi quantitative determination. Despite such limitation, the results of the current study can be used as the basis for future research on the type of tobacco and variation among batches. It is challenging to conduct the pyrolysis experiment in a routine environment but it is useful to apply this technique for the purpose of understanding the compounds formed during the burning process of tobacco, as such information is important for product regulation.
Conclusion
For the purpose of tobacco product regulation, application of a puff-by-puff pyrolysis technique is a simple method to simulate combustion processes occurring during cigarette smoking. From relatively small amounts of tobacco, pyrolysate can be generated in single puff or puff-by-puff mode, and subsequently analysed. As such, it is a suitable method for research purposes, although it may need further improvement to be applied in routine settings. The results show that typical compounds emitted, besides nicotine, which is addictive, from raw tobacco leaves were acetaldehyde and toluene, which are known to have harmful effects when inhaled. With this technique it was possible to determine and compare the pyrolysis of the three most commonly used tobacco leaves in the production of cigarettes. Further studies using these techniques are warranted on processed tobacco. In processed tobacco, ingredients such as humectants and flavours are generally added during the manufacturing process to improve product attractiveness. Such compounds may affect the composition of the smoke.
Acknowledgments
We would like to thank the Netherlands Food and Consumer Product Safety Authority (NVWA project V/090071/14 Tabakswet) for financing this project, and Health Sciences Authority Singapore for the support provided to make this work possible.
Conflict of Interest
The authors declare that they have no conflict of interest. The content is solely the responsibility of the authors and does not necessarily represent the views of National Institute of Public Health, The Netherlands (RIVM), Netherlands Food and Consumer Product Safety Authority (NVWA) or Health Sciences Authority (HSA), Singapore.
References
- Stivoro (2012) Percentage rokers in de Nederlandsebevolking 1958-2011. TrendPublicatiePercentage Rokers.
- Hoffmann DHSS (1989) Advances in tobacco carcinogenesis. Springer, p: 94.
- Connolly GN (2000) How cigarette additives are used to mask environmental tobacco smoke. Tob Control, p: 9.
- Rodgman A,Perfetti TA (2013) The Chemical Components of Tobacco and Tobacco Smoke. New York: CRC Press, Taylor and Francis Group.
- Talhout R (2011) Hazardous compounds in tobacco smoke. International Journal of Environmental Research and Public Health 8:613-628.
- Davis LD, Nielsen MT (1999) Tobacco - Production, Chemistry and Technology. Blackwell Science, Oxford, UK.
- Leffingwell JM (1999) Tobacco: Production, Chemistry and Technology. World Agriculture Series. Blackwell Publishing.
- Geissm (2007) Tobacco, Cigarettes and Cigarette Smoke. Institute for Health and Consumer Protection.
- Adam T (2009) Investigation of Tobacco Pyrolysis Gases and Puff-by-puff Resolved Cigarette Smoke by Single Photon Ionisation (SPI) - Time-of-flight Mass Spectrometry (TOFMS). BeiträgezurTabakforschung International 23: 203-226.
- ISO, ISO 4387:2000/Amd(2008) Cigarettes-Determination of total and nicotine-free dry particulate matter using a routine analytical smoking machine International Organization for Standardization.
- Bodnar JA (2012) Mainstream smoke chemistry analysis of samples from the 2009 US cigarette market. Regulatory Toxicology and Pharmacology: RTP 64: 35-42.
- Busch C (2012) Pyrolysis and combustion of tobacco in a cigarette smoking simulator under air and nitrogen atmosphere. Anal BioanalChem 403: 419-430.
- Lee JG (2007) Fast analysis of nicotine in tobacco using double-shot pyrolysis--gas chromatography--mass spectrometry. J Agric Food Chem 55: 1097-1102.
- Baker RR, Pereira da Silva JR, Smith G (2004) The effect of tobacco ingredients on smoke chemistry. Part I: Flavourings and additives. Food ChemToxicol 42: S3-37.
- Baker RR (2004) The pyrolysis of tobacco ingredients. Journal of Analytical and Applied pyrolysis. 71: 223-311.
- Zhou S (2011) Pyrolysis behavior of pectin under the conditions that simulate cigarette smoking. Journal of Analytical and Applied Pyrolysis 91: 232-240.
- Moldoveanu SC (2010) Analysis of Acrylonitrile and a-Methacrylonitrile in Vapor Phase of Mainstream Cigarette Smoke Using a Charcoal Trap for Collection. beitra¨gezurTabakforschung International/Contributions to Tobacco Research 24: 145.
- Bassilakis R, Carangelo RM, Wójtowicz MA (2001) TG-FTIR analysis of biomass pyrolysis. Fuel 80: 1765-1786.
- ISO, ISO 3308 (2012) Routine analytical cigarette-smoking machine -- Definitions and standard conditions. International Organization for Standardization.
- BakerB (2004) The pyrolisis of tobacco ingredients. Analytical and Applied Pyrolysis 71: 223-311.
- http://legacy.library.ucsf.edu/documentStore/k/r/a/kra98e00/Skra98e00.pdf
- Adam T (2005) Discrimination of three tobacco types (Burley, Virginia and Oriental) by pyrolysis single-photon ionisation-time-of-flight mass spectrometry and advanced statistical methods. Analytical and Bioanalytical Chemistry 381: 487-499.
- Kuusimaki L, Peltonen K, Vainiotalo S(2007) Assessment of environmental tobacco smoke exposure of Finnish restaurant workers, using 3-ethenylpyridine as marker. Indoor Air 17: 394-403.
- Johnsson T (2006) Environmental tobacco smoke in Finnish restaurants and bars before and after smoking restrictions were introduced. Ann OccupHyg50: 331-41.
- Wagner KA, Higby R, Stutt K (2005) Puff-by-Puff Analysis of Selected Mainstream Smoke Constituents in The Kentucky Reference 2R4F Cigarette. BeitrzurTabakforschInt 21: 273.
- Baker RR (2006) The generation of formaldehyde in cigarettes--Overview and recent experiments. Food ChemToxicol 44: 1799-1822.
- Baker RR (1977) The Diffusion of Carbon Monoxide out of Cigarettes. BeiträgezurTabakforschung International/Contributions to Tobacco Research, p: 3.
- Baker RR (1990) Tobacco combustion-the last ten years. Recent Advance Tobacco Science 16: 3-101.
- Adam T (2006) Quantitative Puff-by-Puff-Resolved Characterization of Selected Toxic Compounds in Cigarette Mainstream Smoke. Chemical Research in Toxicology 19: 511-520.
Citation: Cheah PN, Hendrickx LPA, Borst SE, Cremers JWJM, Jansen EHJM, et al. (2017) A New Technique to Determine Emissions of Burley, Virginia and Oriental Tobacco Using Single Puff and Puff-By-Puff Pyrolysis. J Anal Bioanal Tech 8: 394. DOI: 10.4172/2155-9872.1000394
Copyright: © 2017 Cheah PN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 7849
- [From(publication date): 0-2017 - Dec 06, 2025]
- Breakdown by view type
- HTML page views: 6844
- PDF downloads: 1005