Research Article Open Access
A Pilot Study of the Use of Blood Markers of Alcohol Use and Brief Intervention during Pregnancy
| Joan M Stoler1,2*, Peter W Forbes3, Marla J Burton2, Amy Rubin4, Elaine M Heffernan5, Martha T Kane6, Jeffrey Ecker7, Ronald Iverson7,8 and Lewis B Holmes2 | |
| 1Division of Genetics, Department of Medicine, Boston Children’s Hospital, Boston, USA | |
| 2Medical Genetics Unit, Mass General Hospital for Children | |
| 3Clinical Research Center, Boston Children’s Hospital, Boston, USA | |
| 4Boston University School of Medicine, Boston, USA | |
| 5Department of Obstetrics and Gynecology, MGH Charlestown HealthCare Center, Charlestown, USA | |
| 6Center for Addiction Medicine, Department of Psychiatry, Massachusetts General Hospital, Massachusetts General Hospital, Boston, USA | |
| 7Department of Obstetrics and Gynecology, Massachusetts General Hospital, Boston, USA | |
| 8Department of Obstetrics and Gynecology, Boston Medical Center, Boston, USA | |
| Corresponding Author : | Joan M Stoler Division of Genetics, Department of Medicine Boston Children’s Hospital, Boston, USA Tel: 781 828-0837 E-mail: Joan.Stoler@ childrens.harvard.edu |
| Received: September 21, 2015; Accepted: October 09, 2015; Published: October 16, 2015 | |
| Citation: Stoler JM, Forbes PW, Burton MJ, Rubin A, Heffernan EM, et al. (2015) A Pilot Study of the Use of Blood Markers of Alcohol Use and Brief Intervention during Pregnancy. J Preg Child Health 2:199. doi:10.4172/2376-127X.1000199 | |
| Copyright: © 2015 Stoler JM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at Journal of Pregnancy and Child Health
Abstract
Objective: To assess the effectiveness of feedback of maternal blood markers in decreasing alcohol intake and improving neonatal outcomes.
Method: Pregnant women were screened using a validated alcohol-screening questionnaire, the TWEAK, and blood markers of alcohol use (hemoglobin-associated aldehyde, mean red blood cell volume, carbohydrate deficient transferrin and gamma glutamyl transpeptidase). There were two independently recruited cohorts: 1) a reference group without feedback of the blood marker results, and 2) a subsequent feedback group with feedback of the blood results. Self-reported alcohol use, marker results over time, infant size and gestational age of the infant of the mothers with a positive alcohol screening questionnaire and at least one positive marker (Ns=53 and 38, respectively) were compared between the two cohorts.
Results: Feedback of the blood marker results was an incentive to decrease alcohol intake in the majority of women with positive markers, as after the initial positive assay, there were fewer subsequent positive markers. More women in the feedback group had normalization of the markers compared to those in the reference group who did not have feedback (p=0.005). Furthermore the reported average daily absolute ounces of alcohol use before the feedback was 0.17 versus 0.01 ounces after feedback (p=0.004). In contrast the reported average daily absolute ounces of alcohol use in the reference cohort at the time of first blood draw was 0.042 compared to 0.031 at the time of the second blood draw (not statistically significant). There was no difference between groups in the birth size of the infants.
Conclusion: These blood markers were useful as identifiers of at-risk women and motivational and monitoring tools in the care of alcohol abusing pregnant women. The women who received this intervention reported significantly reduced alcohol use and had fewer positive markers.
| Keywords |
| Alcohol use during pregnancy; Brief intervention; Alcohol biomarkers |
| Introduction |
| Alcohol use during pregnancy has a significant impact on both maternal and fetal health. However, the CDC 2002 Behavioral Risk Factor Surveillance System survey showed that 10% of pregnant American women continue to consume some alcohol [1]. The fetal impacts include “full” Fetal Alcohol Syndrome (FAS), as well as permanent disorders of memory function, impulse control and judgment in otherwise healthy children [2]. In addition, 27% of women who had given birth to at least one child with FAS had died 6 years after the birth of the child [3]. While treatment of alcohol-abusing pregnant women is difficult, brief intervention strategies have shown some success [4]. |
| Failure to identify women using alcohol during pregnancy is a major obstacle to intervention. Under-reporting is common, even with the use of brief questionnaires that have been specifically validated in obstetric populations, such as T-ACE and TWEAK [5,6]. Several laboratory tests have been used as markers of alcohol use. Experience with these has shown that the accuracy of self-report increased when there is known laboratory corroboration [7,8]. The markers serve, also, as useful monitoring tools and motivators for patients in treatment [9]. However, no single blood test is sufficiently specific and sensitive in the obstetric population by itself [10]. Therefore, attention has focused on the use of multiple tests, which reflect different aspects of alcohol metabolism, with the hope that at least one would be positive in an alcohol-abusing woman. In a previous study, we showed that a positive value of at least one of four blood markers (hemoglobin acetaldehyde (HAA), carbohydrate deficient transferrin (CDT), mean red blood cell volume (MCV) and gamma glutamyl transpeptidase (GGT)), was more predictive of infant outcomes than any of the self-reporting measures used [11]. Similarly, Sillanaukee et al. [12] use CDT, MCV and GGT together, identified 76% of female heavy drinkers in a primary health care setting. |
| There have been several studies on the use of brief intervention to decrease alcohol use in pregnant women [4,13-15]. While the women’s reported drinking did decrease in the first three studies, the differences between the control and the intervention groups were not significant statistically. However, the women who received the brief intervention in the study by O’Connor and Whaley [13] were five times more likely than the controls to be abstinent by the end of the pregnancy. There was improved infant outcome in the brief intervention group (as measured by higher birth weights, higher birth lengths and decreased fetal mortality rates). |
| Since feedback of laboratory results can be a motivator of change, several studies have combined brief alcohol intervention with feedback of a marker of alcohol use. For example, Fleming et al. [16] studied the effect of a brief intervention combined with feedback on the CDT blood marker versus a control group who received only a general health booklet (including information on seat belt use, exercise, nutrition, smoking, alcohol, drugs, immunizations and sex) in a group of men and women being treated for type 2 diabetes and/or hypertension. The patients in the brief intervention and feedback group decreased their alcohol intake and decreased their CDT levels compared to the control group. |
| We report here on an approach in which we combined the use of blood markers with feedback of the results and brief motivational intervention (BI) in pregnant women. The goals were to determine whether using the blood markers as treatment motivators and monitoring tools in conjunction with BI led to the changing of drinking patterns, detection of relapse, and improvement of infant outcome. |
| Materials and Methods |
| This study consisted of two independent cohorts who were both screened for alcohol use and some of whom had blood markers drawn: 1) a reference cohort who received brief counseling and no feedback of the marker results, and 2) a feedback cohort who received feedback of the blood marker results and brief motivational counseling (Figures 1 and 2). IRB approval was obtained at the Massachusetts General Hospital, Brigham and Women’s Hospital, Boston Medical Center, Cambridge Health Alliance, Somerville Hospital and St. Elizabeth Medical Center. |
| The women in each group were given a health behaviors questionnaire which contained the TWEAK questions, which have been validated in an obstetric population [5,6]. The TWEAK consists of the following questions: 1. How many drinks can you hold? (More than three drinks are considered a “positive” response). 2. Do your boyfriend, husband or parents ever express worry or complain about your drinking? 3. Have you ever had a drink first thing in the morning to steady your nerves or to get rid of a hangover? 4. Have you ever awakened the morning after some drinking the night before and found that you could not remember a part of the evening before? 5. Have you ever felt you ought to cut down on your drinking? Positive answers to questions 1 and 2 score 2 points each, and positive answers to questions 3, 4 and 5 score one point each. A positive overall score is two or more. |
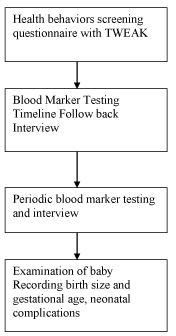
| Reference Cohort (Figure 1). |
| The subjects in the reference cohort (n=647) were recruited in the years 1993-1996 from the obstetric clinics at five Boston area hospitals: Massachusetts General Hospital, Boston Medical Center (including a clinic for pregnant women with substance abuse problems), Cambridge Health Alliance, Somerville Hospital, and St. Elizabeth Medical Center. Details were reported by Stoler et al. [11]. |
| Blood samples for the four markers, HAA (or WBAA, whole blood associated acetaldehyde), MCV, GGT, CDT, were obtained from as many of the pregnant women as possible. Each woman was interviewed about her medical and obstetric history and her use of alcohol and other substances, using a modification of the Timeline Follow Back Procedure [17] before each blood draw. The Timeline Follow Back Procedure is a method used to facilitate recall of alcohol use using a calendar and cues such as birthdays, holidays, medical appointments and routine behavior. Information about the use of other substances of abuse was obtained through self-report and urine toxicology screens. Maternal weight gain was recorded and nutrition was assessed using a 24-hour dietary recall. Information about age, ethnicity, educational level and type of insurance was recorded. Ethnicity was determined by the woman’s own assessment and the origin of her four grandparents, if known. The participant was also asked about the ethnicity of her partner and recorded, if known. The insurance categories were private, public assistance, and no insurance. Each woman received a brief intervention by the Research Coordinator about the dangers of alcohol use and were given the National Institutes of Alcoholism and Alcohol Abuse pamphlet, “Alcohol: A Women’s Health Issue”, but were not given feedback of the results of the alcohol markers. |
| Birth weight, head circumference, birth length, gestational age, and neonatal complications were recorded for each infant born after the monitored pregnancy. Gestational age was determined using first trimester ultrasonographic dating, if available, or assessment of the gestational age after birth by experienced pediatricians. |
| The analysis of the results showed that the four blood markers correlated with infant size outcomes and were better than self-reporting at predicting infant outcome [11]. Because of these findings, we began an interventional study to provide feedback of the blood marker results to the women. |
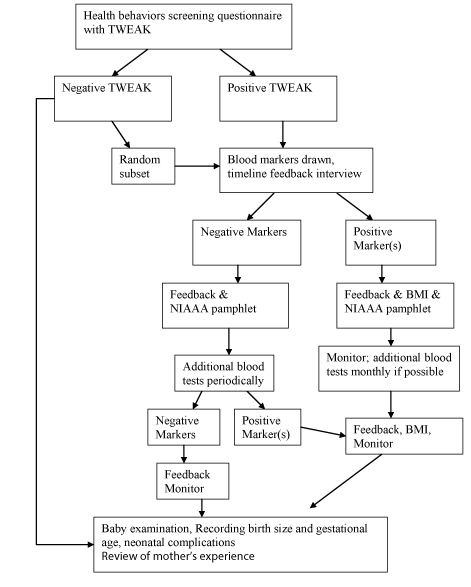
| Feedback Cohort (Figure 2). |
| The feedback cohort consisted of women recruited between 2000-2006 from the general obstetric clinics of four area hospitals, Massachusetts General Hospital, Boston Medical Center (including the substance abuse clinic), Cambridge Health Alliance, Brigham and Women’s Hospital and two affiliated community health clinics. The recruitment methods were similar to those used for the reference cohort, but limited the subjects to those below 36 weeks of gestation in order to provide time to obtain test results and provide feedback and brief motivational intervention. Most women were not recruited at the first prenatal visit because the health care providers at the different sites decided that the first visit was too hectic a time for the women to be approached. Information about health problems, other substances of abuse, medications, ethnicity, educational level, type of insurance, maternal weight gain and nutrition were obtained, as had been done in the reference cohort. The enrolled women were asked to complete a health behaviors questionnaire, which contained the TWEAK. Patients with positive TWEAK scores or who reported using alcohol during the pregnancy were then asked to have blood drawn for alcohol markers and to attend brief intervention sessions with feedback based on results of the alcohol markers measured. |
| Each woman who had blood drawn was interviewed using a modification of the Timeline Follow Back Procedure (TLFB). The clinical staff were blinded to the TWEAK and blood marker results. Each woman was seen at the next visit for feedback of their results. Women who were negative on both the TWEAK and the blood tests were informed of this by research staff. Women who were positive on the TWEAK, or the blood tests, or who reported ongoing alcohol use were seen by an Alcohol Intervention Specialist (either EMH or AR). Monthly study staff meetings were held with the addictions psychologist (MTK) to review cases. |
| The motivational monitoring techniques included brief intervention and monitoring over time, including techniques adapted from Motivational Interviewing by Miller and Rollnick [18], such as empathy, reflective listening, feedback of information, and enhancement of self-efficacy to support motivation to change. During the first interventional session, positive answers on the TWEAK were explored and women were given the results of their blood tests. Women were given information on the potential fetal effects of drinking during pregnancy. After this discussion, each woman was asked if she would be willing to take another blood test. Each woman was given the National Institutes of Alcoholism and Alcohol Abuse pamphlet, “Alcohol: A Women’s Health Issue”. After each blood test, the intervention specialist met with each woman for feedback and motivational counseling. Referral and support for abstinence was offered and the patient was monitored over time. Additional interventional sessions were held to enhance motivation, refer to additional treatment or social services, or support abstinence as appropriate for each patient. Repeat blood tests were performed every 6 weeks, if possible, depending upon the gestational age. |
| Laboratory methods: Determination of HAA (also known as WBAA), GGT, CDT and MCV values were performed using the methods described in our previous paper [11], and by Halvorson et al. [19]. |
| Based on the findings in our previous study, positive values for the blood markers were defined as: HAA > 9.5 μmol/L; GGT>45 U/L; MCV > 100 fL; CDT varied according to the assay used. The cut-off value of HAA was >9 μmol/L had been used in the previous study. This was raised to > 9.5 μmol/L because it appeared that there were too many false positives with the lower cut-off. The other chosen cutoff values were at the 99th percentile for non-pregnant women reporting no alcohol use at all [19-21]. A woman was considered to have a positive screen if she had at least one positive value for any of the samples obtained. Blood was sent immediately to the laboratory for analysis. |
| Statistical analysis |
| This was a cohort study comparing the amount of alcohol use, decline of markers and infant outcome among women positive for the alcohol screening questions and at least one marker between the cohorts. T-tests and chi-squared tests were used to compare the reference and feedback cohorts on pregnancy, demographic, socioeconomic, and drug and alcohol use variables. A chi-squared test was used to compare the reference and feedback cohorts on change over time in the markers. A paired-t test was used to compare the average daily amount of alcohol consumed before and after the intervention among feedback cohort women with any alcohol use during Pregnancy. Linear regression was used to compare infant outcome of women who had both a positive TWEAK score and a positive marker result in order to better test the effect of intervention on pregnant mothers who were drinking during pregnancy. Covariates in the adjusted models were maternal weight gain, drug use (any vs. none), and smoking status (three-levels: none; 1-9/day; 10+/day). |
| Results |
| Characteristics of the women |
| The characteristics of the women in both cohorts are detailed in Table 1. There were 647 women in the reference cohort and 610 in the feedback cohort. There were some differences between the two groups in timing of enrollment as the women were not recruited at the first visit in the feedback group. There were also differences in the sites at which the women were recruited. More women in the reference cohort reported alcohol and cigarette use than did the women in the feedback cohort. However, there was no difference in the groups for reported drug use and for the number of women who had positive markers. |
| Fifty-six of the women in the reference cohort had one or more positive markers (Table 2). Forty women in the reference cohort had a second sample drawn, fourteen of which were positive and three had a third blood sample drawn, which was all negative. |
| Thirty-one of the women in the feedback cohort had positive markers. Fifty-six had a second sample drawn, thirteen (23%) of which were positive. Fifteen had a third sample drawn, of which 3 (20%) were positive, and one had a fourth sample drawn (which was negative). Forty women had one positive marker and only one had two positive markers. |
| Response of the women to the feedback |
| All of the women in the feedback cohort who had blood drawn had feedback of the marker results. Two women, one of whom had two positive markers, withdrew after the initial intervention meeting. Ten women had positive markers, but negative TWEAK scores, and did not report any alcohol use during the pregnancy. This may have been because of a previous history of alcohol use. The women who received the feedback stated that they were very eager to see that their blood tests became normal at the next visit. Twenty-three women had 2 feedback sessions, 8 had 3 feedback sessions and 2 had 4 feedback sessions. |
| The majority of the women with positive markers had a decline in the marker levels over time with subsequent blood samples. This was more pronounced in the feedback cohort as compared to the reference cohort (Table 3). Of the women with positive initial samples (Reference n=16 (40%); Feedback n=22 (39%), a greater proportion in the Feedback cohort than the Reference cohort were negative on their second test (Reference 6 of 16 (38%); Feedback 18 of 22 (82%); p=0.005). Third samples were not included because there were so few subjects with 3 samples. |
| The maternal blood markers for four women in the feedback group remained elevated over time and three of these four women reported alcohol use at some time during the pregnancy. Nine women had elevated values on their second or third samples after having had normal values. The elevations in three of these women were associated with a binge (defined as five or more drinks at a sitting); one woman reported using alcohol earlier during the pregnancy and 5 women denied ongoing alcohol use although one of them had a TWEAK of four. In the reference cohort, four of the twenty-four women with initial negative levels had an elevated level on subsequent testing. |
| The decrease in drinking was also documented by self-report in the feedback cohort. The average daily amount of alcohol (converted to ounces of absolute alcohol) reported in the women who received the intervention in the Feedback cohort prior to the intervention was 0.17 versus 0.01 after the intervention (p=0.004). In contrast the reported average daily absolute ounces of alcohol use in the reference cohort at the time of first blood draw was 0.042 compared to 0.031 at the time of the second blood draw (p=not statistically significant). |
| Deterrent effect of the markers |
| Each woman in the Feedback cohort was interviewed at the end of the pregnancy about her experiences during the study and her use of other resources. Most of the women stated that their aim was to make sure that the blood tests were normal at the subsequent blooddrawing. One woman withdrew at the end, stating that it had made her feel as if she was an alcoholic and did not want to participate. None of the women reported seeking additional services outside of the brief intervention. Of note was the experience of three women who reported a history of heavy drinking before the pregnancy and, then reported that they stopped drinking after they became pregnant. Each had a positive TWEAK, but, in fact, had negative markers on enrollment. These women had negative markers throughout their pregnancies and reported that the desire to keep these blood tests within the normal range motivated them to continue to abstain from drinking alcohol. |
| Analysis of infant size between the reference and feedback cohorts |
| In order to assess the effect of feedback of the markers between the two groups, on infant size and gestational age, the analysis was limited only to women who had both a positive TWEAK score and a positive marker result. No effect of intervention was observed for any outcome in unadjusted or regression-adjusted analysis and observed differences in means between the two groups were small (Table 4). |
| Discussion |
| This study shows the positive effect of having objective laboratory tests available for monitoring during pregnancy. Certainly pregnancy itself is a great motivator to decrease or stop drinking during pregnancy [22]. However, these markers can be used as additional motivators. We were able to show that several women, who had problems with alcohol in the past, were quite conscious of having the blood tests performed and this motivated them to remain abstinent. The number of our patients who had positive markers or reported alcohol use during the pregnancy was small. However, we were able to show some deterrent effects. 82% of the women with positive initial markers in the feedback cohort had a decrease in subsequent values and most of them were quite conscious of making this happen. This is in contrast to the reference cohort, who did not receive behavioral intervention with feedback of their results, where only 38% of the women with positive initial markers had decrease in their marker levels on subsequent samples. In addition, we showed that there was a decrease in the self-reported alcohol use after the intervention and this was not so in the reference cohort. |
| We were able to show that this panel of blood markers is a good adjunct to the self-reported questionnaires. In the study by Chang et al. [4] on brief intervention in pregnant women, there was a subgroup of women who continued to drink and there was no way to monitor their intake other than by self-report. In our study we were able to correlate resumption of drinking with the finding of positive values later in the pregnancy in several cases. Thus, there is the potential for these markers to be used as monitoring tools (in those that test positive initially). In addition, the markers were able to detect some women who were not drinking initially upon enrollment, but began drinking later in the pregnancy. There were also women whose markers remained elevated throughout the pregnancy. These women were candidates for further referrals, intervention, and more intensive treatment in some cases. |
| There are several limitations of our study. 1) Due to HIPAA regulations, we were not able to obtain information on women who did not consent to the study or who withdrew from the study. Thus, we cannot compare the characteristics of the women who did not participate or who withdrew to those who did participate. Approximately 90% of the women approached at the different sites agreed to participate. |
| 2) The two cohorts were not enrolled concurrently. Given the findings of our initial study, we felt that it was important to share the information about the marker results with the women. There were some significant differences between the two groups as outlined in Table 1. Some of these differences, such as the difference in the ethnic distribution, age and type of insurance may be due to the difference in the recruitment sites. More women were recruited at Massachusetts General Hospital in the Feedback cohort which has a more highly educated, affluent population than at the other sites. |
| 3) Another limitation of our study is that the sample sizes were small. The small samples reflected the difficulty in recruiting and following atrisk women. Many of these women did not keep appointments, did not have reliable methods of transportation or methods of being contacted, transferred their care or withdrew from the study. In addition, many at-risk women showed up quite late to prenatal care and, therefore, were not able to participate in the study. |
| Furthermore, the small sample size precluded adjustment for additional socio-economic differences between cohorts. The higher socio-economic status of the feedback cohort is a potential source of bias, since improved socio-economic status is associated with better child outcomes. |
| The ultimate goals are to improve both maternal and fetal health. The studies by both Tzilos [14] and O’Connor [13] did show some improvement in fetal outcome in the intervention groups. We were not able to show a positive impact on infant birth size, but the sample size was small. In addition, in some of the cases, intervention may have been too late in the pregnancy to affect significant change in body size at birth. |
| Intervention during pregnancy has been shown to decrease alcohol use [4,13] but the women need to be identified and monitored. We propose that our blood markers could help to identify those pregnant women at risk, and can provide additional motivation and monitoring of how well the women are doing. It would be ideal if pregnant drinkers could be identified earlier in pregnancy so that intervention could be started earlier. Further study is needed to corroborate and expand on these findings and also to monitor the long-term effects on the children of women who received such intervention. |
References
- Centers for Disease Control and Prevention (CDC) (2004) Alcohol consumption among women who are pregnant or who might become pregnant--United States, 2002. MMWR Morb Mortal Wkly Rep 53: 1178-1181.
- Coles CD, Brown RT, Smith IE, Platman KA, Erickson S, et al. (1991) Effects of prenatal alcohol exposure at school age. Physical and cognitive development. Neurotoxicology and Teratology 13: 357-376.
- May PA (1995) A multiple-level comprehensive approach to the prevention of fetal alcohol syndrome (FAS) and other alcohol-related birth defects (ARBD). The International Journal of the Addictions 30: 549-1602.
- Chang G, McNamara TK, Orav EJ, Koby D, Lavigne A, et al. (2005) Brief intervention for prenatal alcohol use: a randomized trial. ObstetGynecol 105: 991-998.
- Bradley KA, Boyd-Wickizer J, Powell SH, Burman ML (1998) Alcohol screening questionnaires in women: A critical review. Journal of the American Medical Association 280: 166-171.
- Russell M, Martier SS, Sokol RJ, Mudar P, Jacobson S, et al. (1996) Detecting risk drinking during pregnancy: a comparison of four screening questionnaires. Am J Public Health 86: 1435-1439.
- Lowe JB, Windsor RA, Adams B, Morris J, Reese Y (1986) Use of a bogus pipeline method to increase accuracy of self-reported alcohol consumption among pregnant women. Journal of Studies on Alcohol 47: 173-175.
- Hingson R, Zuckerman B, Amaro H, Frank DA, Kayne H, et al. (1986). Maternal marijuana use and neonatal outcome: Uncertainty posed by self-reports. American Journal of Public Health 76: 667-669.
- Borg S, Helander A, Voltaire Carlsson A, Högström Brandt AM (1995) Detection of relapses in alcohol-dependent patients using carbohydrate-deficient transferrin: improvement with individualized reference levels during long-term monitoring. Alcohol ClinExp Res 19: 961-963.
- Rosman AS, Lieber CS (1992) An overview of current and emerging markers of alcoholism: R.Z. Litten& J.P. Allen, Measuring alcohol consumption: psychosocial and biochemical Methods Totowa, NJ: Humana Press.
- Stoler JM, Huntington KS, Peterson CM, Peterson KP, Daniel P, et al. (1998) The prenatal detection of significant alcohol exposure with maternal blood markers. J Pediatr 133: 346-352.
- Sillanaukee P, Aalto M, Seppa K (1998) Carbohydrate-deficient transferrin and conventional alcohol markers as indicators for brief intervention among heavy drinkers in primary healthcare. Alcoholism:Clinical and Experimental Research 22: 892-896.
- O'Connor MJ, Whaley SE (2007) Brief intervention for alcohol use by pregnant women. Am J Public Health 97: 252-258.
- Tzilos GK, Sokol RJ, Ondersma SJ (2011) A randomized phase I trial of a brief computer-delivered intervention for alcohol use during pregnancy. J Womens Health (Larchmt) 20: 1517-1524.
- Nilsen P1 (2009) Brief alcohol intervention to prevent drinking during pregnancy: an overview of research findings. CurrOpinObstetGynecol 21: 496-500.
- Fleming M, Brown R, Brown D (2004) The efficacy of a brief alcohol intervention combined with %CDT feedback in patients being treated for type 2 diabetes and/or hypertension. J Stud Alcohol 65: 631-637.
- Sobell LC, Sobell MG (1992) Timeline follow back. A technique for assessing self-reportedalcohol consumption: R.Z. Litten& J.P. Allen JP Measuring alcohol consumption: Psychosocial and biochemical methods Totowa, NJ Humana Press.
- Boardman T, Catley D, Grobe JE, Little TD, Ahluwalia JS (2006) Using motivational interviewing with smokers: do therapist behaviors relate to engagement and therapeutic alliance? J Subst Abuse Treat 31: 329-339.
- Halvorson MR, Campbell JL, Sprague G, Slater, K, Noffsinger JK et al (1993) Comparative evaluation of the clinical utility of three markers of ethanol intake: the effect of gender. Alcoholism: Clinical and Experimental Research 17: 225-229.
- Helander A, Vabo E, Levin K, Borg S (1998) Intra- and inter-individual variability of carbohydrate-deficient transferrin, glutamyltransferase and mean corpuscular volume in teetotalers. Clinical Chemistry 44: 2120-2125.
- Ylikorkala O, Stenman UH, Halmesmäki E (1987) gamma-Glutamyl transferase and mean cell volume reveal maternal alcohol abuse and fetal alcohol effects. Am J ObstetGynecol 157: 344-348.
- Rosett HL, Ouellette EM, Weiner L, Owens E (1978) Therapy of heavy drinking during pregnancy. ObstetGynecol 51: 41-46.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 |
Figures at a glance
 |
 |
| Figure 1 | Figure 2 |
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 9638
- [From(publication date):
December-2015 - Aug 23, 2025] - Breakdown by view type
- HTML page views : 8809
- PDF downloads : 829
