A Prospective Cohort Study on Less Invasive Surfactant Administration (Lisa) in Preterm Neonates at 34 Weeks and Below with Respiratory Distress Syndrome
Received: 01-Apr-2023 / Manuscript No. nnp-23-97047 / Editor assigned: 03-Apr-2023 / PreQC No. nnp-23-97047 / Reviewed: 17-Apr-2023 / QC No. nnp-23-97047 / Revised: 22-Apr-2023 / Manuscript No. nnp-23-97047 / Published Date: 29-Apr-2023 DOI: 10.4172/2572-4983.1000300
Introduction
Modified natural surfactants have been in clinical use for the treatment of respiratory distress syndrome (RDS) for about 30 years. [1,2] Replacement therapy with exogenous surfactants preparations dramatically reduced RDS related morbidity and mortality and became the most effective evidence-based therapy for RDS. In the first decade of replacement therapy, surfactant bolus administration during mechanical ventilation was the method of choice. In 1990s, continuous positive airway pressure (CPAP) became increasingly popular and small catheter surfactant delivery method was described [3]. However, it was believed that at least a short interval of positive pressure ventilation was needed to move surfactant from the trachea to the lung periphery, resulting in the development of the Intubate, Surfactant, Extubate to CPAP (INSURE) technique [4]. However, with INSURE, laryngoscopy and intubation with a standard large diameter endotracheal tube are performed accompanied by analgesia and sedation. Following surfactant bolus administration, the infants are either ventilated with bag-tube ventilation (BTV) and/or connected to a mechanical ventilator delivering gas volumes with the help of positive pressure at least for a short interval. With evolving management paradigms, CPAP became more popular for premature infants. However CPAP failure because of RDS especially in the most immature infants prompted search for a method for delivering surfactant in a less invasive manner [5]. This resulted in the development of the less invasive surfactant administration (LISA) technique with the aim to effectively provide an adequate dose of surfactant while the infant is breathing spontaneously with the support of CPAP [6,7]. Thus the INSURE technique was further modified to avoid even this brief positive pressure ventilation. Kribs et al. used minimally invasive surfactant therapy (MIST), also called less invasive surfactant administration (LISA) with the aim to prevent intubation for surfactant delivery [8]. This technique ensures continuous uninterrupted nasal CPAP throughout the entire procedure. Hence, temporary loss of functional lung capacity and atelectasis during the intubation can be prevented. Surfactant is administered into the trachea by direct laryngoscopy, via a thin tube (5 FG feeding tube) through the vocal cords, while the infant remains on nasal CPAP. After surfactant administration, the tube is immediately removed. LISA can avoid the need for sedation and tracheal intubation; and has shown promising results with reduced need for mechanical ventilation, improved oxygenation, and decreased duration of ventilation [8-10]. Hence this study is being undertaken to assess the feasibility of less invasive surfactant administration (LISA) in preterm neonates with RDS.
Aims and Objective
Does less invasive surfactant administration (LISA) in preterm neonates at 34 weeks and below period of gestation with RDS result in better short-term respiratory outcomes compared to neonates managed with InSurE or surfactant administration and continued ventilation? Here, aim is to determine short-term respiratory outcomes in preterm neonates at 34 weeks and below with RDS given intra-tracheal surfactant by LISA technique.
Primary objective
To determine the duration of respiratory support in hours.
Secondary objectives
The secondary objectives of this study will be as described below:-
To determine the required number of doses of surfactant per patient
To determine the failure of LISA technique
To determine the procedure success rate
To find out the incidence of preterm neonatal morbidities: BPD, PDA, IVH, ROP and NEC.
Materials and Method
This study was a prospective cohort study carried out at a level 3 Neonatal Intensive Care Unit (NICU) of a tertiary care hospital in Maharashtra. In this study, the data collected prospectively. The populations under study were neonates born at less than 34 weeks gestation diagnosed with RDS who required surfactant therapy for management of Respiratory Distress Syndrome admitted to NICU. The study was conducted during a period of 18 months from 1 Jan 2021 to 30 June 2022.
The preterm neonates of less than 34 weeks gestation with RDS requiring surfactant therapy were a cohort for this study. Within this group of preterm neonates (less than 34 weeks with RDS requiring surfactant therapy), one group of preterm with RDS received the surfactant by Less Invasive Surfactant Administration (LISA) technique and the other group received the surfactant therapy by Intubate- Surfactant Administration-Extubation (InSurE) technique. They were then followed up to evaluate the various outcomes of interest.
Delivery of surfactant by LISA technique was the exposure of interest. The participants (neonates less than 34 weeks with RDS requiring surfactant therapy) were divided into the LISA group or InSurE group based on the technique used to deliver/administer the surfactant. Participants were followed up during their stay in NICU to assess the outcome of interest namely the short term respiratory and other outcomes as already described above. Data was collected and variables likely to affect outcome were collected and evaluated including likely confounding factors like maternal co-morbidities, gestational age, weight mode of delivery etc. The data so collected was tabulated using Microsoft excel and analyzed for the outcome of interest in these study groups. Approval from the Institutional l ethical committee was obtained.
Inclusion Criteria
Preterm neonate ≤34 weeks of gestational age with moderate to severe degree of RDS requiring surfactant administration followed by respiratory support in the form of CPAP or other forms of ventilator support.
Exclusion Criteria
Preterm neonate ≤34 weeks of gestational age with mild RDS not requiring surfactant.
For LISA group (babies who required early intubation and continued ventilation).
Neonate with congenital anomalies.
The collected data were analyzed with SPSS statistics software version 20.0 (IBM SPSS Statistics). To describe about the data descriptive statistics frequency analysis, percentage analysis was used for categorical variables and the mean & SD were used for continuous variables. To find the significance in categorical data Chi-Square test was used, similarly if the expected cell frequency is less than 5 in 2×2 tables then the Fisher’s Exact was used. In both the above statistical tools the probability value .05 is considered as significant level.
Results
During the study period of 18 months from January 2021 to June 2022, a total 331 neonates were admitted in NICU of that 129 preterm neonates (< 34 weeks gestation with respiratory distress syndrome) were assessed for this study. Of the 129 preterm babies (< 34 weeks gestation with respiratory distress syndrome) admitted in NICU in the Tertiary care hospital during the study period, 69 neonates had mild RDS not requiring surfactant administration and were managed with CPAP only. These neonates were excluded from the study for not meeting the inclusion criteria (Preterm neonate ≤ 34 weeks of gestational age with moderate to severe degree of RDS requiring surfactant therapy). The remaining 60 neonates who fulfilled the inclusion criteria (gestational age < 34 weeks gestation with RDS) in whom surfactant therapy was administered were included in the study. Of the 60 preterm neonates, who were administered surfactant 20 fulfilled the inclusion criteria for surfactant administration by the LISA technique while, 40 neonates who were preterm less than 34 weeks but did not meet the criteria to be included in the group delivered surfactant (alveofact) by LISA, were delivered surfactant (alveofact) using the intubation- surfactant administration – extubation ( InSurE technique). The data collected was tabulated using Microsoft excel worksheet and the results were analysed for Clinico-epidemological characteristics and for outcome parameters (Tables A&B).
| Characteristics | InSurE | LISA | P value | ||
|---|---|---|---|---|---|
| 1. | Gender | n (%) | n (%) | 0.275 | |
| |
Male | 17 (57.50 %) | 12 (60.00 %) | ||
| Female | 23 (42.50 %) | 08 (40.00 %) | |||
| 2. | Birth weight | n (%) | n (%) | 0.317 | |
| <750 gm | 06 (15.00 %) | 00 (00.00 %) | |||
| 751-1000 gm | 11 (27.50 %) | 06 (30.00 %) | |||
| 1001-1250 gm | 09 (22.50 %) | 06 (30.00 %) | |||
| 1251-1500 gm | 04 (10.00 %) | 05 (25.00 %) | |||
| 1501-1750 gm | 05 (12.50 %) | 02 (10.00 %) | |||
| >1750 gm | 05 (12.50 %) | 01 (5.00 %) | |||
| 3. | Period of gestation | n (%) | n (%) | 0.073 | |
| < 26 weeks | 07 (17.50 %) | 00 (00.00 %) | |||
| 26-28 weeks | 07 (17.50 %) | 02 (10.00 %) | |||
| 29-31 weeks | 18 (45.00 %) | 13 (65.00 %) | |||
| 32-34 weeks | 08 (20.00 %) | 05 (25.00 %) | |||
| 4. | Growth status | n (%) | n (%) | 0.084 | |
| AGA | 33 (82.50 %) | 20 (100.0 %) | |||
| SGA | 07 (17.50 %) | 00 (00.00 %) | |||
| 5. | Mode of delivery | n (%) | n (%) | 0.014 | |
| NVD | 11 (27.50 %) | 12 (60.00 %) | |||
| LSCS | 29 (72.50 %) | 08 (40.00 %) | |||
| 6. | Antenatal steroid | n (%) | n (%) | 0.780 | |
| Complete course | 26 (65.00 %) | 12 (60.00 %) | |||
| Incomplete course | 14 (35.00 %) | 08 (40.00 %) | |||
| 7. | Probable cause of prematurity | n (%) | n (%) | 0.384 | |
| PPROM | 26 (7.50 %) | 17 (00.00 %) | |||
| Pre-eclampsia | 08 (65.00 %) | 03 (85.00 %) | |||
| Twins | 03 (20.00 %) | 00 (15.00 %) | |||
| Chorioamniotis | 03 (7.50 %) | 00 (00.00 %) | |||
| 8 | Downes score | n (%) | n (%) | 0.054 | |
| 04-06 | 24 (60.00 %) | 06 (30.00 %) | |||
| >06 | 16 (40.00 %) | 14 (70.00 %) | |||
Table A: Comparison of baseline characteristics of two groups.
| Parameters | InSurE | LISA | p-value | |
|---|---|---|---|---|
| Primary outcome | ||||
| 1. | Duration of respiratory support | n (%) | n (%) | 0.009 |
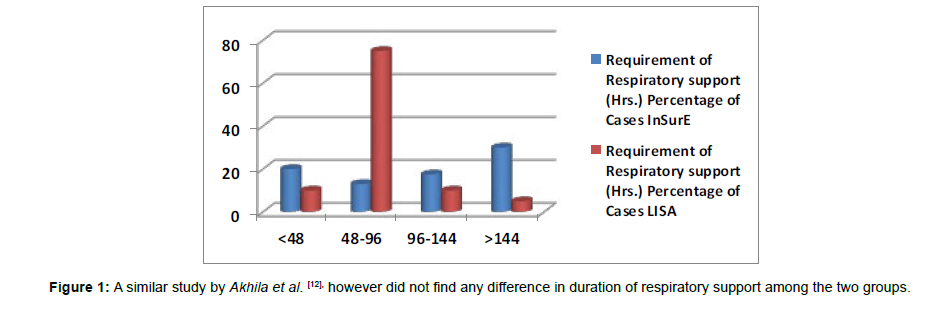
| <48 | 08 (20.00 %) | 02 (10.00 %) | ||
| 48-96 | 13 (32.50 %) | 15 (75.00 %) | ||
| 96-144 | 07 (17.50 %) | 02 (10.00 %) | ||
| >144 | 12 (30.00 %) | 01 (05.00 %) | ||
| Secondary outcome | ||||
| 2. | No. of surfactant Doses | n (%) | n (%) | 0.020 |
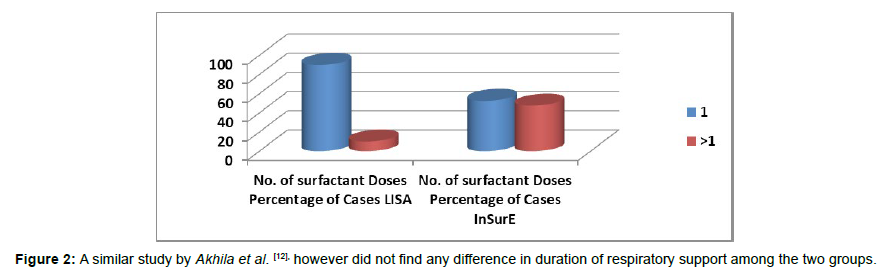
| 1 | 35 (87.50 %) | 12 (60.00 %) | ||
| > 1 | 05 (12.50 %) | 08 (40.00 %) | ||
| 3. | Nature Of Respiratory Support | n (%) | n (%) | 0.000 |
| CPAP | 18 (45.00 %) | 18 (90.00 %) | ||
| CPAP /MV | 22 (55.00 %) | 02 (10.00 %) | ||
| 4. | CPAP Requirement | n% | n % | 1.000 |
| 05-06 | 12 (30.00 %) | 07 (35.00 %) | ||
| >6 | 28 (70.00 %) | 13 (65.00 %) | ||
| 5. | FiO2 Requirement | n% | n% | 0.082 |
| <0.3 | 03 (07.50 %) | 00 (00.00 %) | ||
| 0.3-0.4 | 26 (65.00 %) | 14 (70.00 %) | ||
| >0.4 | 11 (27.50 %) | 06 (30.00 %) | ||
| 6. | Severity Of RDS | Frequency | Percent | 1.000 |
| Mild | 03 (07.50 %) | 01 (05.00 %) | ||
| Moderate | 17 (42.50 %) | 09 (45.00 %) | ||
| Severe | 20 (50.00 %) | 10 (50.00 %) | ||
| 7. | Neonatal morbidities | n (%) | n (%) | 0.218 |
| BPD | 8(20.00%) | 2(10.00%) | ||
| PDA | 6(15.00%) | 4(20.00%) | ||
| NEC | 2(5.00%) | 0(00.00%) | ||
| ROP | 5(12.50%) | 1(05.00%) | ||
| IVH | 0(00.00%) | 0(00.00%) | ||
| 8. | Duration of NICU stay | n (%) | n (%) | 0.002 |
| <20 | 03(7.5%) | 00(00.00%) | ||
| 20-40 | 09(22.5%) | 06(30.00%) | ||
| 41-60 | 26(65.00%) | 12(60.00%) | ||
| >60 | 2(5.00%) | 02(10.00%) | ||
Table B: Outcome parameters of infants with RDS after surfactant administration.
(Table 1) Duration of respiratory support The mean duration of respiratory support in the LISA group was 87 hours (SD=43.04) while in the InSurE group the mean duration of respiratory support was 187 hours (SD=230.97). The duration of respiratory support between the LISA and InSurE groups was statistically significant (p = 0.009). Similar study done by Jena et al. [11], observed a significant reduction in the need for MV in the MIST group (MIST: 19%, INSURE: 40%, p<0.01, risk ratio=0.49).
| Objective | Outcome Variables | Definition of Outcome Variables | Method of measurement |
|---|---|---|---|
| Duration of respiratory support in hours | Duration of nasal CPAP or non-invasive ventilation (NIV) in hours | Duration of respiratory support is duration of nasal CPAP or NIV after surfactant administration by LISA procedure | Hours (mean ± SD) |
Table 1:Primary outcome.
Correlation of birth weight, period of gestation and antenatal steroids on duration of respiratory support with mode of surfactant delivery
The multiple variables of birth weight, POG and antenatal steroids were analysed together for their statistical significance on the duration of respiratory support in both the LISA and the InSurE groups. Where, in the LISA group the multiple variables of birth weight (p=0.34), POG (p=0.29)and Antenatal steroid therapy(p=0.31) were not found to have a statistically significant influence on the duration of respiratory support required by the preterm neonate whereas, in the InSurE group, the variables of birth weight (p=0.002) and POG(p=0.003) were found to have a statistically significant (p<0.05) correlation with the duration of respiratory support required, while antenatal steroid therapy(p=0.99) was not found to have a statistically significant effect on the duration of respiratory support(p>0.05) in our study.
(Table 2) Number of doses of surfactant
| Objective | Outcome Variable | Definition of Outcome Variable | Method of Measurement |
|---|---|---|---|
| Required number of doses of surfactant per patient | Doses of surfactant | Number of repeat doses of surfactant required assessed every 6 to 8 hourly for a maximum of 3 doses | Numbers (%) Median (IQR) |
| Failure of LISA technique | LISA technique failure | Inability to oxygenate i.e. SpO2 < 92% on FiO2 0.7, presence of tachycardia or apnea necessitating intubation within first 48 hours | Yes/ No; Numbers (%); Relative Risk (RR) |
| Procedure Success Rate | Procedure success | LISA procedure completed satisfactorily in 2 attempts | Yes/ No; Numbers (%); |
| Incidence of preterm morbidities BPD, ROP, NEC, IVH,PDA |
BPD, ROP, NEC, IVH, PDA | Standard contemporary definitions as given in literature | Numbers (%); Relative Risk (RR) |
Table 2: Secondary outcomes.
Both the groups (LISA / InSurE) were also analyzed for the number of doses of Surfactant delivered for management of preterm with RDS. In the LISA group, the mean surfactant doses was 1.10 (SD=0.308) while, in the InSurE group the mean surfactant doses was 1.605 (SD=0.778). This difference was found to be statistically significant (p = 0.020). Akhila et al. in their study, [12] did not find any difference in doses of surfactant needed when they compared LISA and InSurE techniques. In a study by Kanmaz et al. [13], an almost equal percentage of both groups (about 20%) required the second dose of surfactant. In the study, by Aguar et al. [14] the infants in the MIST group received a 100 mg/kg dose of Curosurf and those in the InSurE group received a 200 mg/kg dose, and about 36% of the MIST (LISA) group and only 6.5% of the InSurE group needed the second dose of. From these, it was observed that initial dose of surfactant appears to dictate the need for a second administration of surfactant regardless of the technique used. In our study, preterms with severe respiratory distress that were intubated during delivery room resuscitation were excluded from the LISA group. This confounder could have resulted in statistically significant difference in the number of surfactant doses given to the preterm in these groups.
Failure of LISA Technique
Failure rate of LISA technique was 13.04 % (3/23). The failure rate could be due to reduced level of expertise in a relatively new procedure as compared with an older technique like InSurE (Figures 1and 2).
Figure 1: A similar study by Akhila et al. [12], however did not find any difference in duration of respiratory support among the two groups.
Figure 2: A similar study by Akhila et al. [12], however did not find any difference in duration of respiratory support among the two groups.
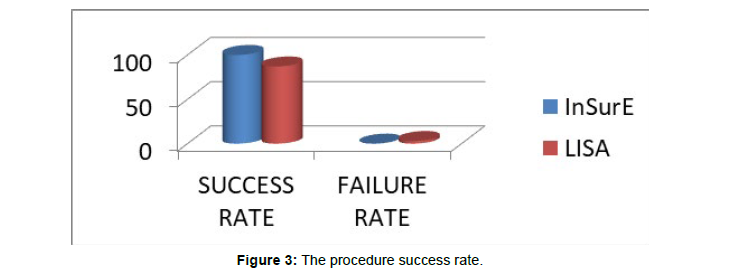
(Figure 3) The procedure success rate In our study we had a procedure success rate of 86.96% (20/23 cases) with the LISA technique while the success rate with InSurE technique was 100% (40/40).
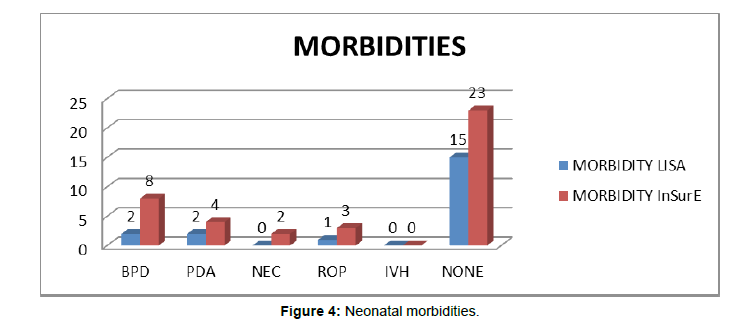
(Figure 4) Neonatal morbidities
In babies who received LISA 2 (10.00%) developed BPD; 2 (10.00%) neonates developed PDA, 1 (05.00%) developed ROP primarily; while, 15 (75%) were remained normal during the course of NICU stay, although most of the time other associated morbidities were also there in these neonates. In babies who received InSurE 8(20.00%) developed BPD primarily but other morbidities (PDA, ROP) in milder form were also developed, 4(10.00%) neonates developed PDA with other comorbidities, 2(5.00%) developed NEC with other comorbidities, 3(07.50%) developed ROP with other co morbidities, while 23(57.5%) were remained normal.
Other Significant Parameters
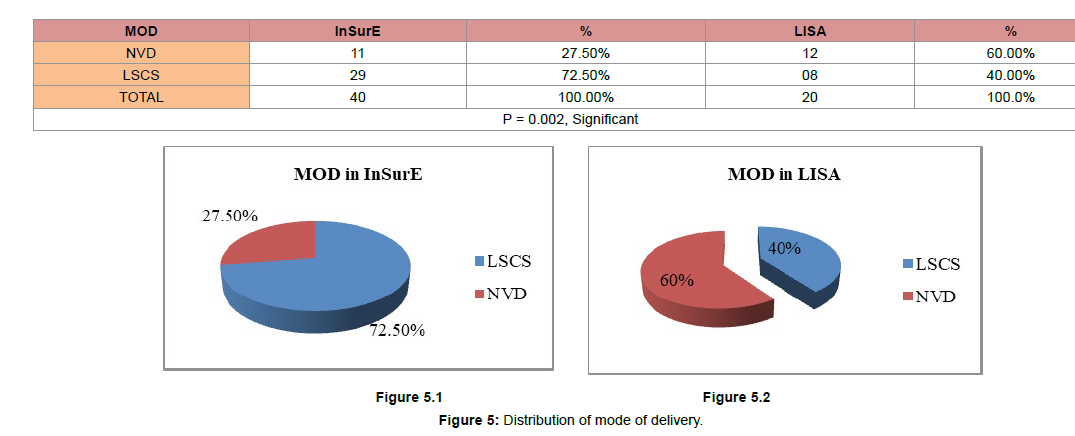
(Figure 5) Distribution of mode of delivery
In the LISA group, mode of delivery Normal Vaginal Delivery (NVD) were 12 (60.00%) while, LSCS delivery was 08 (40.00%).
Whereas, In InSurE group, NVD delivery mode was 11 (27.50%) and, LSCS mode of delivery 29 (72.50%).
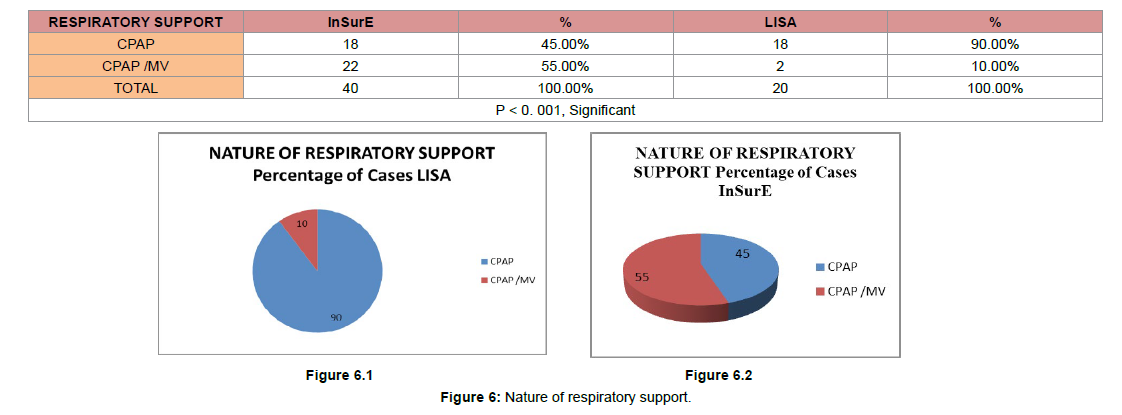
(Figure 6) Nature of respiratory support
In LISA groups only 2 (10.00%) neonates needed the respiratory support in form of mechanical ventilation and CPAP while 18 (90.00%) neonates needed the respiratory support in form of CPAP only. In InSurE groups, 22 (55.00%) neonates needed the respiratory support in form of mechanical ventilation and CPAP while 18 (45.00%) neonates needed the respiratory support in form of CPAP only.
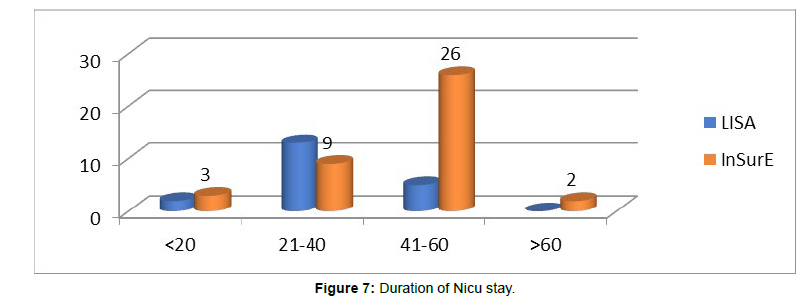
(Figure 7) Duration of Nicu stay
Duration of NICU stay was also a criterion for outcome related evaluation between the LISA and InSurE groups. The mean duration of stay in the LISA group was 29.90 (SD=11.192) while, in the InSurE group the mean duration of NICU stay was 44.40 days (SD=15.556). The variation in duration of NICU stay in the two groups was statistically significant (p=0.005). A study by Akhila et al. also came across similar statistically significant reduction in duration of NICU stay in the LISA group as compared with the InSurE group (p=0.017).
A study by Jena et al. [11], the NICU stay in the LISA and InSurE groups were 11 and 28days respectively (p<0.01). Also, came to similar conclusions showing reduced duration of NICU stay in preterms (11days) delivered surfactant by LISA method as compared with preterms delivered surfactant by InSurE method had a mean NICU stay of 28 days. A possible reason for reduction in NICU stay could be due to lesser lung injury and lesser oxygen support in the LISA group as compared with the InSurE group. In our study, exclusion of preterms requiring delivery room intubation from the LISA group could also have added to the difference in NICU stay between the two groups.
Discussion
In recent times LISA as a method for Surfactant delivery in neonates is being increasingly accepted. This study was carried out in a level 3 NICU of a tertiary care hospital in Maharashtra. This prospective cohort study was conducted to determine the short term respiratory outcomes in preterm neonates less than 34 weeks gestation with RDS who were given intra tracheal surfactant using the LISA method.
During the period of study from 01 Jan 2021 to 30 June 2022, a total of 129 preterm neonates under 34 weeks gestation were born who had features of varying severity of RDS. Of the 129 preterm neonates that were born at < 34 weeks gestation and had features of Respiratory Distress Syndrome during the study period, 60 required Surfactant therapy and were included in the study. Of the 60 preterm < 34 weeks with moderate/severe RDS who required surfactant therapy, 20 were given surfactant using the LISA technique, while 40 were administered surfactant using the InSurE technique based on inclusion and exclusion criteria for LISA technique which were clearly established prior to undertaking the study.03 preterm were switched from the LISA to the InSurE group due to failure of procedure.
The outcome parameters were compared with preterm < 34 weeks with RDS who were given surfactant therapy with the InSurE method.
The clinic-demographic parameters analysed included-
Gender
Gestational Age
Weight
Intrauterine growth(AGA/SGA)
Downe’s Score to assess severity of RDS
Antenatal Steroids therapy (Complete /Incomplete)
Underlying maternal Co-Morbidities (PPROM, PIH,Chorioamnionitis, Multiple pregnancy)
The parameters used to measure short term respiratory support and outcome included
FiO2 required
Requirement of ventilation (PEEP/ NIV/ SIMV)
Duration of ventilator support
Neonatal co -morbidities
Duration of NICU
In our study LISA was the initial choice for surfactant delivery in preterm neonates with RDS requiring Surfactant therapy, which satisfied the inclusion criteria. Those preterm with RDS who did not meet with the inclusion criteria for Less Invasive Surfactant Administration were delivered surfactant by InSurE method. The exclusion criteria were namely,
That requiring delivery room resuscitation intubation for resuscitation
Preterm with major congenital anomalies including tracheoesophageal fistula, Congenital Diaphragmatic Hernia etc
Preterm with upper airway abnormalities
Preterm above 34 weeks gestation
Failure of LISA procedure
We found that the Less Invasive method was feasible in 20 preterm neonates in our study group. Both groups were compared for the baseline variables. The analysis of the above clinic-demographic parameters did not reveal any statistically significant difference in the LISA and InSurE groups. We had 17 males (42.5%) and 23 females (57.5%) in InSurE group and 12 males (60%) and 8 females (40%) in the LISA group.
The mean birth weight in the LISA group was 1217 gms with a range of 897 gms and a SD of 266.79. In the InSurE group the mean weight was 1201.58 gms with a range of 1865 gm and SD = 449.54
The mean period of gestation noted in the LISA group was 206.65 days with a SD = 10.059. In the InSurE group the mean POG was 205.20 days with an SD of 17.502 and Range. Thus period of gestation is not a limiting factor in using LISA technique.
In the InSurE group 65 % mothers had received full course of steroids antenatal while 35% had received incomplete course of antenatal steroids while in the LISA group 60 % mothers had received full course of antenatal steroids while 40% had received incomplete doses of steroids
The underlying probable cause for prematurity such as PPROM, PIH, Multiple gestation and Chorioamnionitis in the two groups was analysed and not found to be statistically significant in our study.
The foetal growth status (AGA/SGA) was not statistically significant in the two groups.
Outcome variables were analysed in the two groups (LISA and InSurE) such as--
FiO2 required
Requirement of ventilation (PEEP/ NIV/ SIMV)
Duration of ventilator support
Number of surfactant doses
Neonatal co -morbidities
Duration of NICU stay
The LISA group was seen to require shorter duration of respiratory support. The mean duration of respiratory support in the LISA group was 87 hours (SD=43.04) while in the InSurE group the mean duration of respiratory support was 187 hours. In our study, preterms with severe respiratory distress that were intubated during delivery room resuscitation were excluded from the LISA group and this may have acted as a confounder for duration of subsequent respiratory support.
The mean Downe’s Score in the LISA group was 6.85 (SD=0.813) and in the InSurE group was 6.25 (SD=1.032) The Downe’s score at the time of selection for delivering surfactant was not found to be statistically significant in our study.
The mean FiO2 in the LISA group, was 43.75% (SD=10.867) while, in the InSurE group, the mean FiO2 required was 39.02% (SD=12.27). The difference in range of FiO2 was not seen to be statistically significant.
In our study, the mean PEEP in cm of water in the LISA group was 6.05 cm of water (SD=0.945) while, in the InSurE group, the mean PEEP in cm required was 6.13 cm of water (SD=0.966). This was not found to be statistically significant. Thus requirement of PEEP support was similar in both groups in our study.
Our study revealed statistically significant difference in the level of respiratory support required by the two groups (LISA / InSurE). We observed that, in InSurE group, 22 cases required Mechanical Ventilation (55%) while, 18 cases were managed on CPAP (45%). While in LISA group, 2 cases required Mechanical Ventilation (10%) while, 18 cases were managed on CPAP (90%).
The mean duration of respiratory support in the LISA group was 87 hours (SD=43.04) while in the InSurE group the mean duration of respiratory support was 187 hours. This difference was found to be statistically significant.
In the LISA group, the mean surfactant doses was 1.10 (SD=0.308) while, in the InSurE group the mean surfactant doses was 1.605 (SD=0.778). This difference was found to be statistically significant. However, exclusion of neonates with severe RDS who required intubation during resuscitation could have acted as a confounder while analysing this aspect. There was no difference overall in the occurrence of morbidities like- ROP,NEC, BPD and IVH between the two groups . The mean duration of NICU stay in the LISA group was 29.90 (SD=11.192) while, in the InSurE group the mean duration of NICU stay was 44.40 days (SD=15.556). This reduced duration of NICU stay in the LISA group in our study was found to be statistically significant.
Conclusions
This study says that difference in mean duration of respiratory support, the mean surfactant doses and mean duration of NICU stay were statistically significant between both groups while there was no difference overall in the occurrence of morbidities like- ROP,NEC, BPD and IVH between the two groups .
Our study compares a less invasive alternate method of surfactant administration (LISA) in preterm neonates with RDS with a standard practice and tested method of surfactant administration i.e. InSurE.
Our study, though conducted on a relatively small sample size, indicates that the less invasive method viz LISA is comparable with the more invasive but widely accepted practice of delivering surfactant by the InSurE method especially in the parameters assessing respiratory outcomes in preterm neonates who were administered surfactant for RDS.
Limitation
Part of the study period was during the COVID-19 pandemic thereby affecting the sample size. A larger sample size with equal number of participants would have improved the sensitivity of our study.
The study was conducted in a Level 3 NICU at a single centre. A multicenter study would have increased the sample size and improved the sensitivity of the study.
References
- More K, Sakhuja P, Shah PS (2014) Minimally invasive surfactant administration in preterm infants: a meta-narrative review. JAMA Pediatr 168: 901–908.
- Herting E (2013) Less Invasive Surfactant Administration (LISA) - ways to deliver surfactant inspontaneously breathing infants. Early Hum Dev 89:875–880.
- Verder H, Agertoft L, Albertsen P (1992) Surfactant treatment of newborn infants with respiratory distress syndrome primarily treated with nasal continuous positive air pressure. A pilot study. Ugeskr Laeger 154:2136–2139.
- Verder H, Robertson B, Greisen G (1992) Surfactant therapy and nasal continuous positive airway pressure for newborns with respiratory distress syndrome. Danish-Swedish Multicenter Study Group. N Engl J Med 331:1051–1055.
- Dargaville PA, Gerber A, Johansson S (2016) Australian and New Zealand Neonatal Network. Incidence and outcome of CPAP failure in preterm infants. Pediatrics 138: 985.
- Kribs A, Pillekamp F, Hunseler C (2007) Early administration of surfactant in spontaneous breathing with nCPAP: feasibility and outcome in extremely premature infants (postmenstrual age 27 weeks). Paediatr Anaesth 17:364–369.
- Herting E, Hartel C, Gopel W (2019) Less invasive surfactant administration (LISA): chances and limitations. Arch Dis Child Fetal Neonatal Ed 104: F655–F659.
- Kribs A, Vierzig A, Hunseler C, Eifinger F, Welzing L, et al. (2008) Early surfactant in spontaneously breathing with nCPAP in ELBW infants: a single centre four-year experience. Acta Paediatr 97:293-298.
- Dargaville PA, Aiyappan A, Cornelius A, Williams C, De Paoli AG (2011) Preliminary evaluation of a new technique of minimally invasive surfactant therapy. Arch Dis Child Fetal Neonatal Ed 96: 243-248.
- Dargaville PA, Aiyappan A, Kuschel CA, Kamlin CO, De Paoli AG, et al. (2013) Minimally invasive therapy in preterm infants on continuous positive airway pressure. Arch Dis Child Fetal Neonatal Ed 98: 122-126.
- Jena SR, Bains HS, Pandita A (2019) Surfactant therapy in premature babies: SurE or InSurE. Pediatric Pulmonology. 54(11): 1747-1752.
- Akhila A, Ghoshal B, Mahapatra CN (2021) A study on the comparison of minimally invasive surfactant therapy with insures technique of surfactant therapy in preterm babies with respiratory distress in a tertiary care hospital – A prospective cohort study. Indian J Child Health. 8(10): 362-366.
- Kanmaz HG, Erdeve O, Canpolat FE, Mutlu B, Dilmen U (2013) Surfactant administration via thin catheter during spontaneous breathing: Randomized controlled trial. Pediatrics 131(2): 502-509.
- Aguar M, Cernada M, Brugada M, Gimeno A, Gutierrez A, et al. (2014) Minimally invasive surfactant therapy with a gastric tube is as effective as the intubation, surfactant and extubation technique in preterm babies. Acta Paediatr 103: 229-233.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Bhondele SK, Tewari VV, Rajan AR (2023) A Prospective Cohort Studyon Less Invasive Surfactant Administration (Lisa) in Preterm Neonates at 34 Weeksand Below with Respiratory Distress Syndrome. Neonat Pediatr Med 9: 300. DOI: 10.4172/2572-4983.1000300
Copyright: © 2023 Bhondele SK. This is an open-access article distributed underthe terms of the Creative Commons Attribution License, which permits unrestricteduse, distribution, and reproduction in any medium, provided the original author andsource are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2475
- [From(publication date): 0-2023 - Dec 19, 2025]
- Breakdown by view type
- HTML page views: 2120
- PDF downloads: 355