Accuracy of Combined Tru Cut and FNAC in Preoperative Sampling of Ovarian Tumors
Received: 20-Mar-2014 / Accepted Date: 01-May-2014 / Published Date: 02-May-2014 DOI: 10.4172/2161-0681.1000168
Abstract
Background: Preoperative histologic confirmation of malignant ovarian tumors before chemotherapy is mandatory. There are few reports about combined use of tru cut and FNAC as methods of preoperative sampling of ovarian tumors.
Patients and methods: This is a prospective study conducted on 50 female patients presented with advanced ovarian tumors, during the period between 1/6/2011 and 31/10/2013. Trans-abdominal ultrasound guided tru cut biopsy and FNAC were performed for the patients at the same setting. The results were compared with the postoperative histopathology reports which considered as the gold standard.
Results: Of the 50 tru cut procedures; 46 cases (92%) revealed diagnostic tissue and 4 specimens were rendered non representative. The diagnostic accuracy of tru cut biopsy is 95.7% with sensitivity is 94.7% and specificity 100%. The diagnostic accuracy of FNAC is 95% with sensitivity is 94.3% and specificity 100%. The Positive predictive value is 100% for both techniques. The negative predictive value for tru cut and FNAC is 80% and 71.4% respectively. When tru cut was combined with FNAC the rate of inadequate samples have fallen to 0. The diagnostic accuracy of combined tru cut and FNAC was 95.5% with sensitivity is 94.9% and specificity 100%. The positive predictive value was 100% and the negative predictive value was 71.4%.
Conclusion: Both tru cut and FNAC are complementary to each other in achieving adequate samples for preoperative histologic diagnosis of ovarian tumors. Even if the diagnostic accuracy wasn't changed markedly, obtaining adequate samples can justify the beneficial combination of both techniques that will reduce the hazards and costs when a single technique has to be repeated to obtain sufficient tissues for diagnosis.
Keywords: Ovarian tumors; Tru cut; FNAC
313675Introduction
Worldwide, ovarian cancer is the sixth most common female cancer and the seventh most common cause of cancer deaths. There are about 204,000 new cases and 125,000 deaths annually [1]. Owing to the anatomic location of the ovary that make it inaccessible for screening, and the non-specific symptoms of its tumors, the majority of the patients present with advanced disease, making prognosis poor [2].
Introduction of neoadjuvant chemotherapy followed by interval debulking was found to improve the cytoreduction and reduce surgery related morbidity in such advanced cases. In addition, neoadjuvant chemotherapy is now gaining popularity in improving physical and emotional trauma associated with initial ovarian cancer treatment [3]. This highlighted the need for preoperative pathological diagnosis of ovarian tumors. Freedman et al. [4] reported the superiority for histology and/or cytology over clinical factors (CA125 and radiology) for diagnosis of epithelial ovarian tumors prior to neoadjuvant chemotherapy.
Image-guided percutaneous biopsy is the ultimate means of establishing the nature of a lesion demonstrated at imaging short of surgical biopsy and is often required in clinical practice. The ideal percutaneous biopsy should always provide sufficient tissue for a histological diagnosis with no discomfort to the patient, no complications, and as little use of resources as possible with respect to training of the operator, time required for the procedure, use of imaging modalities for guidance and disposable items [5]. On the other hand, Bandyopadhyay et al. [6], concluded from their study that ultrasound and CT guided Fine Needle Aspiration Cytology (FNAC) can be an optimum modality for the diagnosis of primary and metastatic ovarian neoplasms and evaluation of recurrent malignant tumors, which has great impact on patient management consequently.
To the best of our knowledge, there are few reports about combined use of tru cut and FNAC as methods of preoperative sampling of ovarian tumors. So, we conducted this prospective study.
Patients and Methods
This is a prospective study conducted on 50 female patients presented with ovarian tumor and submitted either to obstetrics and Gynecology Department or Oncology Center in Mansoura University Hospital, during the period between 1/6/2011 and 31/10/2013.
The patients were exposed to initial evaluation by history taking, general, abdominal and pelvic examination. Routine laboratory investigations were done. The diagnosis was confirmed by ultrasound, CT scan and/or MRI.
A written consent was obtained from all cases. The study was approved by the local ethical committee of Faculty of Medicine, Mansoura University.
Inclusion criteria
•Advanced ovarian malignancy (according to clinical and/or radiological criteria).
•Recurrent malignant ovarian tumor.
•Ovarian tumor of doughtful origin (primary or metastatic).
Exclusion criteria
•Early stage ovarian tumors (to avoid dissemination).
•Patients with coagulation disorders.
•Patients who were unfit for surgery.
Procedure
After patient consent, they are directed to ultrasound guided transabdominal needle biopsy. The procedure was performed by a radiologist either at Radiology department or by a gynecologist at Obstetrics and Gynecology department. A prior CT or MRI was checked for accurate localization of the site of introduction of the needle. Patients were lying in supine position. The skin at site of introduction of the needle was disinfected with alcohol, then 2% xylocaine was injected into the skin and subcutaneous tissue. An ultrashort incision is made using a scalpel tip. This help to minimize the patient discomfort at time of needle introduction.
The shortest puncture route is chosen. Real time monitoring is a major advantage as the exact location of the tip of the needle can be seen during biopsy that can allow adjusting its position to increase the accuracy of sampling. When the tip of the needle reaches the tumor site, the pistol is retracted so that tissue is sampled then the needle is gently removed. We used 16-18 guage needle was to obtain the tissue core which was immediately placed in 10% buffered formalin for fixation.
FNAC was obtained at the same time by using 22 guage spinal needle. When the needle position is satisfactory, the needle was rotated few times clockwise and anticlockwise around its longitudinal axis, a maneuver that help to detach cells near the needle tip. Then the stylet was removed and 10 ml disposable plastic syringe was connected to the hub with constant suction while the needle is gently removed in and out several times with 1 cm excursion, followed by withdrawal of the needle while maintaining suction on the plunger of syringe. Specimens were either immediately smeared or smeared after centrifugation on glass slides, fixed in 95% alcohol, and were stained by haematoxylin and eosin (H&E) stain.
Patients were closely observed in their wards for 120 minutes after the procedure. No antibiotic therapy was given. No major complications were encountered from the procedure.
The tissue core was processed into a paraffin block that was cut into 5um thick sections stained with H&E (by which a histopathologic diagnosis was achieved in all cases except for 3 cases). The results were compared with the postoperative histopathology reports which considered as the gold standard.
Statistical analysis
The statistical analysis of data was done using Excel program and SPSS (statistical package for social science) program (version 10, SPSS Inc., Chicago, IL, USA)). The description of data was done in form of mean (+/-) standard deviation for quantitative data and frequency & proportion for qualitative data.
The analysis of data was done to test statistical significant difference between groups. For quantitative data, student t-test was used to compare between 2 groups. One way ANOVA test was used to compare more than 2 groups. Chi square test and the two-tailed Fisher’s exact test was used to compare qualitative data.
Quantitative data were presented as number and percentage. Spearman correlation coefficient was used to calculate correlation between variables. Univariate regression analysis was done for estimation of predictors for tru cut and FNAC adequacy. P value was considered significant if it was = 0.05 at confidence interval 95%.
Results
The study was conducted prospectively on 50 cases having ovarian masses during the period from 1/6/2011 to 31/10/2013 (28 months). The mean age of our cases was 52.7 years, (range: 11-63 years). The mean BMI was 28.8 (range: 22-36). The criteria of ovarian tumors were summarized in Table 1.
| Laterality | Unilateral : 41 (82%) Bilateral: 9(18%) |
| Size | Range : 4-25 cm Mean : 11.66 cm |
| Ultrasound* | Solid 4 (8%) |
| Multilocular-solid (8%) | |
| Ascites 42 (84%) | |
| Prior CT or MRI | 35 with Prior CT or MRI (70%) 15 without Prior CT or MRI (30%) |
| CA125 | Range :11-876 u/ml Mean :2.034 u/ml |
| Surgical stage | Ic5 (10%) IIIc 36 (72%) Benign 9 (18%) |
| Ultrasound*: According to the International Ovarian Tumor Analysis (IOTA) group classification system | |
Table 1: Clinical characters of ovarian tumors
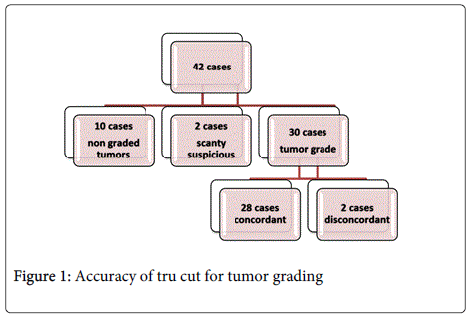
Considering tru cut adequacy, samples containing diagnostic tissue sufficient to perform IHC were available in 42 out of 50 cases (84%). Detailed data was presented in Figure 1.
In univariate regression analysis of factors affecting adequacy of tru cut biopsy; size of tumor and prior CT/MRI were significant positive predictors (p value: 0.0001 & 0.001 respectively). Age of patients, presence of ascites, and CA125 levels were not correlated to adequacy of the specimens (p value: 0.55, 0.17, and 0.48 respectively). Body Mass Index (BMI) was a negative predictor for adequacy in the current study.
The accuracy of tru cut biopsy as compared with the excisional biopsy was clarified in Table 2.
| Tru cut | Postoperative | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis | Number | Inflammatory | Benign | Borderline | HGS | LGS | Mucinous carcinoma | Endometroid carcinoma | Undifferentiated carcinoma | Fibroma | Yolk sac | Embryonal carcinoma | Krukenberg | Lymphoma | DSRCT |
| Inadequate | 4 | 1 | 1 | 1 | 1 | ||||||||||
| Suspicious | 2 | 2 | |||||||||||||
| Inflammatory | 1 | 1 | |||||||||||||
| Benign | 2 | 2 | |||||||||||||
| Borderline | 2 | 1 | 1 | ||||||||||||
| HGS | 16 | 16 | |||||||||||||
| LGS | 2 | 2 | |||||||||||||
| Mucinous carcinoma | 4 | 4 | |||||||||||||
| Endometroid | 1 | 1 | |||||||||||||
| Undifferentiated | 4 | 1 | 3 | ||||||||||||
| Fibroma | 6 | 1 | 5 | ||||||||||||
| Yolk sac | 1 | 1 | |||||||||||||
| Krukenberg | 2 | 2 | |||||||||||||
| Round cell tumor | 1 | 1 | |||||||||||||
| Desmoplastic Round cell tumor | 1 | 1 | |||||||||||||
| Negative for malignancy | 1 | 1 | |||||||||||||
| HGS: High Grade Serous LGS : Low Grade Serous DSRCT: DesmoplasticRound Cell Tumor |
|||||||||||||||
Table 2: Tru cut diagnosis versus final histopathology diagnosis
As can be noted; 4 cases were non representative (scanty tissues unfit for evaluation), such cases were excluded from statistical analysis. 8 cases were true negative for malignancy, 2 cases were false negative for malignancy, while 36 cases were true positive for malignancy.
No false positive cases were encountered in the present study.
Data considering diagnostic accuracy were highlighted in Table 3.
| Diagnostic accuracy | Sensitivity (95% CI) | Specificity (95% CI) | Negative predictive value (NPV) (95% CI) | Positive predictive value(PPV) (95% CI) | |
|---|---|---|---|---|---|
| Tru cut | 95.7% | 94.7 | 100 | 80 | 100 |
| FNAC | 95 | 94.3 | 100 | 71.4 | 100 |
| Combined tru cut and FNAC | 95.5 | 94.9 | 100 | 71.4 | 100 |
Table 3: Diagnostic accuracy of tru cut and FNAC
Concordance between tru cut biopsy and final histopathologic diagnosis regarding tumor type was statistically significant (p=0.045). Typing was available in 39 cases (92.9%). Totally of these 39 cases, 37 cases revealed tumor type concordant with the resection specimen (94.9%). It was possible to accurately diagnose 79.2% of serous tumors, 83.3% of mucinous tumors, and 100% of other tumor types.
Regarding accuracy of tru cut biopsy in detection of tumor grade; out of 42 cases true positive cases, 30 cases have tumor grade 28 cases concordant (93.3%) and 2 cases disconcordant. The details are demonstrated in Table 4.
| Tru Cut grading | Total | |||||
|---|---|---|---|---|---|---|
| Excluded | No grading | concordant | Disconcordant | |||
| Serous | 3 | 2 | 19 | 1 | 25 | |
| Mucinous | 1 | 0 | 5 | 1 | 7 | |
| Endometroid | 0 | 0 | 1 | 0 | 1 | |
| Undifferentiated | 0 | 0 | 3 | 0 | 3 | |
| Krungberg | 2 | 0 | 0 | 0 | 2 | |
| Fibroma | 6 | 0 | 0 | 0 | 6 | |
| Inflammatory | 1 | 0 | 0 | 0 | 1 | |
| Complicated cyst | 1 | 0 | 0 | 0 | 1 | |
| Embryonal carcinoma | 1 | 0 | 0 | 0 | 1 | |
| yolk sac | 1 | 0 | 0 | 0 | 1 | |
| Lymphoma | 1 | 0 | 0 | 0 | 1 | |
| Desmoplastic small round | 1 | 0 | 0 | 0 | 1 | |
| Total | 18 | 2 | 28 | 2 | 50 | |
| X2 = 4.075 P=0.04 | ||||||
Table 4: Accuracy of tru cut biopsy for tumor grading
Pitfalls in Tru Cut Diagnosis
High grade tumors with more undifferentiated areas may be source for discrepancy in tumor typing between tru cut biopsy and final histopathological diagnosis. Mixed tumors as sero-mucinous type whether benign or malignant may be a cause of discrepancy since the sampled area can contain one type only resulting in misdiagnosis as serous or mucinous tumor instead of mixed one.
Low grade serous and sero-mucinous carcinomas are sources for discrepancy in grading due to the presence of adjacent borderline focus that can be the only sampled area in tru cut biopsy
Regarding FNAC, Aspirates were available for 44 cases. 5 cases were hemorrhagic and 3 cases revealed scanty malignant epithelial cells so they were rendered suspicious. In 2 of these 3 cases papillary structures could be identified. Adequacy of FNAC was 81.8%. None of the predictor factors was statistically significant for FNAC adequacy.
Correlation between FNAC and final histopathological diagnosis was summarized in Table 2b. As can be seen 4 cases are inadequate (hemorrhagic), 2 cases are false negative, 5 cases are true negative, 33 are true positives. The diagnostic accuracy of FNAC was 95% with detailed data in Table 5.
| FNAC | Postoperative | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis | Number | Inflammatory | Hemorrhagic | Mucinous cystadenoma | HGS | LGS | Mucinous carcinoma | Endometroid carcinoma | Undifferentiated carcinoma | Fibroma | Yolk sac | Embryonal carcinoma | Krukenberg | Lymphoma | DSRCT |
| Haemorrhagic | 5 | 1 | 1 | 1 | 1 | 1 | |||||||||
| Suspicious | 3 | 3 | |||||||||||||
| Inflammatory | 5 | 1 | 1 | 1 | 2 | ||||||||||
| Benign cystic lesion | 1 | 1 | |||||||||||||
| Papillary serous carcinoma | 8 | 6 | 2 | ||||||||||||
| Malignant smear not specified | 17 | 11 | 3 | 1 | 2 | ||||||||||
| Embryonal carcinoma | 1 | 1 | |||||||||||||
| Mucinous carcinoma | 1 | 1 | |||||||||||||
| Krukenberg tumor | 1 | 1 | |||||||||||||
| Lymphoma | 1 | 1 | |||||||||||||
| Desmoplastic Round cell tumor | 1 | 1 | |||||||||||||
Table 5: FNAC diagnosis versus final histopathology diagnosis
Considering accuracy of FNAC for tumor typing, 33 cases were malignant on FNAC, among which, concordant typing with the final histopathological diagnosis was available in 13 cases only (39.4%) while 20 cases were diagnosed without typing (60.6%). The full data was presented in Table 6.
| FNAC_Typing | Total | |||||
|---|---|---|---|---|---|---|
| Excluded | concordant | No type specified | ||||
| Histotype | Serous | Count | 2 | 8 | 14 | 24 |
| % within histotype | 8.3% | 33.3% | 58.3% | 100.0% | ||
| Mucinous | Count | 2 | 1 | 4 | 7 | |
| % within histotype | 28.6% | 14.3% | 57.1% | 100.0% | ||
| Endometroid | Count | 0 | 0 | 1 | 1 | |
| % within histotype | .0% | .0% | 100.0% | 100.0% | ||
| undiffrentiated | Count | 1 | 0 | 2 | 3 | |
| % within histotype | 33.0% | .0% | 67.0% | 100.0% | ||
| Krungberg | Count | 0 | 1 | 0 | 1 | |
| % within histotype | .0% | 100.0% | .0% | 100.0% | ||
| Fibroma | Count | 2 | 0 | 0 | 2 | |
| % within histotype | 100.0% | .0% | .0% | 100.0% | ||
| Inflammatory | Count | 1 | 0 | 0 | 1 | |
| % within histotype | 100.0% | .0% | .0% | 100.0% | ||
| complicated cyst | Count | 1 | 0 | 0 | 1 | |
| % within histotype | 100.0% | .0% | .0% | 100.0% | ||
| embryonal carcinoma | Count | 0 | 1 | 0 | 1 | |
| % within histotype | .0% | 100.0% | .0% | 100.0% | ||
| yolk sac | Count | 1 | 0 | 0 | 1 | |
| % within histotype | 100.0% | .0% | .0% | 100.0% | ||
| Lymphoma | Count | 0 | 1 | 0 | 1 | |
| % within histotype | .0% | 100.0% | .0% | 100.0% | ||
| desmoplastic small round | Count | 0 | 1 | 0 | 1 | |
| % within histotype | .0% | 100.0% | .0% | 100.0% | ||
| Total | Count | 10 | 13 | 21 | 44 | |
| % within histotype | 22.7% | 29.5% | 47.7% | 100.0% | ||
Table 6: Accuracy of FNAC for tumor typing
Combined Tru Cut and FNAC Diagnosis
When tru cut was combined with FNAC the rate of inadequate samples have fallen to 0, resulting in 100% adequacy. This was due to the presence of adequate tru cut biopsies in cases with hemorrhagic FNACs and vice versa. On the other hand there were still 2 false negative cases, 5 true negative cases and 37 true positive cases. Table 5 shows that the diagnostic accuracy of combined tru cut and FNAC was 95.5% with sensitivity is 94.9% and specificity 100%. The positive predictive value was 100% and the negative predictive value was 71.4%.
Safety of tru cut and FNAC
No major complications like bleeding, shock, or intestinal injury were encountered from any of both techniques during the study. Only minor complications like pain and discomfort during the technique were encountered in some cases.
Discussion
Preoperative histologic diagnosis of ovarian tumors is essential for proper tailoring of treatment plan for each patient. There has been studies that were conducted on tru cut biopsy alone either in ovarian tumors as Zikan et al. [7], Faulkner et al. [8], and Fischerova et al. [9], in pelvic masses as Yarram et al. [10], or in peritoneal carcinomatosis as Spencer [11] and Hewitt et al. [12].
Considering the role of FNAC alone, it was addressed in more studies some of which were specific for ovarian masses including Bandyopadhyay et al. [6], Mehdi et al. [13], Sood et al. [14], Goel et al. [15], and Gupta et al. [16]. Others like Hemalatha et al. [17] studied the role of FNAC in abdomino-pelvic masses in general including ovarian masses as part of their studied population. Similarly, Guo et al. [18], Khan et al. [19], and Jha et al. [20] studied the role of FNAC in pelvic masses not addressing the ovaries specifically. Schwartz and Zheng [3] assessed the role of FNAC from ascites (not from ovarian mass) in pre treatement diagnosis of patients prior to neoadjuvant chemotherapy.
There are few reports about combined use of tru cut and FNAC as methods of preoperative sampling of ovarian tumors [4,21,22].
Our study was conducted prospectively on 50 female patients having ovarian masses that appear on ultrasound to be solid or heterogeneous aiming at investigating the diagnostic accuracy of tru cut biopsy and FNAC, each singly and in combination.
Although ascites and CA125 levels reflect large tumor loads and a larger tumor is easier to access, we didn't find them to achieve a statistically significant value to be considered as positive predictors for adequacy in our study. On the other hand, both parameters were positive predictors for adequacy in the study conducted by Zikan et al. [7] on 190 cases. This statistical difference can be attributed to the small sample size in our study. Instead size of the mass and presence of CT or MRI prior to the technique were our positive predictors in our study. These two parameters haven't been studied in other trials. CT or MRI prior to the technique allows accurate localization of the tumor and its solid component making it easier for radiologists to introduce the needle in shorter way and more targeting.
Moreover, BMI was a negative predictor for adequacy in our study this could be explained by the fact that obesity can hinder the performance of tru cut biopsy (especially by the abdominal approach). This again in contrast to the findings reported by Zikan et al. [7].
The high reliability and safety of tru cut biopsy as a minimally invasive method have been confirmed from the high diagnostic accuracy (95.7%), sensitivity (95.5%), and PPV (100%) that we have encountered in our study. In addition, our study didn't score any false positive diagnoses resulting in 100% specificity. On the other hand, presence of false negative cases has resulted in NPV of 60%.
The adequacy rate we have achieved for the tru cut biopsy (84%) was nearly similar to that reported by Faulkner et al. 8 that was (85.7%), higher than that of Larsen et al. [21] that was (78%), but lower than that reported by Zikan et al. [7] that was (91.3%), Malmstorm et al. [22] that was (88%), Fischerova et al. [9] that was (93.02%), Yarram et al. [10] that was (95.2%), and Spencer [11] that was (92%). Adding to differences in experience as well as the smaller number of cases enrolled in our study, we have adopted only the trans-abdominal approach for obtaining tru cut biopsy while Zikan et al. and Fischerova et al. [7,9] have adopted both trans-abdominal and transvaginal routes. A higher adequacy of transvaginal biopsies is probably due to the proximity of the biopsy lesion to the probe and a better capacity for guiding the biopsy probe more precisely into the vascularized vital parts of the tumor.
The relatively low adequacy rate and false negative diagnoses can be attributed to the new experience as well as presence of cystic degeneration and wide areas of necrosis particularly in high grade tumors which may be the cause of non-sampling of the tumor.
Despite being considered safe, effective and patient friendly procedure, FNAC was found to be slightly less than tru cut biopsy in adequacy (81.1%), diagnostic accuracy (95%), and sensitivity (94.3%). However, these slight differences between tru cut and FNAC were more intensified by the high accuracy for grading and typing in the tru cut biopsy that wasn't coupled with similar figures in FNAC.
On the other hand, combining both tru cut and FNAC has resulted in marked increase in the adequacy rate (100%) that can be explained by the fact that cases with non-representative biopsies for each needle were compensated for by the other one. Tru cut and FNAC were complementary to each other in providing sufficient diagnostic tissues. However, there wasn't improvement in the diagnostic accuracy that represented 95.5%, sensitivity (94.9), specificity (100%), PPV (100%), and NPV (71.4%).
We have achieved the highest sensitivity for tru cut when compared to the reported figures by Malmstorm et al. [22] that was (73%), Yarram et al. [10] that was (91.4%). However, these studies yielded 100% specificity as ours. The same is true regarding the PPV which was 100% in our study similar to results of Yarram et al., Larsen et al., and Malmstorm [10,21,22]. On the contrary our NPV was nearly similar to that of Yarram et al. (78.1%) but lower than that of Larsen et al. (94%). A difference that can be attributed to the false negative case we have encountered.
While Zikan et al. [7], Fischerova et al. [9], and Hewitt et al. [12] reported presence of complications from tru cut biopsy we didn't encounter any major complication. Since the technique was a new experience in our hospital associated with controversial acceptance from senior staff we have been meticulous in the choice of patients that can be candidate for the procedure.
Subtyping was available in 90.3% of cases obtained by tru cut biopsy in the current study with 97.4% concordance with the final histopathological diagnosis, an issue which haven't been discussed in any study apart from that of Freedman et al. [4], who reported less figures; 77% subtyping with 80% concordance.
We have discussed the accuracy of tru cut biopsy for tumor grading and we have reported it to be 93.3%, again this issue wasn't addressed in any of the previously mentioned studies.
Considering FNAC adequacy; Goel et al. [15] reported that it was (83.3%), Malmstrom [22] reported (86%), Larsen et al. [21] reported (75%), and Jha et al. [20] reported (77%) which were comparable to our result which was (81.1%) while Guo et al. [18] reported a higher value (97.1%). Higher values for FNAC adequacy reported by Guo et al. [18] may be due to the presence of cytopathologist and cytotechnologist attending the procedure for all studied population. This allowed preparing direct smears and rendered initial judgment of adequacy and preliminary diagnosis on air dried smears, so that inadequate smears can be repeated at the same setting to obtain sufficient ones.
On the other hand, the diagnostic accuracy for FNAC in our study was within the range reported by most of the studies like that of Bandyopadhyay et al. [6], Sood et al. [14], Freedman et al. [4], Hemalatha et al. [17], and Gupta et al. [16], that were (97.06%, 98%, 96.2%, 96.3%, 92% respectively). But it was slightly higher than that reported by Khan et al. [19] that was (89.9%) and Stewart et al. [23] that was (86.5%), and prominently higher than that reported by Mehdi et al. [13] that was (80.9%).
Sensitivity, specificity, PPV and NPV that the current study achieved were within the range reported by nearly all the studies addressing FNAC. Similarly, we didn't encounter complication from the procedure like all these studies.
Only Freedman et al. [4], and Sood et al. [14] discussed the accuracy of FNAC for tumor typing similar to our study. In both studies ability for estimation of tumor type (83.8% for Sood et al., and 55% for Freedman et al.) was higher than that in our study (39.4%). This may be due to the fact that most of our cases were surface epithelial tumors mostly high grade serous carcinomas. Typing on FNAC is mostly difficult in high grade tumors when compared to low grade ones. Differentiation of serous from endometroid carcinomas may be impossible on FNAC. Also fibromas are among the tumors that are mostly nontyped by FNAC. It may be of importance to mention that germ cell as well as sex cord stromal tumors namely granulosa cell tumor are more likely to be typed on FNAC than surface epithelial ones. A view supported by Mathur [24] and Gupta et al. [16]. In our study we didn't have cases diagnosed as sex cord stromal tumor. In addition, germ cell tumors were represented in our study by only two cases, one of which was accurately typed as embryonal carcinoma from cell block and the other one with final diagnosis of yolk sac tumor had a hemorrhagic aspirate.
When tru cut biopsy was combined to FNAC, the inadequate samples have vanished resulting in adequacy 100% similar to Larsen et al. [21], who found the marked reduction in the amount of inadequate samples to 4% when both techniques were combined. However, there wasn't significant change as regard diagnostic accuracy, sensitivity, specificity, PPV or NPV also similar to Larsen et al. [21] and Stewart et al. [23].
Results from the current study support the safety and beneficial use of tru cut biopsy as well as FNAC in preoperative diagnosis of ovarian tumors whenever indicated. Although tru cut biopsy was slightly more accurate and sensitive than FNAC, the difference wasn't significant. However, tru cut has the advantage of significant predictive power for tumor typing and grading. Histological subtype identification, in the emerging era of personalized medicine may be of relevance both for clinical trial design and for future decision making in clinical practice [4]. As proved from our study, both techniques are complementary to each other in achieving adequate samples.
Conclusion
Both tru cut and FNAC are complementary to each other in achieving adequate samples for preoperative histologic diagnosis of ovarian tumors. Even if the diagnostic accuracy wasn't changed markedly, obtaining adequate samples can justify the beneficial combination of both techniques that will reduce the hazards and costs when a single technique has to be repeated to obtain sufficient tissues for diagnosis.
References
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, et al. (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127: 2893-2917.
- van Niekerk CC, Bulten J, van Dijck JA, Verbeek AL (2011) Epithelial ovarian carcinoma types and the coexistence of ovarian tumor conditions. ISRN ObstetGynecol 2011: 784919.
- Schwartz PE, Zheng W (2003) Neoadjuvant chemotherapy for advanced ovarian cancer: the role of cytology in pretreatment diagnosis. GynecolOncol 90: 644-650.
- Freedman OC, Dodge J, Shaw P, Oza AM, Bernadini M, et al. (2010) Diagnosis of epithelial ovarian carcinoma prior to neoadjuvant chemotherapy. GynecolOncol 119: 22-25.
- Diederich S, Padge B, Vossas U, Hake R, Eidt S (2006) Application of a single needle type for all image-guided biopsies: results of 100 consecutive core biopsies in various organs using a novel tri-axial, end-cut needle. Cancer Imaging 6: 43-50.
- Bandyopadhyay A, Chakraborty J, Chowdhury AR, Bhattacharya A, Bhattachrya P, et al. (2012) Fine needle aspiration cytology of ovarian tumors with histological correlation. J Cytol 29: 35-40.
- Zikan M, Fischerova D, Pinkavova I, Dundr P, Cibula D (2010) Ultrasound-guided tru-cut biopsy of abdominal and pelvic tumors in gynecology. Ultrasound ObstetGynecol 36: 767-772.
- Faulkner RL, Mohiyiddeen L, McVey R, Kitchener HC (2005) Transvaginal biopsy in the diagnosis of ovarian cancer. BJOG 112: 991-993.
- Fischerova D, Cibula D, Dundr P, Zikan M, Calda P, et al. (2008) Ultrasound-guided tru-cut biopsy in the management of advanced abdomino-pelvic tumors. Int J Gynecol Cancer 18: 833-837.
- Yarram SG, Nghiem HV, Higgins E, Fox G, Nan B, et al. (2007) Evaluation of imaging-guided core biopsy of pelvic masses. AJR Am J Roentgenol 188: 1208-1211.
- Spencer JA (2005) A multidisciplinary approach to ovarian cancer at diagnosis. Br J Radiol 78 Spec No 2: S94-102.
- Hewitt MJ, Anderson K, Hall GD, Weston M, Hutson R, et al. (2007) Women with peritoneal carcinomatosis of unknown origin: Efficacy of image-guided biopsy to determine site-specific diagnosis. BJOG 114: 46-50.
- Mehdi G, Maheshwari V, Afzal S, Ansari HA, Ansari M (2010) Image-guided fine-needle aspiration cytology of ovarian tumors: An assessment of diagnostic efficacy. J Cytol 27: 91-95.
- Sood T, Handa U, Mohan H, Goel P (2010) Evaluation of aspiration cytology of ovarian masses with histopathological correlation. Cytopathology 21: 176-185.
- Goel S, Agarwal D, Goel N, Naim M, Khan T (2011) Ultrasound Guided Fine Needle Aspiration Cytology In Ovarian Neoplasms: An Assessment Of Diagnostic Accuracy And Efficacy And Role In Clinical Management. The Internet Journal of Pathology 11.
- Gupta N, Rajwanshi A, Dhaliwal LK, Khandelwal N, Dey P, et al. (2012) Fine needle aspiration cytology in ovarian lesions: an institutional experience of 584 cases. Cytopathology 23: 300-307.
- A L H, Sindhuram V S, S S, J K S, I V, et al. (2013) Ultrasound guided fnac of abdominal-pelvic masses-the pathologists' perspective. J ClinDiagn Res 7: 273-277.
- Guo Z, Kurtycz DF, De Las Casas LE, Hoerl HD (2001) Radiologically guided percutaneous fine needle aspiration biopsy of pelvic and retroperitoneal masses: a retrospective study of 68 cases. DiagnCytopathol 25:43-49.
- Khan N, Afroz N, Aqil B, Khan T, Ahmad I (2009) Neoplastic and nonneoplastic ovarian masses: Diagnosis on cytology. J Cytol 26: 129-133.
- Jha BM, Shah R, Patel J (2013) Effectiveness of image guided fine needle aspiration cytology in cases of deep seated lesions. Int J Med Sci Public Health 2: 439-442.
- Larsen T, Torp-Pedersen ST, Ottesen M, Bostofte E, Sehested M, et al. (1993) Abdominal ultrasound combined with histological and cytological fine needle biopsy of suspected ovarian tumors. Eur J ObstetGynecolReprodBiol 50: 203-209.
- Malmström H (1997) Fine-needle aspiration cytology versus core biopsies in the evaluation of recurrent gynecologic malignancies. GynecolOncol 65: 69-73.
- Stewart CJ, Coldewey J, Stewart IS (2002) Comparison of fine needle aspiration cytology and needle core biopsy in the diagnosis of radiologically detected abdominal lesions. J ClinPathol 55: 93-97.
- Mathur SR (2007) Ovarian cancer: role of cytology. Indian J Med PaediOncol 27: 5-6.
Citation: Naguib R, Hemida R, Wageh A, Elkhiary M, Shabana A, et al. (2014) Accuracy of Combined Tru cut and FNAC in Preoperative Sampling of Ovarian Tumors. J Clin Exp Pathol 4:168. DOI: 10.4172/2161-0681.1000168
Copyright: © 2014 Naguib R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 16478
- [From(publication date): 6-2014 - Jul 09, 2025]
- Breakdown by view type
- HTML page views: 11848
- PDF downloads: 4630