Acrylamide in Crisps (Reducing Acrylamide in Crisps)
Received: 24-Jan-2017 / Accepted Date: 20-Mar-2017 / Published Date: 26-Mar-2017
Abstract
The aim of the report is to understand what factors influence Maillard reactions and mechanisms involved in Maillard reaction, why acrylamide is toxicity, carcinogenetic and how does it cause cancer. To study this, we first understood how acrylamide is exactly formed and what levels of acryl amide in various food products. And then Characterization of several different technical solutions which I was studied in the scientific and patent literature and understood how they work and what extent they reduce acryl amide levels. Lastly, comparison of various several techniques and choose the best techniques that is suited most to reduce acrylamide levels in crisps was studied.
Keywords: Acrylamide; Crisps; Reaction; Maillard reaction; Consumer
76776Maillard Reaction
The name of Maillard Reaction is based on the French scientist Louis Camille Maillard. The reaction is a complex series of reactions between reducing sugars and amino acids, usually at increased temperatures.
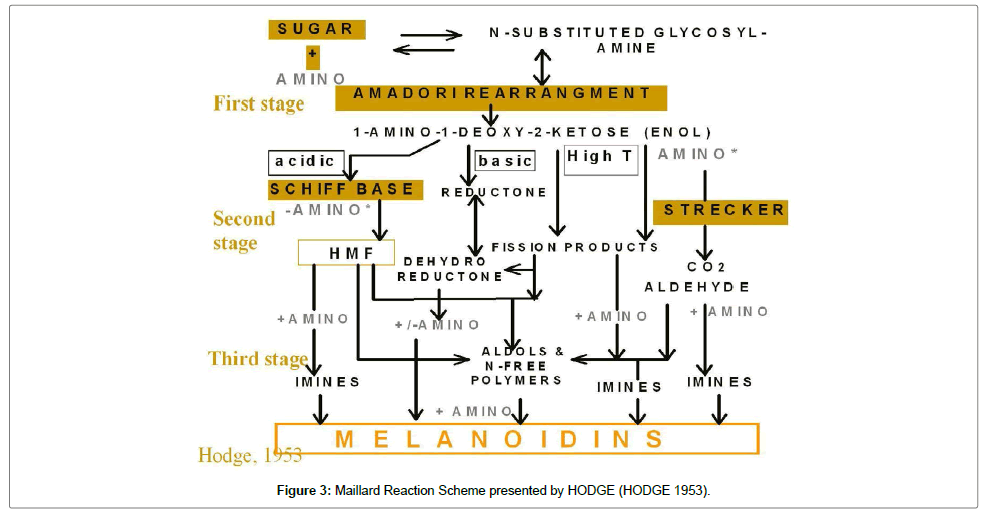
It is a very important reaction for frying, baking or heating of nearly all foods. Maillard reaction is responsible for the flavor of cakes, meat, beer, popcorn and cooked rice and also responsible for creating the baked food characteristics that people are familiar with and enjoy, like the brown color, smell, and flavor. They are quite important to the nutritional value of foods as many of its products, like anti-oxidants, can enhance it while other products can decrease it or even worse, prove to be harmful to the consumer. During the Maillard reaction, there are several compounds being formed by the condensation of reducing sugars like glucose, with amino acids like lysine and terminal amino acids. The product is an N-glycoside which usually rearranges to the Amadori rearrangement product (ARP) which in turn, degrades to another product depending on the pH of the system. This vague description of the products is a testament to the complexity of the Maillard reaction as it one of the most difficult reaction networks to control and characterize.
End-products of the Maillard Reaction may be also toxic or carcinogenic. One of the products of Maillard Reaction is acrylamide, a toxic compound only formed when a temperature at above 180°C, in baked or fried products (French fries). A temperature of frying below 180°C acrylamide is not formed in food.
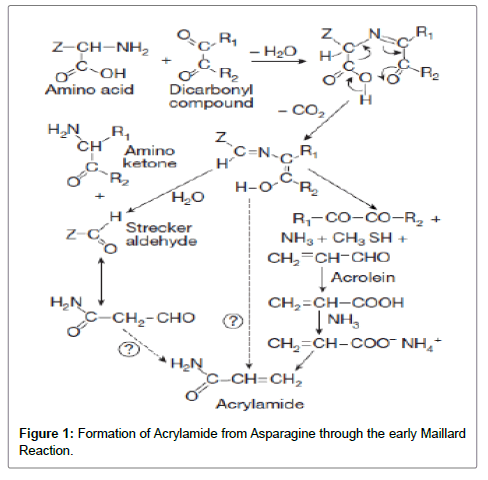
The first step of the Maillard reaction, the N-glycosides and their products like Amadori Rearrangement Product, then undergo so many different steps such as enolizations, condensations, Strecker degradations, cyclizations, dehydrations, retroaldolisations, rearrangements, and isomerizations. All of these reactions produce the nitrogen containing polymers and copolymers that produce that produce the roasted brown color in food called melanoidin. A specific reaction step would be the decarboxylation of the Schiff base that produces the Maillard intermediates that later on lead to acrylamide formation (Figure 1).
The most important factors that control which products are formed and when, is the temperature, the reaction time, the pH of the system, the levels of humidity and finally and most importantly, the nature of the food in process which defines the variety and type of the reducing sugars that take part in the Maillard reaction. Depending on the food process involved these parameters vary independent of the Maillard reaction products but when examining the formation of only one product, acrylamide, it is easier to specify how the reaction conditions affect its formation [1].
Acrylamide
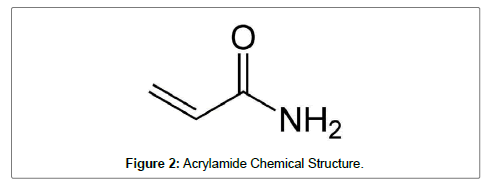
Acrylamide (prop-2-enamide) is useful chemical in the industry for making polymers and other products. It is a crystalline, white odorless solid, soluble in water that decomposes in the presence of acids, bases, oxidizing agents, and iron or iron salts. When heated it decomposes thermally to form carbon monoxide, carbon dioxide, and nitrogen oxides (Figure 2).
In 2002, A Scientific group at Sweden National Food Administration in cooperation with Stockholm university researchers were findings of acrylamide in baked and fried food products that cause massive concern in the food industry because acrylamide has been labeled as a potential carcinogenic. There are several studies with rats or mice that prove that acrylamide can act as a carcinogen in mammalian cells either with genotoxic, or clastogenic effects. These effects are largely caused by glycinamide, which is a bio-byproduct of acrylamide and it is a more potent carcinogenic [2].
Acrylamide is formed in fried and baked products through the Maillard reactions, but the exact mechanistic framework that produces the total amount of acrylamide detected has not been completely elucidated yet even though our understanding of it has grown over the past years [3].
The food products with the highest levels of acrylamide are potato chips, potato based products, and cereal. There are many different steps that take place, from the harvest of the raw materials to the end product and attempt to reduce the level of acrylamide can focus on different parts of the production. The most important part is when the product is baked or fried because the elevated temperature along with other conditions facilitates the Maillard reaction (Figure 3).
Carcinogenicity of acrylamide
Acrylamide has been classified as a potential carcinogen mostly because of studies was done on the laboratory scale that proves the carcinogenic effects of acrylamide intake in mammals. Direct correlation between acrylamide exposure and human cancer has not been established. Several epidemiologic studies have suggested the correlation with various types of cancer but it could not be proven in a concrete manner and there is quite a number of members of the scientific and medical community that are quite a skeptic about the validity of such studies [4].
To begin with, acrylamide chemically is reactive towards nucleophiles which in a cell could be found in the amino- or thiol groups of amino acids in proteins. Acrylamide reacts by a Michael addition to its double bond. Compared to other vinyl monomers it is less reactive but when it comes to its presence in a living organism even that reactivity can be harmful. Acrylamide has the potential to react with the N-terminal valine residue of hemoglobin, and these acrylamide hemoglobin adducts have been proven to be a very useful biomarker in experiments with animals and humans. Finally, acrylamide has been shown to bind to DNA and generate adducts with the ring nitrogen atoms and the amino groups of adenine and guanine DNA bases [5].
When examining the carcinogenic effects of a substance there is more to look at than its chemical reactivity. Glycidamide has been reported to be 100-1000 times more reactive with DNA than acrylamide and adducts with the purine bases of DNA have been observed in animal tissues. Acrylamide and its metabolites (like glycinamide) have been proven to cause both mutagenic and clastogenic events in mammalian cells. For example, it has been shown to induce chromosomal aberration in germ cells of mice and in somatic cells of rodents in vivo. Extensive research has produced results that show that more DNA adducts were formed by glycinamide treatment after than after acrylamide treatment at all doses tested and DNA adduct formation from acrylamide was saturable while the formation of most DNA adducts from glycinamide was dose dependent at the doses tested. This means that glycinamide is more mutagenic than acrylamide at any given dose and that the mutagenicity of acrylamide in human and mice cells is predicated on the capability of its metabolite, glycinamide, to form pro-mutagenic DNA adducts.
The clastogenic of acrylamide has been demonstrated in studies, where they showed micronucleus formation in the bone marrow of rats and mice. They used both hemoglobin adducts and micronucleus formation as biomarkers. These acrylamide- induced markers showed that in mice the effects are dose-dependent but no such trend was observed in rats. This difference indicates that rats may be less sensitive than mice to the effects of acrylamide that are caused by its metabolite glycinamide which agrees with the previous result, that showed that mice are more efficient in this harmful bio-conversion. The potent effects of glycinamide were equal, regardless of the method of administration to the animal which is a strong argument for the case that glycinamide is the most formidable clastogenic agent for mice treated with acrylamide [6-8].
Acrylamide formation
In food, acrylamide is formed via the reaction of asparagines and reducing sugars (both naturally occurring in potatoes)
• At the temperature higher than 120°C acrylamide is formed.
• The amount of acrylamide formed depends on:
• Temperature
• Cooking Time
• Amounts of reducing sugars and asparagines in the potato.
The main components are mainly responsible for acrylamide formation in cooked foods after condensation with reducing sugars or carbonyl source are amino acids and asparagines. Moreover, the sugarasparagines adduct, N-glycosylasparagine and then generates high amounts of acrylamide.
In addition, decarboxylated asparagines (3-amino propionamide), when heated, can also generate acrylamide in the absence of reducing sugars [9,10].
N-glycosides are favored in conditions of high temperature, elevated pH, and low humidity and on the contrary the N-glycosidic bond is more labile in the presence of water or acid and can be easily hydrolyzed. That the type of the reducing sugar and specifically the functional group in the β-position to the nitrogen atom play a very important role in the final acrylamide yield, highlighting the importance of the β-position on both sides of the nitrogen atom in carbonyl-asparagine condensation products [11].
Acrylamide Levels in Food
Below a table with data from the EU database is presented. The EU requested data from official and private food laboratories on acrylamide levels all over Europe. The data was collected between 2002 and 2006. The most data was collected for potato chips followed by baked good (biscuits, gingerbread) (Table 1).
| Food | Number of Records | Number of Accepted Records | Minimum (µ kg-1) | First Quartile (µkg-1) | Median (µkg-1) | Third Quartile (µkg-1) | Maximum (µkg-1) |
|---|---|---|---|---|---|---|---|
| Potato chips (French Fries) | 1666 | 1408 | 5 | 85 | 16 | 363 | 4653 |
| Bakery are, biscuits | 1459 | 1074 | 4 | 53 | 14 | 350 | 3324 |
| Gingerbread | 1131 | 1007 | 5 | 138 | 303 | 668 | 7834 |
| Potato crisps | 920 | 843 | 5 | 314 | 528 | 938 | 4215 |
| Cereals | 410 | 274 | 5 | 30 | 40 | 153 | 1649 |
| Potato pancake | 133 | 118 | 10 | 177 | 385 | 730 | 3072 |
| Bread and Toast | 313 | 151 | 5 | 15 | 50 | 133 | 1987 |
Table 1: Overview of the most abundant food items/food categories in the European Union database on AA levels in food.
Solutions
After the reviewed of Various Research journals and Patent application finally, I reached the stage where I select most appropriate Methods according to me for minimization and control of Acrylamide from Potato Crisps following methods are:
Storage conditions of potato cultivar: It is an important factor for the formation of acrylamide. But we know that acrylamide is never present in raw potato (before cooking or processing), asparagines and reducing sugar content both are different cultivars emphasizing the importance of the starting raw material on the acrylamide forming potential in later processing stages. Acrylamide formation is mainly depending on free asparagines and reducing sugars, the limiting factor being the sugar as asparagines are usually more abundant in potatoes than reducing sugars. The molar ratio of reducing sugars to asparagines content is greater than two, meaning that there is an abundance of reducing sugars than the asparagines content might be the limiting factor for acrylamide formation. This is a better indication of the right time of harvesting at the point of chemical maturity to reduce the potential of high acrylamide percentage formation during processing. If Storage of potato tubers in low temperatures (<8°C) causes an increase in reducing sugar content a phenomenon known as “lowtemperature sweetening”. This will increase result in an enhancement of the brown pigment during processing of chips and French fries and hence in higher acrylamide production. Potato tubers for chip processing should be stored at 8-12°C in order to avoid this increase in sugar content.
Cooking temperature and time effects: Acrylamide formation in potato chips has resulted that the major factors that contribute to the acrylamide production are frying temperatures and time. When the frying temperature is very high (180-190°C), the acrylamide formation levels increased at the end of frying. This was most likely due to the fact that acrylamide formation occurs mainly at the surface of potatoes when the temperature is likely to rise to >120°C when acrylamide formation is believed to form.
Effects of different frying oils: The frying oil could play the crucial role in the formation of acrylamide in French fries but their conclusions were not so good. In one study when was the six different oil types were examined it was shown that palm oil produces much higher acrylamide formation, compared to the different varieties of deep-frying oils. In another study, it was also found that olive oil shows higher formation of acrylamide compared to corn oil.
Use of additives: Amino acids addition has resulted as a mitigation strategy to reduce or low the levels of acrylamide in crisps, flatbreads, and bread crust, while glycine has particular attention as an additive that could potentially reduce or low acrylamide formation. Other amino acids including cysteine, glycine, alanine, lysine, glutamate and glutamic acid have been found to reduce acrylamide formation in heated potatoes. When the herb Rosemary (Rosemarinus Officinalis) added to olive oil as frying oil resulted in 25% reduction in acrylamide formation. Acid treatments most probably with ascorbic acid or citric acid resulted in most reductions of acrylamide in cooked potatoes. Due to the fact that acrylamide formation is minimized at low pH values (<pH 5).
Soaking and rinsing: For at least 15 minutes simple soaking and rinsing of potatoes before frying was found to be very profitable in reducing acrylamide up to 63%. This is an attributed to low or cut-out sugars and asparagines from the surface of potatoes. Another reduction in acrylamide was up to 75% can be examined by acidifying the soaking solution with vinegar (1:3 with water) and or citric acid (2%). The action of acids will lower the pH of the solution.
Technical Solutions for Reduction of Acrylamide
Another approach and focused on modifying the frying process for potatoes and potato chips. The introduction of low-vacuum frying has great potential as it pertains to acrylamide formation because of it is generally performed in lower temperature which is an invaluable asset when considering the temperature dependent behavior of acrylamide formation.
Various potato cultivars were tested, each having different acrylamide contents because of their different reducing sugar content. Some cultivars are better suited for frying while others are better for boiling yet in this experiment all cultivars were examined under the same conditions in order to provide a clear picture. The potatoes were fried in the traditional manner (165°C, 4 min) and with vacuum frying (118°C, 10 Torr, 8 min) and the acrylamide content were always lower in the vacuum fried fries than in the traditional fried ones. The amounts would vary depending on the cultivar of course but the comparison of the two ways of frying shows great potential for vacuum frying even with the evidence showing that there was a small increase in acrylamide content when the time of frying was extended.
Next, the quality characteristics of different potato chips produced with both frying methods were tested. Slight deviations between the products from the different frying processes were occasionally but not consistently observed and they are always dependent upon the particular potato cultivar. The color lightness, color yellow-blue chromaticity, and texture were examined and concluded that vacuum frying was capable of producing chips with desirable color and texture but much lower acrylamide content without a compromise to the flavor of the product.
Finally, the temperature dependence of acrylamide in potato chips was investigated. For traditional frying when ranging from 150°C to 180°C the higher the temperature the higher the acrylamide content and the same applies for the duration of the frying process. Compared to frying at 180°C the chips that were fried at 165°C and 150°C had 23% and 51% less acrylamide. In vacuum frying the temperature dependence is less profound because of the inherently lower temperature of the process. Compared to frying at 140°C the chips that were fried at 125°C and 118°C had 56%-63% less acrylamide [12-14].
When the two processes are compared the vacuum method is clearly a superior method for having a lower acrylamide content as it shown that it can produce up to 94% less acrylamide in the end product without any compromises to the sensory quality of the product.
Other researched solutions have been proposed as well. Investigated the effects of lowering the pH during the frying as a method to stop the formation of the Schiff base (the nucleophilic amine group –NH2 is converted to the non-nucleophilic protonated –NH3+) that leads to the formation of acrylamide. The acid used was the citric acid which is a well-known and safe additive and the concentrations tested were 0.1% and 0.2% which translated to a lowering of the pH by 1.2 and 1.5 units respectively. The results showed a 50% and 82% inhibition of acrylamide formation but in the case of 0.2% of citric acid a slight change in the color of the chips was observed. The same experiment, albeit with higher citric acid concentrations (0, 1% and 2%) was done with French fries instead of potato chips and similar results were obtained. The reduction of acrylamide by 25% was also observed when potatoes were soaked in distilled water. Unfortunately, the fries treated with 2% citric acid had a sour taste and harder texture indicating that for French fries the limit of acid to be used in this kind of process should not exceed 1%.
Explored the beneficial effects towards acrylamide reduction in potato chips of blanching (in hot water 85°C for 3.5 min), asparaginase immersion treatments (at 50°C for 20 min) and rinsing in distilled water. Potato slices were treated in several different ways:
a) Rinsing in distilled water,
b) Rinsing in distilled water and then blanching,
c) Rinsing in distilled water followed by immersion in asparaginase solution,
d) Rinsing in distilled water plus blanching plus immersion in asparaginase solution and rinsing in distilled water followed by blanching followed by immersion in distilled water.
Results showed immersion in asparaginase without any pretreatment was as effective as blanching, reducing the acrylamide content by 17%. The most effective process was blanching the potatoes before the asparaginase immersion which reduced the acrylamide content by almost 90%.
It is clear from this short description that a lot of ways have been suggested toward the reduction of acrylamide in heat treated food and in particular in potato chips. The most compelling aspect of this literature review is that all of these research projects have had the same goal, (to reduce the levels of acrylamide in cooked food) yet almost every solution proposed is unique and approaches a different aspect of the Maillard reaction mechanisms.
This fact might showcase the versatility and creativity of the scientist that worked on those projects but it also indicates the huge potential for combinatorial solutions that encompass the positive attributes of the individual solutions and hopefully make them work in an additive or even better in a synergistic manner. From the solutions presented thus far the most elegant one seems to be the low vacuum frying option but it has the disadvantage that it may not be compatible with the existing plant facilities meaning it would require the large investment in order to incorporate such a process to the existing one. Companies with factories that have the ability to incorporate this solution should adapt it as soon as they can [15].
Companies that do not possess the ability to adapt this solution should try and adapt a combinatorial solution by combining two of the suggested solutions that could potentially be employed simultaneously in the potato chip process. These are the dipping in a solution of divalent cations and the blanching of potatoes before being immersed in asparaginase. Each technique has a lot of potential in its own right as results have shown the reduction of acrylamide over 70% in both cases. One can assume with some certainty that blanching the potato chips in a solution of cations instead of water before immersing them in asparaginase will have even more pronounced results in the reduction of acrylamide levels. This certainty stems from the fact that there is no complicated mixing of overlaying solutions. It is as simple as replacing the water where the chips get blanched in with a properly mineralized water which does not raise the complexity of the process nor does it harbor any potential harmful effects.
Conclusions
The various strategies for the reduction of acrylamide in potato chips in a market, but here some most important techniques are in which I believe after did this report:
1. Selection of low content in reducing sugars and asparagines potato cultivars.
2. The concentrations of sugars are at a low level when the harvesting time at the end of the growing season.
3. Potato tubers should be stored at the temperature not lower than 8-12°C.
4. Avoidance of high (>180-190°C) frying and baking (>250°C) temperatures.
5. Avoidance of long frying and baking times. Use the browning of chips as an indicator of “doneness” of the frying process.
6. Mostly in an acidic environment soaking of the peeled potatoes, at least 15 minutes before the start of processing to remove the acrylamide percentage formation in potatoes.
7. Consider using some additives during frying like the herb Rosmarinus officinalis.
References
- Stadler RH, Blank I, Varga N, Robert F, Hau J, et al. (2002) Acrylamide from Maillard reaction products. Nature 419: 449-450.
- Food and Agriculture Organization of the United Nations. World Health Organization. Summary report of the sixty-fourth meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA).
- Mottram DS, Wedzicha BL, Dodson AT (2002) Acrylamide is formed in the Maillard reaction. Nature 419: 448-449.
- European Commission Recommendation of 3 May 2007 on the monitoring of acrylamide levels in food (2007/331/EC).
- Kita A, Brathen E, Knutsen SH, Wicklund T (2004) Effective ways of decreasing acrylamide content in potato crisps during processing. Journal of Agricultural and Food Chemistry 52: 7011-7016.
- Becalski A, Lau BPY, Lewis D, Seaman SW, Hayward S, et al. (2004) Acrylamide in french fries influence of free amino acids and sugars. J Agric and Food Chem 52: 3801-3806.
- Brem BS, Noti A, Grob K, Imhof D, Bazzocco D (2003) How much reducing sugar may potatoes contain to avoid excessive acrylamide formation during roasting and baking? Eur Food Res Technol 217: 369-373.
- Wilde DT, Meulenaer DB, Mestdagh F, Govaert Y, Vandeburie S, et al. (2005) Influence of storage practices on acrylamide formation during potato frying. J Agric and Food Chem 53: 6550-6557.
- Elmore JS, Koutsidis G, Dodson AT, Mottram DS (2005) Measurement of acrylamide and its precursors in potato, wheat and rye model systems. J Agric and Food Chem 53: 1286-1293.
- Erikson S (2005) Acrylamide in food products: Identification, formation and analytical methodology. Doctoral thesis, Stockholm University.
- Gertz C, Klostermann S (2002) Analysis of acrylamide and mechanisms of its formation in deep-fried products. Eur J Lipid Sci Technol 101: 762-771.
- Haase NU, Lindhauer MG (2005) Minimization strategies in potato food. Research Association of the German Food Industry Forschungskreis der Ernährungsindustrie eV (FEI).
- Isherwood FA (1973) Starch-sugar interconversion in Solanum tuberosm. Phytochem 12: 2579-2591.
- Kita A, Lisinska G (2005) The influence of oil type and frying temperatures on the texture and oil content of French fries. J Sci Food Agric 85: 2600-2604.
- Matthäus B, Haase NU, Vosmann K (2004) Factors affecting the concentration of acrylamide during deep fat frying of potatoes. Eur J Lipid Sci Technol 106: 793-801.
Citation: Chauhan R (2017) Acrylamide in Crisps (Reducing Acrylamide in Crisps). J Cell Sci Apo 1: 104.
Copyright: ©2017 Chauhan R. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 5876
- [From(publication date): 0-2017 - Aug 23, 2025]
- Breakdown by view type
- HTML page views: 4837
- PDF downloads: 1039